Review Article
Volume 1 Issue 1 - 2018
A reminder about covalent bonded quantum sized atomic silver clusters in an aqueous electric suspension and an effective biocide and able to enhance the immune system prior to penetration of the body by foreign antigens.
Independent Nanotechnology Professional Ipswich Australia 4305, Australia
*Corresponding Author: Hans Laroo, Independent Nanotechnology Professional Ipswich Australia 4305, Australia.
Received: August 06, 2018; Published: October 05, 2018
A reminder about covalent bonded quantum sized atomic silver clusters in an aqueous electric suspension and an effective biocide and able to enhance the immune system prior to penetration of the body by foreign antigens.
Preamble
Immunology is defined as that branch of medical science by the way the immune system of humans and animals produces antibodies in order to effectively deal with foreign antigens that enter the body and would otherwise cause disease. The way to apply such a strategy still needs to be worked out. One possible way of early intervention would be its use on the skin of burn victims.
The title of this paper accurately describes the subject matter “quantum sized silver”. Whilst there is no direct relationship with immunology, i.e. that part of biological medicine, concerned with immunity, nano silver is expected to be seen in a similar light as an antibiotic. Unfortunately when our own immune system fails, new alternatives must be found. The most likely candidate is electro-photochemically produced ‘reconstituted quantum silver’. It is an inorganic substance endowed with a property referred to as the “oligo-dynamic effect” and capable of destroying pathogens by its unpaired valence electron action similar to that of free radicals.
Abstract
Technically and scientifically, too much is wrong with how we express our understanding of so-called “Colloidal Silver” and the water it is contained in. Already silver and water being complex substances themselves become even more difficult to understand when their individual properties are combined. Nevertheless, it has proven its bio-efficacy in a number of ways, albeit only anecdotal so far. Presented here are some examples worth mentioning: One such case involved a horse suffering from a severely infected right eye. An antibiotic had already been administered. This however caused distress to the animal. The antibiotic did not help and the owner was considering putting the horse down. A spray of a low concentration of pure nano metre sized neutral silver (not ionic silver) into the infected eye for two days removed the infection and irritation altogether. Sometime later a dog, also with an infected eye was cured the same way. In April, 2017, a team at a local university proved in a clinical trial that all species of Enterococcus faecalis (in the mouth) were successfully eradicated. It was subsequently made public at a Medical convention held in Brisbane shortly after.
Research coupled with experimentation and augmented with downloading a large number of papers on the subject, has made it obvious that most scientists (in particular those involved in Wet Chemistry) have little or no concept of reconstituted nano silver. In this form it is no longer considered metallic. This ignorance, in both the physics and quantum physical aspects of nano silver is substantiated by the use of erroneous concepts and identities given to the substance such as: “colloidal silver, silver particles or just AGNPs.
With the exponential increase in drug resistance by pathogens due to antibiotics being overprescribed and overused, more of these antibiotics cease to be effective. This acquired immunity by pathogens is prompting science to seek alternative solutions. One of the most promising antidotes is nano metre sized (non-metallic) neutral atomic silver clusters suspended in water. It is able to kill pathogens indiscriminately by its unpaired valence electrons and its biocidal property referred to as the ‘oligo-dynamic effect’. It has this in common with the elements copper and gold. Each of these three metals have increased electrical conductivity properties due to having an unpaired valence electron in their outer shell. By scientific definition, atomic silver clusters qualify as a potent inorganic free radical. It is well known that the surfaces of copper, silver and gold have surfaces that are hostile to bacteria and some fungi. Silver also stands out with its unique photo-sensitive properties. To make full use of what silver has to offer, scientists and other researchers involved in clinical trials with nano silver, must also cease with their FOLLY of not operating to an acceptable standard when conducting clinical trials and no longer accept sample materials without a comprehensive description. To do otherwise would be counter-productive and meaningless. An equally serious shortcoming in the science of quantum characterised silver is the absence of suitable instrumentation for measuring parameters such as the Zeta potential, concentration and the application of quality control. Such a strategy is a must to enable adherence to the requirement for minimum inhibitory concentration (MIC).
This paper will demonstrate various strategies available to thorough research into the properties of neutral nano silver as an effective biocide and identity and determine material suitability as a means to surpass conventional antibiotics.
Key words: Pico and Nano metre sized atomic silver clusters; Ionic detection; Electro-photochemistry Quantum local plasmon resonances (LPR) and free radical action
Introduction
The science of immunology works both ways. First of all, there is the immunology provided by our own immune system. Unfortunately it sometimes fails and we will need help. Immunity can also be to our detriment when the immunity is owned by the drug-resistant species of bacteria when conventional antibiotics fail and pathogens have grown immune and resistant.
As indicated in the abstract, there has been far too much ignorance and complacency for decades. This has resulted in a progress, no standard to go by as a well as lack of knowledge as to what actually constitutes so-called colloidal silver. This is after some 80 or so years of experimentation. To this date there is little record and reference about any of the experiments in nano silver production and trials to go with that. That equates to: no real knowledge gained about scientific and technical facts learned. In particularly missing is a real description relating to the so-called colloidal silver, which incidentally is not a colloid. For all intents and purposes this lack of identification can be construed as a material that appears to not even exist. To understand this 'state of affairs’, it must be realised that many different ways have been sought and tried in order to produce the so-called colloidal silver. The variations in these attempts are too numerous to explain and only the most common production methods are listed as hereunder, each with a short description: [2-7].
Note! In most cases a small AC/DC power supply was used, i.e. mains voltage to 12 volt AC.
1. The electrolysis method. Two silver electrodes are inserted into a container filled with distilled water. The electrodes are subjected to an alternating current and occasionally a rectified DC version. Since water on its own impedes the flow of current, it requires salt in the water, hence electrolysis.
1. The electrolysis method. Two silver electrodes are inserted into a container filled with distilled water. The electrodes are subjected to an alternating current and occasionally a rectified DC version. Since water on its own impedes the flow of current, it requires salt in the water, hence electrolysis.
2. The electrochemical way. This is very similar to the method in 1, but for a severely restricted current flow at the onset and rising slowly over time.
Note! When the voltage potential on the electrodes exceeds 1.23 volt DC, the anode is stripped of its metallic atoms at a rate decided by the level of current in ampere per hour. In that process, the atoms thus removed lose their outer electron and become a positively charged cation. This cation is attracted by the cathode and if close enough will discharge itself by forming a neutral metallic atom or a compound once again. The problem however is the fact that the method used in 1 and 2 lacks any form of controlled conditions as explained further down.
3. High voltage ablation. Here the two electrodes, designated as cathode and anode and are here subjected to high voltages and in many cases by extra high tension voltages in potentials of 10 or even hundreds and thousands of volts and allowing the electrodes to touch each other and cause sparking. Like the other methods, ionic silver is produced.
4. Use of Nitric acid. Metallic silver is dissolved in nitric acid and converted into Silver nitrate (AgNO3), a positive cation that requires reduction into neutral silver (Ag°). One method of doing so is through the use of Sodium Borohydride, an alkaline substance. Incidentally, the process of converting silver into an ion is referred to as oxidation.
Note! What all of these production methods have in common is inconsistency and unpredictability as to its quality, i.e.:
(a) All atomic clusters that have been formed are all of all different sizes, perhaps ranging in sizes from 20 to 100nm. At very large dimensions, nano silver becomes useless. It is thought that it are the small clusters of silver measuring 10nm or less and possessing an astronomical ratio of surface to volume that makes for a potent biocide. Very often this material can be identified by presenting a yellowish tinge. It means that the concentration is too high and/or the clusters are too large and too numerous. The yellowish effect is created through the absence of the spectral colours violet and indigo, because silver absorbs these colours. The opposite occurs with the Raleigh effect, i.e. our blue sky!
(a) All atomic clusters that have been formed are all of all different sizes, perhaps ranging in sizes from 20 to 100nm. At very large dimensions, nano silver becomes useless. It is thought that it are the small clusters of silver measuring 10nm or less and possessing an astronomical ratio of surface to volume that makes for a potent biocide. Very often this material can be identified by presenting a yellowish tinge. It means that the concentration is too high and/or the clusters are too large and too numerous. The yellowish effect is created through the absence of the spectral colours violet and indigo, because silver absorbs these colours. The opposite occurs with the Raleigh effect, i.e. our blue sky!
(b) Not all silver has been reduced from its oxidative ionic state to a stable neutral state. More often or not, most of the silver will retain its ionic state. In method 1 for instance the added salt (Sodium chloride) and ionic silver (Ag+) will form a compound known as Silver chloride, a rather insoluble material and difficult to remove from the body if taken orally.
(c) Silver ions in the mix with suspended neutral silver can cause electrical breakdown. As a result, the entire mixture will aggregate into ever larger clusters. At some point complete breakdown occurs and the suspended nano silver will return to metallic silver at the bottom of the container.
5. The use of commercially produced finely pulverised metallic silver. The problem with this is the retention of its metallic state and unlike other electrochemically formed products. Even when dispersed in water, it remains a metal. Coupled with silver’s propensity to the oligo-dynamic effect, it has common with gold and copper, it will kill body cells as well as bacteria. Its use is not recommended.
Note! All five methods are considered to be in the realm of classical chemistry and in that way restricted to be controlled. Our introduction of ‘Electro-photochemistry’ has been found to offer a radical improvement over any other method of production. Its principle of production is explained as follows:
6. Electro-photochemistry. With this method, no classical chemistry is involved. Instead it is based on Physics and Quantum physics at that. Its main requirements for a predictable product are to use only controlled direct current for flow consistently. For the reduction to small atomic clusters in dimensions of 10nm and smaller, photonic light from a large number of LEDs radiating at 420nm are used. 420nm is used, as it possesses an ionising potential of around 2.95eV and able to reduce ionic silver to neutral. This is also a suitable wavelength as it is not impeded by the water through which it has to travel. Water at this wavelength becomes transparent and will not absorb the energy used. It is simply a way to produce quantum nano silver in a controlled fashion. That is provided that the production process is done in a refrigerator at a temperature between 4 and 100 Centigrade and no other light but the 420nm is available. Just a switch for on and off when the required concentration has been reached.
Working toward a detailed and acceptable standard
With no standard available and those involved in its research coping with less than a basic experience in what the properties of water suspended quantum nano silver clusters actually are, proper wording and descriptions to positively identify the material are not available either. To remedy this situation will require a whole new nomenclature for this particular science and associated technology. ‘Buzzwords’ like colloidal silver and AgNPs must be refrained from being used. First order of the day must include the introduction of a proper nomenclature, i.e. proper names and descriptions on anything relating to full and accurate use for describing all aspects of nano metre suspended silver. This will certainly rid the science and technology of confusing language and understanding as listed below:
With no standard available and those involved in its research coping with less than a basic experience in what the properties of water suspended quantum nano silver clusters actually are, proper wording and descriptions to positively identify the material are not available either. To remedy this situation will require a whole new nomenclature for this particular science and associated technology. ‘Buzzwords’ like colloidal silver and AgNPs must be refrained from being used. First order of the day must include the introduction of a proper nomenclature, i.e. proper names and descriptions on anything relating to full and accurate use for describing all aspects of nano metre suspended silver. This will certainly rid the science and technology of confusing language and understanding as listed below:
*The terms Colloidal silver, AGNPs etc when in fact nano silver is actually reconstituted non-metallic neutral atomic silver clusters (not particles) valence bonded and kept in a repelling negatively charged electrical suspension by a hydrophobic interfacial potential. It cannot be identified as a true colloid.
*Thinking that so-called colloidal silver is no different from ionic silver or even that both must exist together. As one manufacturer in the USA puts it: “Colloidal silver, guaranteed 100% ionic”.
*Thinking that the only methods available to a proper production is by either using silver dissolved in nitric acid, high voltage ablation, electrolysis or electro-chemistry.
*More often than not, other chemistry is added in an attempt to facilitate ionic reduction into neutral silver. Such is the case with Silver Nitrate (AgNO3). Nitric acid has a pH of 3.01, and the claimed reducing chemistry of Sodium borohydride with a pH of 14. Simply placing freshly made Silver nitrate in the sun for 10 minutes will do a better job. An even better way of accomplishing this task is through the use of physics such as electro-photochemistry. This method is very effective and economical yet generally not as well-known and understood as it should be.
*Testing of the product and its watery base is only done by a relatively few practitioners. The testing equipment itself, for some form of quality control is equally limited to the inappropriate use of instrumentation use such as a pH meter, a Conductance meter, a spectrophotometer and a mass spectrophotometer (for concentration). None of these instruments are suitable for the purpose of maintaining either a standard or quality control. Commercial instrumentation available claiming to be able to determine particle size and Zeta potential, albeit only collectively, are not considered up to the task of quality control either. The problem is exacerbated by the use of distilled water that is too low in its resistance to current flow and thus suffering from high conductivity.
*The inability or unwillingness to fully describe the properties of so-called colloidal silver and the many other details that can prove the material’s quantity and quality or not. In at least two cases a lack of description of the material was checked out and the material in question found to have been finely pulverised metallic silver instead of a more acceptable form of so-called colloidal silver. One was at Purdue University in the US. It was used in trials on the fathead minnow fish and subsequently reporting death and malformation of offspring (Sepulveda 2010). A second incidence in the concealed use of finely pulverised metallic silver (Kjeldsen 2014) reporting death of human cells in using such material. Their respective reports did not include a mention of what type of silver was actually used [3-81].
*The testing of water using antiquated and considered flawed instrumentation for measuring the conductivity of water. Instead of using current in ampere, resistance in ohms and the associated DC voltage potential as per Ohms Law for direct current, Alternating current at a variety of different frequency is used. The problem with the use of alternating current creates instrumentation inconsistencies between manufacturers. Impedance, also called “alternating current resistance”, changes with different frequencies. That being the case, a conductance meter operating at one frequency, for example 50Hz cannot be compared with the results obtained with an instrument operating at another frequency, i.e. 3,000Hz (3 kHz) [42-59].
Note! In most cases of determining the conditions of how things work, three parameters are used In Ohm’s Law. They are: current in (I), resistance in (R) and voltage potential in (E or V), i.e. at a resistance of 1 Ohm and a voltage potential of 1 volt, 1 ampere of current will flow. Change the value of one of the three, the other two will be effected. Change the stated resistance value from 1 to 2 Ohm and only half of the current will flow. If the 1 ohm resistance value is reduced to 0.5 Ohm, the current will be increased to 2 ampere. Many scientific principles have three elements like Ohm’s Law. Another such threesome is the relationship between the speed of light, wavelength and frequency. The speed of light is roughly 300,000 kilometres per second. Divide frequency over the speed of light and you get the wavelength, i.e. 30 MHz, a popular transmission frequency equates to 10 metres in wavelength.
*The questionable use of alternating current versus direct current: For a number of years I questioned this anomaly and was given reasons such as direct current causes polarization of the water molecules due to the dipolar character of water. The ultimate answer for the actual reason proved to be a technical tradition from the early years of 1900 when transformers were used for measurement. Direct current is unable to traverse through a transformer, only alternating current is able to do so. With the advent of sophisticated solid state technology since the 1960s it begs the question “why persist in old technology when a better and more precise direct current technology is available at a lower cost?” A further argument for the use of direct current comes from the early 1800s by an Englishman by the name of Michael Faraday. He was the first to determine the atomic weights of Hydrogen, Silver and Copper using direct current and formulated his First and Second Law of Electrolysis. He also gave us, by using very basic instrumentation, the equilibrium of water as 1.25 volts DC. Today with our sophisticated equipment it has been determined to be 1.23 volt DC. Faraday’s determination was only 0.02 out. It is said that Faraday produced the first Colloids and all of it using Direct Current. Why wet chemistry proponents still use versions of use alternating current instead of maintaining the ‘status Quo’ of the more accurate method of Direct Current, is a mystery [1-60].
Other aspects of Ohm’s Law that needs to be known when dealing with water
A. The parallel resistance factor
Water itself can be considered to have an infinite resistance being classed as di-electric and thus an insulator. The highest practical resistance used in Industry lies in the Tera Ohm region, i.e. 1012 Ohm. Some commercial solid state operational amplifiers boast an input level of 1.5 Tera Ohm. This enables these devices to measure extremely high resistances, i.e. Tera Ohms (1012 Ohm) as well as extremely low currents in the Pico ampere regions, i.e. (10-12 ampere/h). However it is debatable if water actually offers an electrical resistance, i.e. impede the flow of current as when we measure current in the water. Instead it are the positive ions (charge carriers) that we measure and resistance does not come into the equation. Nevertheless a thought experiment converted into a practical experiment referred to as “Testing the resistance of water without testing the resistance of water” did show that the parallel resistance factor of Ohm’s Law can be used in measuring some sort of resistance artefact. Parallel resistance can be explained by taking two identical resistors, both measuring 1,000 Ohm. If connected end to end in parallel, the combination will measure 500 Ohm, because the current (electron flow) will have a choice of two paths. If however one of the resistors has a lower resistance then the other, more current will flow through the lower value resistor. This is called “current hugging”.
A. The parallel resistance factor
Water itself can be considered to have an infinite resistance being classed as di-electric and thus an insulator. The highest practical resistance used in Industry lies in the Tera Ohm region, i.e. 1012 Ohm. Some commercial solid state operational amplifiers boast an input level of 1.5 Tera Ohm. This enables these devices to measure extremely high resistances, i.e. Tera Ohms (1012 Ohm) as well as extremely low currents in the Pico ampere regions, i.e. (10-12 ampere/h). However it is debatable if water actually offers an electrical resistance, i.e. impede the flow of current as when we measure current in the water. Instead it are the positive ions (charge carriers) that we measure and resistance does not come into the equation. Nevertheless a thought experiment converted into a practical experiment referred to as “Testing the resistance of water without testing the resistance of water” did show that the parallel resistance factor of Ohm’s Law can be used in measuring some sort of resistance artefact. Parallel resistance can be explained by taking two identical resistors, both measuring 1,000 Ohm. If connected end to end in parallel, the combination will measure 500 Ohm, because the current (electron flow) will have a choice of two paths. If however one of the resistors has a lower resistance then the other, more current will flow through the lower value resistor. This is called “current hugging”.
The experiment involved the following items:
- A 10 litre tank almost filled with deionised water and two partially submerged silver electrodes.
- A variable high Ohm potentiometer (resistance) of a 1 Million Ohm resistance.
- A high voltage combined with a low controlled and limited current DC power supply. 300 Volt and deliberately limited to 50 micro ampere full scale deflection.
- Two identical analogue panel meters rated at 50 micro ampere full scale deflection (FSD).
Both the electrodes in the tank as well as the potentiometer have the power supply in common and each a panel meter is in series connected. When power is applied to both systems it is likely to draw more current as compared to the other with less current. However by adjusting the variable resistor, a point is reached when the current through both system is equal and both panel meters show a half scale deflection at 25 micro ampere each. The resistance in this particular instance was 170,000 Ohm, just as the thought experiment had predicted. The water thus represents a resistance of 170,000 Ohm to the current flowing through it. Below is a picture of the experimental set-up and a composite picture of the comparisons between the settings of the parallel resistance factor:
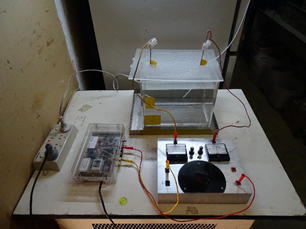
Figure 1: This show the entire set-up, i.e. The 300 Volts DC @ 50 micro ampere (limited) power supply unit to the left, the actual measuring instrument at the bottom right and a 10 litre tank filled with 9 litres of deionised water and containing two pure silver electrodes at the rear.
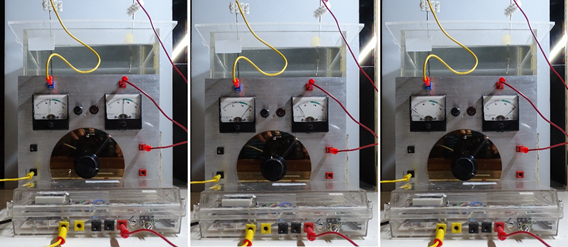
Figure 2: This shows the same instrument three times. Behind the instrument, the upper part of the tank filled with deionised water can be seen. The instrument itself contains two panel meters and a large dial plate housing a 1 million Ohm variable resistor, referred to as a potentiometer. In front of the instrument is the actual DC power supply providing 300 volts @ 50 micro ampere. The left panel meter represents the panel meter in series with the two submersed electrodes in the tank. The panel meter on the right is in series with the variable resistance. The picture on the far left shows an equal current in both panel meters at a half-scale deflection at 25 micro ampere, the picture in the middle shows a full scale deflection for the tank electrodes and the far right picture a maximum current of 50 micro ampere. Whenever current is biased in favour of one circuit, the other circuit will show less current. That is called current hugging. Since the variable resistance is ‘Not’ in the water, it can be said that “we are measuring the resistance of water without measuring the resistance of water”. In this particular case the measured resistance proved to be 170,000 ohm approximately shared parallel between the current flow between the electrodes and the variable potentiometer.
B. A variation of the parallel resistance factor known as ‘DC Input resistance’.
DC input resistance is the loading of a measuring device it presents to whatever it is measuring. For alternating current that name is Impedance or Z. For instance, if a multimeter, digital or analogue has an internal input resistance of 1 million Ohm and the particular quantity of water has the same resistance, i.e. 1 million Ohm, the actual measurement will give a result at 500,000 ohm. That is an error factor of 50%. A 10 million ohm would have a 10% error factor and a 100 million Ohm, a 1% error factor. For my experiments I use a self-built 10,000 million Ohm input resistance. Instrument. Compared to that, a vacuum tube voltmeter as used in the mid-1900s, had a maximum input resistance of 1,000 million Ohm, adequate for most purposes when repairing and testing Black & White television sets. An analogue based water conductivity measuring instrument boasting a DC input resistance of 10,000 million Ohm is shown hereunder:
DC input resistance is the loading of a measuring device it presents to whatever it is measuring. For alternating current that name is Impedance or Z. For instance, if a multimeter, digital or analogue has an internal input resistance of 1 million Ohm and the particular quantity of water has the same resistance, i.e. 1 million Ohm, the actual measurement will give a result at 500,000 ohm. That is an error factor of 50%. A 10 million ohm would have a 10% error factor and a 100 million Ohm, a 1% error factor. For my experiments I use a self-built 10,000 million Ohm input resistance. Instrument. Compared to that, a vacuum tube voltmeter as used in the mid-1900s, had a maximum input resistance of 1,000 million Ohm, adequate for most purposes when repairing and testing Black & White television sets. An analogue based water conductivity measuring instrument boasting a DC input resistance of 10,000 million Ohm is shown hereunder:

Figure 3: Analogue panel meter instrument in the background has a DC input resistance of 10,000 million Ohm and loads the water at just 1 volt DC. This loading is so small that a very high accuracy is obtained. There is almost no parallel resistance factor. Three jars are filled with deionised water in the jar on the left, commercially available distilled water in the jar in the middle and a jar filled with ordinary tap water on the right. The meter movement itself has a full scale deflection of 100 micro ampere and the readings obtained are 2.5 micro ampere for the deionised water, around 10 micro ampere for the distilled water (an 4 fold increase in conductivity) and a half scale deflection at around 50 micro ampere for the tap water. A 20 fold increase in conductivity. This instrument is also able to measure the conductivity of urine.
Proviso: Water molecules become unstable when voltage potentials placed in it exceed 1.23 Volt DC. Instruments used must not impose a voltage in excess of the equilibrium voltage of water on the water to avoid splitting up the water molecules. However, it is safe to use 1 volt DC for testing.
The use of Ohm’s Law and its associated measurements
Ohm’s Law, the brainchild of Georg (Simon) Ohm a German mathematics professor, was introduced in 1825. It originated when Ohm realised that direct current flow through a conductor is directly proportional to the potential difference (a DC voltage potential) and inversely proportional to the resistance of the circuit through which this current flows. His name is used to name the unit Ohm.
Ohm’s Law, the brainchild of Georg (Simon) Ohm a German mathematics professor, was introduced in 1825. It originated when Ohm realised that direct current flow through a conductor is directly proportional to the potential difference (a DC voltage potential) and inversely proportional to the resistance of the circuit through which this current flows. His name is used to name the unit Ohm.
Another German by the name of Ernst Werner Siemens, who founded the electrical and telecommunications company Siemens coined a standard of resistance for a length of copper telegraph cable. Out of experiments arising out of setting such a standard, the term ‘Siemens’ was adopted as the SI unit of electrical conductance (1816 to 1892).
Resistivity or Specific resistance
Is the electrical resistance between opposite faces of a 10mm x 10mm x 10mm cube, i.e. 1 cubic cm or cm3 of a given conductive material? It is the reciprocal of electrical conductivity.
Is the electrical resistance between opposite faces of a 10mm x 10mm x 10mm cube, i.e. 1 cubic cm or cm3 of a given conductive material? It is the reciprocal of electrical conductivity.
Ohm, Siemens and resistivity
These terms are directly related to the electrical properties of solid state conductors, in particular that of metallic conductors. It has nothing whatsoever to do with the properties of water and whatever its conductance may be it is unable to conform to the stated conditions nor be measured for its Ohmic resistance. It is still debatable if indeed water, a dielectric and an insulator, does possess a resistance, albeit a very high one and beyond the capability of most instruments. It is also beyond the reasoning of the Author how such values as Ohm, Conductivity in Siemens and Resistivity and its measurement per cm (10mm or cm2) can be introduced for measuring the conductivity of water. That something is amiss with the science of wet chemistry is evidenced by the disagreement on the actual resistance of water quoted as 18.24 million Ohm by one group and a 100 million Ohm by another.
These terms are directly related to the electrical properties of solid state conductors, in particular that of metallic conductors. It has nothing whatsoever to do with the properties of water and whatever its conductance may be it is unable to conform to the stated conditions nor be measured for its Ohmic resistance. It is still debatable if indeed water, a dielectric and an insulator, does possess a resistance, albeit a very high one and beyond the capability of most instruments. It is also beyond the reasoning of the Author how such values as Ohm, Conductivity in Siemens and Resistivity and its measurement per cm (10mm or cm2) can be introduced for measuring the conductivity of water. That something is amiss with the science of wet chemistry is evidenced by the disagreement on the actual resistance of water quoted as 18.24 million Ohm by one group and a 100 million Ohm by another.
According to a recent experiment by the Author, if there is a resistance of water, it will depend to a large extent to the volume of water and other factors such as the tank material and any other conducting media. For the experiment, a large Perspex tank was chosen measuring 1200x 300x 250mm and two electrodes placed 1000mm apart in 40 litres of deionised water with a conductance of 0.022micro Siemens. At 1,000mm distance the Ohmic resistance was 1,000 million Ohm and at 400mm distance it was 300 million Ohm. In each case the test result was compared with an actual 1% resistor of that value and found to be very similar. Using standard 10x10mm silver foils and mounted 10mm (1 cm) apart also measured 300 million Ohm. From this we can conclude what factors are influencing any electrical measurements of a tank of water:
- How it is measured, i.e. at a high or low input resistance and the loading factor involved.
- The actual quantity of the water.
- The size and shape of the tank.
- The material the tank is made of, And if there are conducting metals in close proximity.
- The input resistance of the instrument and its probe
- The contamination level of the water, i.e. the ionic content and uncharged carriers
- The fact that charge carrier flow in water does not flow in a straight line (linea recta) and is also subject to the lines of force [43-57].
Working to a standard
Unless a common standard is developed covering all aspects of any experimentation and trials, results cannot be compared with others. Not doing so will continue the existing chaos. One of these causing the most chaos is the inability of many producers to remove ionic silver content. The second serious flaw is a ‘mixed bag’ of cluster sizes that may range from 10nm to 200nm. The problem with large clusters is it would form the bulk of the concentration and a loss of effectiveness as a biocide.
Unless a common standard is developed covering all aspects of any experimentation and trials, results cannot be compared with others. Not doing so will continue the existing chaos. One of these causing the most chaos is the inability of many producers to remove ionic silver content. The second serious flaw is a ‘mixed bag’ of cluster sizes that may range from 10nm to 200nm. The problem with large clusters is it would form the bulk of the concentration and a loss of effectiveness as a biocide.
To initiate Nano silver production, the basic minimum information should include:
- A proven method of production such as the electro-photochemical process, whereby metallic silver is ionised and immediately reduced/neutralised by intense irradiation of violet light at around 420nm. At that wavelength water offers no impediment to its radiation. It works this way: metallic silver anode and cathode are partially immersed in water and subjected to a voltage potential high enough to allow a certain amount of current to flow. In the case of this new production method, a voltage potential of 300vdc and a current of 500 micro ampere is applied. Using this this particular voltage and controlled/ limited current removes silver atoms from the anode. In this process, the removed atom loses its outer electron and changes into a silver cation (ionic silver with a positive charge). It is attracted to the cathode that is also partially submerged in the water some distance away. In order to stop this from happening the anode and cathode are separated by a distance of no less than 200mm. The electron removed from the silver atom (negative charge) is now held captive by the water molecules with its hydrogen atoms orientating themselves around the intruding hydrated electron. The cation, also in the water is totally dissolved. A simultaneously applied intense irradiation of violet light at 420nm at an electron volt level at around 2.95 and one of the photons colliding with the hydrated electron, will impart its energy. It does so by forming a virtual particle named a photon-electron. A silver cation nearby (positive) accepts such a photon-electron (negative) and is reduced to a neutral silver atom once more. However the process of stability has not been established until it meets up immediately with another newly formed neutral silver atom. This pair of neutral silver atoms each donate the outer electron and form a dimer (two atoms). This valence bond so-made establishes long-term stability. The entire process of reduction by photonic action is often referred to as Photo-Electron Transfer. This method of production requires no more than switching it off when the required concentration is reached. The end result is nano metre sized atomic silver clusters, produced by quantum action in a range of 3 to 10nm with the bulk of the concentration between 5 and 7 nm.
- Atomic silver clusters so formed would be uniform in size within a narrow size distribution, i.e. between 3 and 10nm. The repelling action between the atomic clusters, valence-bonded, would present an equally distributed Zeta potential for good stability.
- Water quality must be adequate for the required current of 500 micro ampere at 300vdc to enable ionic silver formation at a suitable rate. A precise analogue based water purity tester with an input resistance of 10,000 million Ohm will be essential so as not to load the water and thus provide an accurate indication of the water purity. To also determine the extent of both uncharged organic and inorganic matter contamination, a light scattering cross polarization instrument will detect levels of contaminants at nano metre dimensions and also quantify same by measuring the level of obscurity in the water caused by any contamination by means of photo sensitive devices
- Water purity for electro-photochemistry must preferably be rated at 0.1 micro Siemens or better, the equivalent of 10 million Ohm resistivity. A slightly higher conductivity at 0.22 micro Siemens has nevertheless proven workable. Levels of hazardous substances such as lead and arsenic should not exceed 10ppb.
- Neutral atomic clusters produced must be small enough to offer the largest surface area ratio to volume possible. Experimentation has proven that the wavelength used, i.e. approximately 420 nm, produces cluster sizes from 3nm to 10nm. A singular wave length (Mono-chromatic) is bound to produce an even narrower band, perhaps 3nm instead of 7 nm. It is considered that the smaller clusters are able to move in closer to their targets and coupled by the quantum confined electrons they contain ensure a greater success eradicating any pathogens. It should be noted that a number of Fungi such a Candida Albicans also succumb to nano silver’s Oligo-dynamic properties.
- Establishing concentration and Minimum Inhibitory Concentration (MIC), the production of ionic silver and subsequently reconstituted to a neutral silver (no longer metallic) is determined by current over time (in ampere over hours, i.e. ampere/). Attempts have been made to calculate the concentration being produced at the applied current rate, but this has proven unsuccessful so far due to a number of factors which are presently unknown and mainly related to water. Since concentration and MIC are measured in ppm equal to mg/l, it would be prudent to either use 1 litre of water or fractions thereof. The smaller the quantity of water used the quicker the desired concentration or MIC is reached. According to tests conducted at Griffith University 3.3 ppm appeared to be an adequate concentration for the eradication of some pathogen (bacteria and fungi) ‘in vitro’. The use of larger quantities of water would produce only a small concentration over an extended period. However because of needing a specific distance between the anode and cathode to ensure COMPLETE REDUCTION by irradiation, too small a tank would fail in that endeavour.
Warning
There are a number of issues with neutral suspended silver that should be avoided at all costs. These are:
There are a number of issues with neutral suspended silver that should be avoided at all costs. These are:
- Not to apply so-called capping agents. The killing action of silver is the ‘Oligo-dynamic effect’, i.e. the action of electrons such as that of “FREE RADICALS”. Silver is an inorganic element that has been reconstituted to a new material that operates within the realm of Quantum Physics. Certain properties such as ‘not being subject to gravity’, Local Plasmon resonance and the Photo-electric effect are not found in other metals. Capping agents would only insulate silver’s ability to kill pathogens with its outer electrons.
- Do not add water to dilute the produced concentration so as to arrive at the desired MIC. Any additional water would not have gone through the process of the initial ionization and subsequent neutralisation of the silver and the actual ionisation of the water molecules when subjected to the 300 volts DC during production. Instead it would make more sense when arriving at the desired concentration to stop the production process. This way, batches can be made at 3ppm, 5ppm, 10ppm etc. The problem of this practice is that of determining the required concentration. Normally this could be measured on an instrument such as an Inductively Coupled Plasma Mass Spectrophotometer (ICP MS). Unfortunately it would not be able to distinguish between neutral and ionic silver. A relatively less costly electronic device under construction, using the principle of Capacitance Reactance is expected to do so better and more precise.
- Silver is a transitional metal that normally is not used by the body like iron and calcium are. During the transition to neutral atomic clusters in an electrical suspension in water it remains INORGANIC and unrelated to organic matter such as antibiotics and other chemistry. Using this silver in combination with anything at all, will have confusing outcomes. Initially this new silver material should be used on its own before trying it out with other matter. Even a combination of ionic and neutral silver would only provide tainted outcomes. The individual properties of both neutral and ionic silver AND organic matter are so different, they cannot possibly be compared.
- Do not expose neutral silver in water to ambient light, in particular SUNLIGHT as well as temperatures in excess of 15 degrees during use or storage. Also it should not be contained in containers made of soda glass for fear of leaching and contaminating the material with sodium. It will turn the liquid alkaline. Containers made of borosilicate glass or the plastic used for soft-drink bottles are both considered safe for long term storage. The plastic material mentioned is known as PET. This abbreviation stands for Polyethylene terephthalate.
- Do not ingest nano metre sized atomic silver clusters, i.e. ’in vivo’. Too little is known about this material. Most scientists don’t even possess a basic knowledge of quantum nano silver and only identify the product as AgNPs. Its use should be restricted to external uses only, i.e. ears, mouth, skin and some other orifices. It must not reach the blood system as there is a potential threat when very small nano silver clusters reach the brain via the blood supply causing possible electrical interference [19-39].
Instrumentation
Quality control on both the water and silver prior to and during production is the best course of action. It will ensure consistent production. Some of the most suitable instruments for this strategy are listed as follows:
Ionic conductivity meter based on Ohm’s Law using only direct current, resistance and voltage potential
- This instrument operates to the principles of Ohm’s Law by measuring current over time at a voltage level of 1 volt DC or less so as not to exceed the equilibrium voltage of water (1.23 volts DC) that would otherwise break-up the water molecules into Hydrogen and Oxygen gasses.
- It operates exclusively on direct current (DC) and at a very high input resistance of no less than 10,000 million Ohm. It is generally assumed that 1 micro Siemens equals 1 million Ohm and for 0.1 micro Siemens that would be 10 million Ohm. Using an analogue or digital multimeter with an input resistance of 10 million Ohm, the actual reading would be out by 50%. Increasing the input resistance to 100 million Ohm would provide a 10% accuracy and a 1,000 million (mega) Ohm a 1% accuracy. The instrument described offering a 10,000 million Ohm would than give a 0.1% accuracy. This would allow measurement of ionic content without the usual impediment found in most other instrumentation.
- The instrument is based on analogue principles and for most purposes, consistent at most ambient temperatures.
- Accurate readings are instantaneous and repeatable without much change.
- The probe uses very pure silver electrodes that do not corrode like many other electrodes do.
Ionic Conductivity Comparison instrument (parallel resistance factor)
- This instrument measures current flow in water between two partially submersed silver electrodes AND a variable resistor external to the tank. When the entire system is in balance, the two panel meters will each show half of the total current in the system. An out of balance system will also indicate the ‘current hugging’ phenomena.
- It is the only REAL ‘water resistance tester’.
Concentration tester (ppm indicator)
- This instrument uses an electronic bridge system to measure the principle of Capacitance Reactance in pico and nano Farad capacitance ranges from 10-12 and 10-9 Farad respectively. The smaller the quantity of neutral silver, the lower the Farad measurement. More neutral silver and thus a greater concentration in ppm or mg/l, the greater the reactance. If high enough measurements, readings will be in the high nano Farad range.
- The accuracy of the bridge operation will determine the accuracy of the concentration. Note! Few instruments are able to accurately determine concentration in the parts per million (ppm) and generally a Mass Spectrophotometer is used. However it has its short comings as well
A combined updated Nephelometer and Densitometer augmented with linear cross polarised light scattering
Note! Water generally contains a mixture of ionic (charged matter) and a combination of uncharged inorganic and organic matter that, if in sufficient quantity, will cause obstruction to light scattering (turbidity). This can be quantified by sensitive electro-optical means. Enhancing this methodology with linear cross polarization will enable a distinction to be made between materials in the water with a refractive index at visible wavelengths, i.e. between 700 and 400nm and material that does not. Silver has the highest reflectivity of all metals and only absorbs visible light at 420nm, Violet coloured spectral light. It works this way: when both the polarizer and analyser are in phase, light scattering is picked-up from all matter down to nano metre sizes. However when the controlling polarising filter is turned 90 degrees, only the scatter of light from the silver remains visible. The other matter’s scatter will simply disappear from sight.
Note! Water generally contains a mixture of ionic (charged matter) and a combination of uncharged inorganic and organic matter that, if in sufficient quantity, will cause obstruction to light scattering (turbidity). This can be quantified by sensitive electro-optical means. Enhancing this methodology with linear cross polarization will enable a distinction to be made between materials in the water with a refractive index at visible wavelengths, i.e. between 700 and 400nm and material that does not. Silver has the highest reflectivity of all metals and only absorbs visible light at 420nm, Violet coloured spectral light. It works this way: when both the polarizer and analyser are in phase, light scattering is picked-up from all matter down to nano metre sizes. However when the controlling polarising filter is turned 90 degrees, only the scatter of light from the silver remains visible. The other matter’s scatter will simply disappear from sight.
- The instrument also serves as an indicator of turbidity in the water. The higher the turbidity, the higher the contamination level. It is recommended to test all water water prior to nano silver production.
- The instrument also uses it photosensitive properties to determine levels of silver concentration. It will not be accurate but levels in production can be compared.
- The cross polarization part of the instrument can also be extended with a filter option. Introducing a violet light source and a violet pass filter at the absorption factor of silver will make the light scattering of silver disappear as well, proving that the scatter of light from the silver clusters is actually Silver.
- An instrument extended with a variable UV/VIS diffraction system, similar as a spectrophotometer, will enable other metals in the mix also to be identified by their wavelength absorption characteristics. For most metals however that will be in the ultra violet part of the spectrum except for gold, which is red-shifted toward the middle of the visible spectrum at around the spectral colour green at around 560nm.
Instruments for measuring Zeta potential
(a) It is doubtful that an analogue instrument exists capable of measuring the Zeta potential between single atoms or cluster of atoms suspended in water. A Zeta potential is described in the literature as a static electrical interfacial charge between the water and matter which has a hydrophobic relationship with the water it is suspended in. In the case of silver atomic nano sized clusters, the Zeta potential charge is zero at the Iso-electric point and a minus 100mV at its maximum. It is also said, that at around -30mv, the repelling charge is strong enough to negate the attractive forces of the ‘van der Waals’ force. A high Zeta potential is thus an indicator of stability. As stated, the Zeta potential is a potential between an individual particle, a molecule or atomic cluster and the water. Nano-particulate matter ranges from approximate 100nm (10-7) to 1nm (10-9). Diffraction limits the resolution of an optical microscope to about 200nm, a magnification of 1,500 times. Some instruments claim to measure Zeta potential in a collective way. It is questionable if a collective Zeta potential actually exists. However I am working on designing an instrument and probe that is able to measure the Zeta potential at a local concentration of silver clusters. The probe will need to be screened with a miniature Faraday cage, to stop other signals from interfering.
(a) It is doubtful that an analogue instrument exists capable of measuring the Zeta potential between single atoms or cluster of atoms suspended in water. A Zeta potential is described in the literature as a static electrical interfacial charge between the water and matter which has a hydrophobic relationship with the water it is suspended in. In the case of silver atomic nano sized clusters, the Zeta potential charge is zero at the Iso-electric point and a minus 100mV at its maximum. It is also said, that at around -30mv, the repelling charge is strong enough to negate the attractive forces of the ‘van der Waals’ force. A high Zeta potential is thus an indicator of stability. As stated, the Zeta potential is a potential between an individual particle, a molecule or atomic cluster and the water. Nano-particulate matter ranges from approximate 100nm (10-7) to 1nm (10-9). Diffraction limits the resolution of an optical microscope to about 200nm, a magnification of 1,500 times. Some instruments claim to measure Zeta potential in a collective way. It is questionable if a collective Zeta potential actually exists. However I am working on designing an instrument and probe that is able to measure the Zeta potential at a local concentration of silver clusters. The probe will need to be screened with a miniature Faraday cage, to stop other signals from interfering.
Measuring small currents at very high resistances
- It is now possible to measure resistances in the high Giga Ohms, i.e. 1,000 Million Ohm (109) to 100,000 million Ohm (1011) and currents as small as 1 nano ampere (10-9). A number of operational amplifiers (0p-Amps) with input resistances of 1 Tera Ohm and even 1.5 Tera Ohm (10s) are commercially available at relatively low cost. Instruments using such devices will enable other properties of nano metre sized silver to be identified as well.
- The significance of these seemingly extreme levels of current and resistances, is for the benefit of obtaining equally extreme precision in measurement for determining the properties of water. Just like measuring conductivity with a conductivity meter measuring micro Siemens and resistivity using the concept that high conductivity equates to low resistivity and high resistivity being the reciprocal of low conductivity. These high and low values work exactly the same way, albeit adding voltage potential to the equation. All inorganic and organic substances inhibit the flow of electrical current. Copper, silver and gold possess low resistances and are excellent conductors. Water, glass and plastics do not and are considered insulators. With water at least this opposition to current flow can be lowered with an applied voltage potential. Experiments substantiated with actual use has shown that at a DC voltage potential of 300 volts, a current of 500 micro ampere can flow. Increasing this voltage potential to 600 volts DC shows a three-fold increase to 1,500 micro ampere, i.e. 1.5mA.
- Under normal conditions the so-called resistance of water, which is not really a resistance but an opposition to charge carrier flow, i.e. ionic flow. This is in turn is determined by the contamination of the water and any ionic matter also contained therein, is very prone to being loaded by the input resistance factor of the measuring instrument [1-51].
Note! With most of the instruments designed for testing the so-called resistance of water do not provide details of their internal resistance factor and for that reason are not deemed appropriate for their intended use. I have an instrument under design and construction that should be able to measure ionic content, i.e. cations and anions totally independently and distinguishable from one another.
Quantum Nano silver in suspension and immunology
Many things in life present themselves as a friend or foe and often a balance needs to be struck between the benefits and any possible inherent risks. Silver is like that and so are antibiotics and chemotherapy. They may heal but also kill and often indiscriminately. It simply responds to conditions and circumstances at the time. It is all a question of balance and an ideal example of this would be immunity against pathogens invading the body or the reverse as presented by auto-immune diseases where the immune system attacks itself or perhaps some more severe than others. There is also the research done in parts of the British Isles where survivors of the bitter conditions of WW II developed Diabetes II decades later in life and spoken off as a result of an auto-immune disease [76- 88].
Many things in life present themselves as a friend or foe and often a balance needs to be struck between the benefits and any possible inherent risks. Silver is like that and so are antibiotics and chemotherapy. They may heal but also kill and often indiscriminately. It simply responds to conditions and circumstances at the time. It is all a question of balance and an ideal example of this would be immunity against pathogens invading the body or the reverse as presented by auto-immune diseases where the immune system attacks itself or perhaps some more severe than others. There is also the research done in parts of the British Isles where survivors of the bitter conditions of WW II developed Diabetes II decades later in life and spoken off as a result of an auto-immune disease [76- 88].
Free radicals
Free radicals are defined as molecules whose atoms contain an unpaired electron that can easily be removed due to oxidative effects. It is the referred to as a free radical in search of another electron forming part of a stable molecule that itself will become a free radical in search of an electron. After some extensive research and putting known information together, we can draw the conclusion that copper, silver and gold can most likely be considered as having characteristics of being a free radical. Some of the evidence that points that way are:
Free radicals are defined as molecules whose atoms contain an unpaired electron that can easily be removed due to oxidative effects. It is the referred to as a free radical in search of another electron forming part of a stable molecule that itself will become a free radical in search of an electron. After some extensive research and putting known information together, we can draw the conclusion that copper, silver and gold can most likely be considered as having characteristics of being a free radical. Some of the evidence that points that way are:
- Copper has an unpaired electron in its 4th outer electron shell, silver in its 5th and gold in its 6th outer shell. All three are claimed to be endowed with the oligo-dynamic effect.
- An oligo-dynamic effect (by an oligomer) ensures that bacteria and many other bacteria are unable to survive on a copper, silver and gold surfaces. All three metals are worn as ornamental jewellery on the hands and other body parts. Many older doors are fitted handles that are either brass or copper for the same reason.
- In particular silver, which possesses a very much heightened light sensitivity compared to other matter, cannot exist as a single neutral atom for long before returning into an ionic state. So when the opportunity avails itself, it will form a covalent bonded dimer, i.e. each unstable single neutral silver atom donates its unpaired valence electron to ensure relative stability.
- As ionic silver in an aqueous medium it will try and seek out neutrality by attaching itself to substances that have an excess electrons such as sodium chloride and forming silver chloride.
- On its own as a neutral silver atom or cluster of atoms all covalently bonded (in an aqueous medium) and considered hydrophobic will despite its apparent neutrality present an external highly negative charge. This was reported by Professor George Maass from the Colloidal Science Laboratory in the United States on several occasions. It is most likely formed by the Stern Layer, i.e. water molecules orientating themselves by polarization, i.e. hydrogen atoms (positive) turned inward toward the silver atoms and the Oxygen atom (negative) outward as described in the DLVO theory, Derjaquin, Landow, Verwey and Overbeek and the associated Double Layer Concept.
- Oxygen in particular has a tendency to promote free radical production such as the Hydroxyl radical (Lewis structure) which is claimed to contain an unpaired electron. Particularly in metabolism and exercise.
- Silver in any state appears to have Free Radical properties and that no doubt explains its biocidal qualities as well.
We are living in an age where many conventional medications and in particular antibiotics have outlived their usefulness. Many pathogens easily subdued with medicine in the past have learned how to protect themselves by becoming resistant due to over prescribed conventional antibiotics. Such is the case of certain strains of dangerous pathogens developing ‘biofilm’ protection. It simply forms a colony of dormant bacteria protected by a cocoon of simple sugars that the antibiotics cannot penetrate. Even if it could, the dormant colony would not respond, being more dead than alive. There have even been reports that other species are joining such colonies for protection. Other bacteria are changing their DNA to become less vulnerable at potent medication and even exchange their DNA. We must not forget that bacteria have been on this Earth much longer than we ‘Humans’ have.
With the failing of most antibiotics due to bacterial resistance, the race is on to introduce alternative strategies and new ways of dealing with this escalating problem, Quantum behaviour of nano metre sized atomic silver clusters may offer a viable and economical alternative. Nano sized silver at dimensions at 10nm and below possess surface area to volume that can be considered astronomical. Combined with the oligo-dynamic effect of easily catapulted electrons at very close quarter (the average bacteria is sized at around 1 micron = 1000 nano metres), such pathogens of whatever species will unlikely possess strategies to deal with an inorganic material like silver. Unfortunately, insufficient research has been conducted in the area of ‘in vivo’ clinical trials and only external body surfaces and ‘in vitro’ studies have been reported. The reason for this shortcoming has been the omission of a standard protocol in nomenclature, identifying the material used and safety and security features. It is the purpose of this short report to identify the shortcomings of current research. It is to seek remedy by determining many of its properties by setting a standard for its method of production. In addition the testing of relevant properties and other parameters as well as introducing an acceptable nomenclature. For too long Wet Chemistry has avoided taking the steps necessary to move into a new era of discovery and adopt a more direct approach to research and development of pico and nano metre sized atomic silver clusters for use as an effective biocide. This may provide a promise of effectively dealing with failing antibiotics and a broadband drug resistance of pathogens.
Although so-called Colloidal silver has been around for thousands of years, REAL research on this material and its properties has made no real progress. It is all very well to establish how many different species of bacteria it can kill and how fast, to determine the killing factor. It also requires at what Minimum Inhibitory Concentration (MIC) and by what mechanism this killing occurs. This has so far been ignored. Even now, no official attempts have been made to set a standard or instruments designed for conducting clinical trials ‘in Vivo’ in order to establish once and for all the ‘Friend or Foe’ factors in the character of nano silver. Until we do, PROGRESS will be slow or even cease. Much is at stake for mankind!
Conclusion
Water is an important and absolutely necessary substance used in a variety of ways in both biological chemistry trials and Physics experimentation. However it is also a substance often misunderstood on its most effective use. Critical areas are its use in vaccines, injections and other liquid medical preparations.
Note! Colloidal silver has been around for Centuries and much anecdotal evidence of its antiseptic properties have been recorded. Nevertheless, clinical research on reconstituted and water suspended silver has not made any real medical progress. It is all very well to establish how many hundreds of bacteria and even some fungi will succumb to such silver solutions ‘in vitro’, but without any research and accurate findings from clinical trials held ‘in vivo’, any real strategy during an epidemic is still far away. This also applies to the necessary equipment and instrumentation for the exercise of quality control, consistency and in particular concentration in ppm for the fulfilment of a Minimum Inhibitory Concentration (MIC) standards. More important still is the need to establish a ‘Friend or Foe’ character for such quantum sized silver and any side-effects there may be. It may however be one of the substances that is able to thwart a major catastrophe in the near future!
Further Readings
- Bacterial Paper Impregnated with Silver Nanoparticles for Point-of-Use water Treatment. Theresa A. Dankovich and Derek G Gray. McGill University, Montreal, QC Canada.
- Laurier L. Schramm Dictionary of Colloid and Interfacial Science. Petroleum Recovery Institute. An Institute of Alberta Research Council 2001. Published by Wiley-Interscience. ISBN 0-471-39406—8
- Oral Biofilm Architecture on Natural Teeth. Vincent Zijnge et al. Published Plos February 24, 2010.
- Bacterial biofilms: a diagnostic and therapeutic challenge. Christopher A. Fux et al. published by Future Drugs Ltd ISSN 1478-7210 Expert Rev. Anti-infect. Ther. 1 (4), 667-683 (2003).
- Ion channels enable bacterial electrical communication in bacterial communities. Arthur Prindle et al. Published in Nature 15709 doi: 10.1038. Macmillan Publishers 2015.
- Successful treatment of biofilm infections using shock waves combined with antibiotic therapy. Divya Prakash Gnanadhas et al. www. Nature.com/scientificreports.5:17440/DOI: 10.1038/srep 17440.
- Photoexcited quantum dots for killing multidrug-resistant bacteria. Colleen M. Courtney at al. published in Nature Materials (LETTERS) January 18, 2016 DOI: 10.1038.
References
- “NIST, Special Publication260-142, 2004 Ed. Standard Reference Materials: Primary Standards and Standard Reference Materials for Electrolytic Conductivity by R.H. Shreiner & K.W. Pratt”
- Press Release: February 23, 2017. “The plan to Avert our Post-Antibiotic Apocalypse” – The Atlantic, May 19, 2016. Under instructions from the then United Kingdom Prime Minister David Cameron, by Economist Jim O’Neil.
- “Popular nanoparticle causes toxicity in fish, study shows. Sepulveda and Geoff Laban on Fathead Minnow”.
- “An in vitro study on antimicrobial effect of metallic silver colloid against multispecies biofilm. Laurens Walsh et al. School of Dentistry presented at SDRI Solutions for Drug Resistant Infections Conference 2017, Brisbane, Queensland Australia”.
- “Enhanced antibiotic activity through complexation with metal ions: evaluated via ITC and biological studies. Chris Caboche, et al. School of Chemistry and Molecular Bioscience, University of Queensland, Brisbane, Queensland Australia”. (2017)
- “Depolarised light scattering from silver nanoparticles, by Zygmunt Cryczynski, et.al. University of North Texas, Fort Worth, TX76107, USA”.
- “Enhanced Vancomycin Bio-potency by Complexing with Silver by Zyta M. Ziora et al. Institute for Molecular Biosciences, University of Queensland. Brisbane, Queensland, Australia”.
- “An In vitro study of the antimicrobial activity of some endodontic medicaments against Enteroccus faecalis biofilms. B Athanassiadis et al. Private practice I Brisbane Queensland Australia”.
- “Properties of Ordinary Water-Substance, in all its phases: Water-vapor, Water, and all its Ices. Compiled by N. Ernest Dorsey, Physicist National Bureau of Standards, Washington, DC published by Reinhold Publishing Corporation New York USA 1940, Second Printing 1953”.
- “Quantum Mechanics by John L. Powell (University of Physics, Oregon) and Bernd Crasemann (Dept. of Physics, Oregon), published by Addison – Wesley Publishing Company, Inc, Third Printing May, 1965”.
- “Laboratory Manual for Queensland Sugar Mills, Division of Mill Technology, Bureau of Sugar Experiment Stations Queensland Australia”. (1939)0.
- R Das., et al. “Assam University, India. Preparation of Silver Nanoparticles and their Characterization June 17th”.
- Andrew J Frank., et al. “Wilfrid Laurier University Ontario, Canada. Synthesis of Silver Nano-prisms with Variable Size and Investigation of their Optical Properties: A First-Year Undergraduate Experiment Exploring Plasmonic Nanoparticles. Journal of Chemical Education (2010).
- Angela B Javurek., et al. “Various Universities and facilities. Gut Dysbiosis and Neurobehavioral Alterations in Rats Exposed to Silver Nanoparticles”. Published in Nature Scientific Reports 7:2822.
- N. LKHAGVAJAV I., et al. “Ege University Izmir, Turkey. Antimicrobial Activity of Colloidal silver Nanoparticles prepared by Sol-Gel Method. Digest Journal of Nanomaterials and Biostructures, 6.1 (2011): 149-154.
- T Linnert., et al. “Hahn-Meitner-Institut Berlin, FRG. Long-Lived Non-metallic Silver Clusters in Aqueous Solution: Preparation and Photolysis”. 1990 American Chemical Society.
- BG Ershov., et al. “Hahn-Meitner-Institut, Berlin, FRG. Silver Atoms and Clusters in Aqueous Solution: Absorption Spectra and the Particle Growth in the Absence of Stabilizing Ag+ Ions”. American Chemical Society (1993).
- A. Henglein., et al. Hahn-Meitner-Institut, Berlin Germany. Chemistry of Silver Aggregates in Aqueous Solution: Non-Metallic Oligomers and Metallic Particles. 1991. Electrochimica Acta, Vol. 36, No. 11/12, pp. 1743- 1745.
- Shelby Hatch and George Schatz, North-western University, Evenston, IL USA. Synthesis and Analysis of Silver/Gold Nanoparticles.
- Control the Morphology and Optical Properties of Silver Nanoparticles. Journal of American Chemical Society, 2010, 132, 1825-1827. 10.1021/ja910010b. JACS Communications.
- Hangxun Xu., “Water-Soluble Fluorescent Silver Nanoclusters”. ADVANCED MATERIALS 22(2010).
- Adele M Jones., et al. “University of New South Wales, Australia. Superoxide-Mediated Formation and Charging of Silver Nanoparticles". Environmental Science & Technology 45(2011):1428-1434. ACS Publications.
- Ying Chen et al, Northeast Normal University, China. Controlling nano colour and shape with pH adjustments. Nanotechnology 18, 2007 see www.physorg.com/news 105015736.html.
- Isabel Diez Zaragoza University (Spain) and Robin H.A. Ras Aalto University. Fluorescent Silver nanoclusters. Nanoscale, 2011, 3, 1963-1970.The Royal Society of Chemistry.
- J. Mocket al, University of California, San Diego. Shape effects in plasmon resonance of individual colloidal silver nanoparticles. American Institute of Physics 2002.
- Linglu Yang et al. Boston University, Massachusetts, USA. Calibration of Silver Plasmon Rulers in the 1-25nm Separation Range: Experimental Indications of Distinct Plasmon Coupling regimes. Journal of Physical Chemistry C Nanometre Interfaces 114.1 (2010): 4901-4908.
- R Kostecki. “Augustynski, University of Geneva, CH-1211, Switzerland. Photon-driven reduction reactions on Silver”. Journal of Applied Electrochemistry 23 (1993): 567-572.
- Michael A. Duncan and Dennis H. Rouvray, Micro-clusters, Scientific American December 1989 pages 60-65.
- Audrey Moores, Yale University New Haven CT and Frederic Goettmann, Max-Planck Institutes for Colloids and Interfaces. The plasmon band in noble metal nanoparticles: an introduction to theory and applications. Journal of The Royal Society of Chemistry and the Centre National de la Recherce Scientifique 2006. New J. Chem, 2006, 30, pages 1121-1132
- F.Javier Garcia De Abajo, the Instituto de Quimica Fisica "Rocasolano, Madrid, Spain. PLASMONS GO QUANTUM, Nature Vol. 483, March 22. Macmillan Publishers Ltd.
- Igor Sevonkaev et al, Clarkson University, Potsdam, NY, USA. Distribution of density in spherical colloidal particles by transmission electron microscopy. Colloids and Surfaces A: Physicochemical and Engineering Aspects, www.elsevier.com/locate/colsurfa. DOI: 10.1016/j.colsurfa.2009.05.013.
- Dorota KORTE et al, University of Nova Gorica, Vipavska, Slovenia and University of Torino, Italy. Thermal lens spectrometric determination of colloidal and ionic silver in water. 39th Winter School on Wave and Quantum Acoustics.
- Audrey L. Companion, Illinois Institute of Technology MacGraw-Hill Book Co. 1964. Library of Congress Card No. 64-22457. See section 5.9, pages 103-104 Van Der Waals Forces.
- B.G. Ershov Hahn-Meitner-Institut, Berlin, Germany. Growth of Silver Particles in Aqueous Solutions: Long-Lived 'Magic" Clusters and Ionic strength Effects. Journal of Physical Chemistry 1993 No.97, pages 339-343.
- 39. State of the Science Literature Review: Everything Nanosilver and More. Jessica Sanford, Environmental Protection Agency EPA/600/R-10/084 August 2010 Task Order No. 95 Contains 363 pages.
- David D. Evanoff Jr and George Chumanov, Clemson University SC, USA. Synthesis and Optical Properties of Silver Nanoparticles and Arrays. ChemPhysChem 2005, 6, 1221-1231 Wiley-VCH Verlag GmbH & Co. DOI: 10.1002/cphc.200500113
- R. Das et al. Assam University, India. Preparation of Silver Nanoparticles and their Characterization June 17th, 2009 AZ0jono.
- B.G. Ershov Hahn-Meitner-Institut, Berlin, Germany. Growth of Silver Particles in Aqueous Solutions: Long-Lived “Magic” Clusters and Ionic strength effects. Journal of Physical Chemistry 1993 No. 97, pages 339-343
- State of the Sciences Literature Review: Everything Nano Silver and More. Jessica Sanford, Environmental Protection Agency EPA/600/R-10/084 August 2010 Task Order no. 95. Contains 363 pages.
- Dorota Korte et al. University of Nova Gorica, Vipavska, Slovenia & University of Torino, Italy. Thermal lens spectrometric determination of Colloidal and ionic silver in water. 39th Winter School on wave and Quantum acoustics.
- Hans Laroo, private researcher in Ipswich, Queensland, Australia. When a Particle is really a Cluster, a Dispersion a Suspension and there is no colloid in sight, you have the recipe for Colloidal Silver that is not a0
- Hans Laroo, private researcher. Colloidal Nano Silver- Its Production Method, Properties, Standards and its Bio-efficacy as an Inorganic Antibiotic. Published by Physical Chemistry & Biophysics. Volume 3, Issue 2 – 1000130 ISSN 2161- 0398 JPC
- Colin Baras on Coloured Lights sculpt nanoparticles (New Scientist) on Stamplecoskie, Sciaiano and American Chemical Society. February 12 (2010).
- Hans Laroo “private researcher. The Fragile and Flawed existence of Questionable Water Purity as used in Wet Chemistry and in particular where such water is destined for Medical Applications such as Vaccines”. Published by The International Journal of Vaccines and Vaccinations (MedCrave) 4 (2017).
- The structural origin of anomalous properties of liquid water. Review paper by Anders Nilsson & Lars G.M. Pettersson, Dept. of physics, AlbaNova University Centre, Stockholm, Sweden. Nature Communication December 8 (2015).
- Hans Laroo, “private researcher. Testing of metal derived Nanometre sized particle, using analogue Methodologies”. Published by Open Access Text (oat) (2017)
- Dielectric Constant of Water from 00 to 1000 C, by C.G. Malmberg and A.A. Maryott. Published in the Journal of Research of the National Bureau of Standards in January 1956. Measuring the resistance of water with greater accuracy using an electronic bridge (Author’s comment).
- Water with Excess Electric Charge by Leandra P. Santos et al, Institute of Chemistry, University of Campinas, Brazil. Proc. ESA Annual Meeting on Electrostatics 2011. Determining the electrostatic charge on water dropped from an electrically charged needle (Author’s comment).
- Structures of Cage, Prism, and Book Isomers of Water Hexamer from Broadband Rotational Spectroscopy by Cristobal Perez et al. Science 336, 897 and a published article “Chemists Merge Experimentation with Theory in Understanding of Water Molecule” in Science Daily, Includes illustration of the prism. Cage and Book (Author’s comment) (2012).
- Resistivity of Water from The Physics Factbook ™, edited by Glenn Elert. The presentation of the conflicting concepts of the resistivity of water from 18.18MOhm to 40MOhm, when in fact the true resistance of water is substantially higher (Author’s comment).
- “Visible and near-ultraviolet absorption spectrum of liquid water: Comment by Edward S. Fry. Department of Physics, Texas A&M University”. Published in APPLIED OPTICS 39.16(2000).
- “Ultrapure Water – The Standard for Resistivity Measurements of Ultrapure Water by Anthony C. Bevilacqua, Thornton Associates, Inc, Waltham, Massachusetts. Paper presented at the 1998 Semiconductor Pure Water and Chemicals Conference”.
- “Measurement of the Resistivity of Ultrapure Water at Elevated Temperatures by K. R. Morash, C. H. Saunders Anthony C. Bevilacqua and T. S. Light and published in the Ultrapure Water Journal in December of (1994).
- “New UV Technology for Point-of-Use Water Disinfection by Yu. Bilenko et al at Sensor Electronic Technology, Columbia”. Published by Clean Technology ISBN 978-1-4398-3419-0 (2010).
- “Some Thermodynamic Properties of the Hydrated Electrons by Joshua Jortner, Tel Aviv University, Israel and Richard. Noyes University of Oregon, 8(1968).
- GF Reiter., et al. “Evidence of a new quantum state of nano-confined water. G. Reiter’s work was supported by the DOE, Office of Basic Energy Sciences”. 27 (2011).
- Joshua Jortner., “Department of Chemistry, Tel-Aviv University, Israel and Richard M Noyes Department of Chemistry University of Oregon, Some Thermodynamic Properties of the Hydrated Electron. Published in The Journal of Physical Chemistry September 8 (1985).
- Boris P. Gorshunov et al. Quantum Behaviour of Water Molecules Confined to Nano-cavities in Gemstones. A.M. Prokhorov General Physics Institute, Russian Academy of Sciences Moscow, published in The Journal of Physical Letters, ACS Publications American Chemical Society 2015.
- Vlad P., et al. “Signature properties of water: Their Molecular Electronic Origins, National Physical Laboratory, Middlesex, United Kingdom”. Published by www.pnas.org/cgi/doi/10. 1073/pnas. 1418982112 (2015).
- CG Malmberg “Dielectric Constant of Water from 00 to 1000 C”. Journal of Research of the National Bureau of Standards”. 56.1 (1956): 2641.
- Anthony C., “Thornton Associates, Inc in Waltham, Massachusetts USA”. Ultrapure Water – The Standard for Resistivity Measurements of Ultrapure Water, presented in March (1998).
- K RMorash., et al. “Thornton Associates, Inc Waltham, MA 02154. Measurement of the Resistivity of Ultrapure Water at elevated temperatures. Published in the Ultrapure Water Journal in December of 1994 and presented at the Water EXPO”. It was pre-printed with permission of Tall Oaks Publishing.
- Stephen Lower., “A gentle Introduction to Water and its structure”. Canada.
- Water Structure and Science Series by Professor Martin Chaplin, London UK: Anomalous properties of water; Water structure and Science; Water molecule structure; Water clusters: overview; Water absorption spectrum and Water’s Two-State cluster history.
- Robert M., et al. “Accurate Predictions of Water Cluster Formation, (H2O), Department of Chemistry, Bucknell University, Lewisburg, Pennsylvania, USA”. Published by ACS Publications. The journal of Physical Chemistry 30(2010).
- Robin M. Pope., “Absorption Spectrum (380-700nm) of pure water. II. Integrating cavity measurements.” Provides evidence of an absorption minimum at 418nm 36.33 (1997).
- Professor Susan A., et al. “Latent Image Formation. Obtained from the Internet under this description”.
- “Photoelectron Spectroscopy of hydrated electrons by Alexander T. Shreve et al Dept. of Chemistry University of California, Chemical Sciences Division, Lawrence Berkeley National Library, Berkeley, California, USA’. Published in Chemical Physics Letters 493 (2010): 216-219.
- “An Investigation into the Electrical Impedance of Water Electrolysis Cells – With a View to Saving Energy Kaveh Mazloomi et al, Dept. of Electrical and Electronic Engineering, University Putra, Malaysia and in conjunction with Dept”. of Civil Engineering Islamic Azad University, Estahban, Iran. International Journal of Electrochemical Science. 7 (2012): 3466 – 3481.
- Joshua Jortner “Tel-Aviv University, Israel and Richard M. Noyes, University of Oregon and Max-Planck Institut fur physikalische Chemie, Gottingen, Germany”. Some Thermodynamic Properties of Hydrated Electrons. The Journal of Physical Chemistry 8(1965).
- “The Institute of Physics. Fast Processes in Radiation Chemistry and Biology”. John Wiley Sons, 1975. ISBN. 0-471-00475-8
- N Yamamoto., “okyo Institute of Technology, Tokyo. Photon Emission from silver particles induced by a high-energy electron beam”. The American Physical Society Physical Review B, 664.205419 (2001).
- David Abbott MA., “Chemistry through investigation, ARIC 1967 J.M. Dent Ltd London”. See Chapter 7, Electrolysis 98-125.
- Ajoy K Ghatak, “Indian Institute of Technology, New Delhi, India. An Introduction to Modern Optics”. MacGraw-Hill Publishing Co. 1972. See chapter 3, Electromagnetic Character of Light and Polarization, (1972): 95-144.
- Andrew j Frank., et al. “Wilfrid Laurier University Ontario, Canada. Synthesis of Silver Nano-prisms with Variable Size and Investigation of their Optical Properties: A First-Year Undergraduate Experiment Exploring Plasmonic Nanoparticles”. Journal of Chemical Education 87.10(2010).
- Ciraci C., et al. “Probing the ultimate limits of Plasmonic Enhancement””. Duke University. Publisher: Science.
- “Published titled Zeta Potential in Colloid Science: Principles and applications”. Published in London by Academic Press ISBN 0123619602.
- Ian Cock., et al. “Griffith University, Brisbane Australia. Colloidal Silver (CS) as an Antiseptic: Two opposing viewpoints”. Pharmacognosy Communications 1(2012).
- Mahendra Rai., et al.” Dept. Biotechnology, SGB Amravati University, India July 28, 2008, Silver nanoparticles as a new generation of antimicrobials”. Biotechnology Advances 27 (2009): 76-83.
- NLkhagvajav I., et al. “Ege University Izmir, Turkey. Antimicrobial Activity of Colloidal silver Nanoparticles prepared by Sol-Gel Method”. Digest Journal of Nanomaterials and Biostructures, Vol. 6.1(2011): 149-154
- DMBT Disaanayake., et al. “Preventing/Treating Rheumatoid Arthritis? Efficacy of some Colloidal Silver and Silver Salts against Proteus Species (2014).
- Daming Fu., et al. “Evaluation of the Antibacterial Efficacy of Silver Nanoparticles against Enterococcus faecalis biofilm”. American Association of Endodontists. Published by ACS NANO(2013).
- Frank Kjeldsen., et al. “Protein Research Group and “Memphys” Center for Bio-membrane Physics, University of Southern Denmark. Insights into Cellular Response Triggered by Silver Nanoparticles Using Quantitative Proteomics”. February 10, 2014 10.1021/nn4050744 American Chemical Society.
Citation:
Hans Laroo. “A reminder about covalent bonded quantum sized atomic silver clusters in an aqueous electric suspension and an effective biocide and able to enhance the immune system prior to penetration of the body by foreign antigens.” Bulletin of Clinical Immunology 1.1 (2018): 31-49.
Copyright: © 2018 Hans Laroo. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.