Research Article
Volume 2 Issue 5 - 2018
Huntington ’s disease – Unifying Mechanism Involving Antioxidant Therapy: Reactive Oxygen Species, Oxidative Stress, and Phenolics
1Peter Kovacic, Department of Chemistry and Biochemistry, San Diego State University, San Diego, CA, USA
2Wil Weston, Library and Information Access, San Diego State University, San Diego, CA, USA
2Wil Weston, Library and Information Access, San Diego State University, San Diego, CA, USA
*Corresponding Author: Peter Kovacic, Department of Chemistry and Biochemistry, San Diego State University, San Diego, CA, USA.
Received: August 18, 2018; Published: August 28, 2018
Abstract
Reactive oxygen species (ROS) and oxidative stress (OS) play roles in Huntington’s disease (HD), as also in Alzheimer’s disease (AD), Parkinson’s disease (PD), Dementia, Schizophrenia (SCZ), Multiple Sclerosis (MS) and Depression. Various sources, including oxidases, serve as generators of ROS-OS, such as mitochondria, NADPH, cytochromes P450, monoamines, ET metal complexes, G72 gene, and microglia. Diverse types of antioxidants (AOs) exert a positive influence on the harmful effects. There are appreciable numbers of phenol and phenolic ether drugs, as for AD (Kovacic & Weston, 2017a) and PD (Kovacic & Weston, 2017b). A unifying mechanism based on ET-ROS-OS-AO is involved. Other possible influential aspects are discussed in a multifaceted approach.
Keywords: Huntington’s disease; Radicals; Oxidative stress; Reactive oxygen species; Antioxidants
Abbreviations: ET: Electron transfer; ROS: Reactive oxygen species; OS: Oxidative stress; AO: Antioxidant; HD: Huntington’s disease; SCZ: Schizophrenia; AD: Alzheimer’s disease; PD: Parkinson’s disease
Introduction
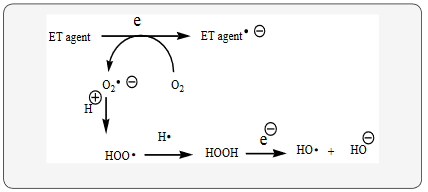
Huntington’s disease (HD) fits into the unifying mechanism, which has been widely applied previously as set forth in an article involving electron transfer (ET), reactive oxygen species (ROS), and oxidative stress (OS) (Kovacic & Weston, 2017c). This unifying mechanism argues that the preponderance of bioactive substances, usually as the metabolites, incorporate ET functionalities. We believe these ET-metabolites play an important role in physiological responses. The main group include quinones (or phenolic precursors), metal complexes (or complexors), aromatic nitro compounds (or reduced hydroxylamine and nitroso derivatives), and conjugated imines (or iminium species). Resultant redox cycling is illustrated in Scheme 1. In vivo redox cycling with oxygen can occur, giving rise to oxidative stress (OS) through generation of reactive oxygen species (ROS), such as hydrogen peroxide, hydro peroxides, alkyl peroxides, and diverse radicals (hydroxyl, alkoxyl, hydroperoxyl, and superoxide) (Scheme 1). Cellular and mitochondrial enzymes can also perform catalytically in the reduction of O2.
In some cases, ET results in involvement with normal electrical effects (e.g. neurochemistry). Generally, active entities possessing ET groups display reduction potentials in the physiologically responsive range. Hence, ET in vivo can occur resulting in production of ROS, which can be beneficial in cell signaling at low concentrations, but produce toxic results at high levels (Kovacic, Ott, & Cooksy, 2013). Electron donors consist of phenols, N-heterocycles or disulfides in proteins, which produce relatively stable radical cations. ET, ROS and OS have been increasingly implicated in the mode of action of drugs and toxins, e.g. anticancer drugs (Kovacic & Osuna, 2000), carcinogens (Kovacic & Jacintho, 2001), cardiovascular toxins (Kovacic & Thurn, 2005), toxins (Kovacic, Pozos, Somanathan, Shangari, & O’Brien, 2005), ototoxins (Kovacic & Somanathan, 2008) and various other categories (Halliwell & Gutteridge, 1999a).
In addition to the above, there is a plethora of experimental evidence supporting the theoretical framework. This evidence includes generation of the common ROS, lipid peroxidation, degradation products of oxidation, depletion of AOs, effect of exogenous AOs, and DNA oxidation and cleavage products, as well as electrochemical data (Kovacic & Weston, 2017c). This comprehensive, unifying mechanism is consistent with the frequent observation that many ET substances display a variety of activities (e.g. multiple-drug properties), as well as toxic effects.
Symptoms
HD causes select brain cells to operate improperly, ultimately leading to mental breakdown and loss of motor control (Rapoza, 2017). Usually, this occurs between ages 35-50. The disease gets worse over time, is genetic, inherited, and rather uncommon. HD can affect the following: intellectual ability (memory loss, dementia, and inattention), uncontrolled motions (twitching, clumsiness, difficulty swallowing, dancing movements, slurred speech, difficulty walking), uncontrollable emotions (depression, irritability, personality changes). Symptoms can change over time, starting subtly and slowly becoming more pronounced. Seizures and rigidity are also common. Regrettably, cure and prevention are currently impossible.
HD causes select brain cells to operate improperly, ultimately leading to mental breakdown and loss of motor control (Rapoza, 2017). Usually, this occurs between ages 35-50. The disease gets worse over time, is genetic, inherited, and rather uncommon. HD can affect the following: intellectual ability (memory loss, dementia, and inattention), uncontrolled motions (twitching, clumsiness, difficulty swallowing, dancing movements, slurred speech, difficulty walking), uncontrollable emotions (depression, irritability, personality changes). Symptoms can change over time, starting subtly and slowly becoming more pronounced. Seizures and rigidity are also common. Regrettably, cure and prevention are currently impossible.
Chorea, characterized by brief, abrupt, irregular, and unpredictable uncontrolled movements, is a distinctive aspect of HD (Barker, Musso, & Gouvier, 2011). Common symptoms include cognitive decline and emotional disturbance. HD is caused by a mutation of a single gene responsible for generation of a protein called huntingtin whose function is not clear. Pathology associated with the illness includes atrophy of neurons in the cerebral cortex and basal ganglion. The important symptoms are motor impairment, cognitive deficits, and psychological problems. Symptoms associated with early stages include abnormal gait, mild chorea, abnormal eye movement, bradykinesia, and emotional problems. With disease progression, motor impairment increases, brain functioning declines, and there is an increase in psychological aspects, such as apathy, depression, and anxiety. The later stages involve motor deficits, language impairment, rigidity, chewing difficulty, incontinence, and psychological impairment. Other symptoms are sleep disorders, dystonia, obsessive-compulsive behavior, hallucinations, and behavioral problems.
ROS
ROS can be beneficial, but at high levels, toxic effects often predominate. There are various sources for these species (Kovacic & Weston, 2017c). NAPDH oxidase is an important producer of the ROS in various organs. The G72 gene increased radical generation in cells. The gene acts as an activator of oxidase. ROS generated by NO synthase have been implicated in an array of harmful behaviors. Mitochondria provide another source of ROS-OS, which appears to contribute to aging. Leakage of electrons occurs in the ET chain, which react with oxygen to produce superoxide, a precursor of other ROS. Other examples of ROS producers are cytochrome P450, metal complexes, monoamine oxidase and microglia.
ROS can be beneficial, but at high levels, toxic effects often predominate. There are various sources for these species (Kovacic & Weston, 2017c). NAPDH oxidase is an important producer of the ROS in various organs. The G72 gene increased radical generation in cells. The gene acts as an activator of oxidase. ROS generated by NO synthase have been implicated in an array of harmful behaviors. Mitochondria provide another source of ROS-OS, which appears to contribute to aging. Leakage of electrons occurs in the ET chain, which react with oxygen to produce superoxide, a precursor of other ROS. Other examples of ROS producers are cytochrome P450, metal complexes, monoamine oxidase and microglia.
It is necessary to recognize the importance of the multifaceted nature of physiological action. In addition to ET-ROS-OS-AO, other factors are at play in HD as indicated in this discussion: cell signaling, mitochondria, receptor binding and enzyme inhibition (Walton., et al. 2013). The literature addresses the basic aspects of these items: cell signaling (Kovacic & Somanathan, 2013a), mitochondria (Kovacic, Pozos, Somanathan, Shangari, & O’Brien, 2005), and receptor binding (Kovacic, Pozos, & Draskovich, 2007). The related articles on PD and AD deal with the role of AOs in decreasing ROS-OS. However, the specific source of the harmful species was not addressed. Information is available on generation of ROS-OS in the brain.
Discussion
HD is a hereditary, neurodegenerative disorder involving motor, psychiatric, and cognitive symptoms (Pérez-Severiano, Montes, Gerónimo-Olvera, & Segovia, 2013). Defects in energy metabolism may cause oxidative damage. Determining the mechanisms inducing oxidative damage may lead to new drugs with AO activities. Alteration in neurotransmitter signaling is known to contribute to the pathophysiological basis of HD and PD (Kovacic & Weston, 2017b). Several mechanisms relating to neurotransmitter alteration have been advanced, including OS, mitochondrial dysfunction (Kovacic., et al. 2005) and neuroinflammation.
Considerable progress has been made in understanding the pathogenic mechanisms of HD (Stack & Ferrante, 2007). Mechanisms have been proposed for the toxicity, namely OS, aberrant apoptosis, mitochondrial dysfunction, excitotoxicity and protein aggregation. A report by Ryu and Ferrante (2005) dealing generally with AOs for treatment of neurodegenerative disorders, including HD, postulate that OS is a hallmark of these disorders. The role of AO protection is summarized and the complications of this strategy are addressed in this 2005 report.
HD, AD and PD are common medical and social problems (Klivényi & Vécsei, 1997). These illnesses are connected with excitotoxins and alterations in mitochondrial ET resulting in free radical generation. Formation of ROS is involved. Impaired energy metabolism can result in excitotoxicity, which may be the final pathway to neuronal death.
Phenols and Phenolic Ethers
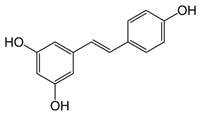
Resveratrol (figure 1) is a naturally occurring AO, which has exhibited promising results versus neurodegenerative diseases (Peñalver. et al. 2018). Alkylated resveratrols were tested with the aim of improving AO and anti-inflammatory activity. A derivative delayed the onset and reduced the severity of the illness. The effects involved AO activity and anti-inflammatory effect.
Resveratrol (figure 1) is a naturally occurring AO, which has exhibited promising results versus neurodegenerative diseases (Peñalver. et al. 2018). Alkylated resveratrols were tested with the aim of improving AO and anti-inflammatory activity. A derivative delayed the onset and reduced the severity of the illness. The effects involved AO activity and anti-inflammatory effect.
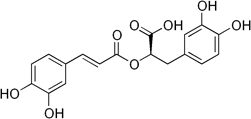
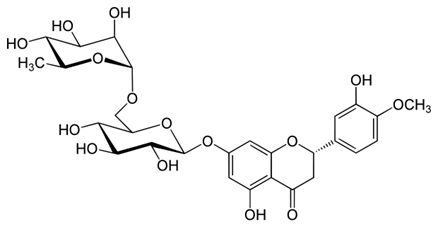
Rosmarinic acid (figure 2), a polyphenol, was investigated as an AO for management of HD (Bhatt, Singh, Prakash, & Mishra, 2015). Treatment improved behavioral abnormalities and attenuated OS. There was significant therapeutic action.
Polyphenols are known to attenuate OS and reduce the risk of neurodegenerative disorders, such as HD, AD, PD, and MS. Signaling pathway and modulation of immune responses are addressed (Bhullar & Rupasinghe, 2013). There is modulation of several therapeutic targets at once.
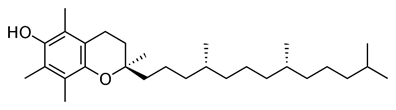
Evidence points to elimination of neurons by OS in HD (Peyser., et al. 1995). AO therapy with d-⍺-tocopherol (figure 3) may slow the rate of motor decline. The AO had a significant effect on neurological symptoms. The AO reduced oxyradical damage to cell membranes, which slow the course of HD.
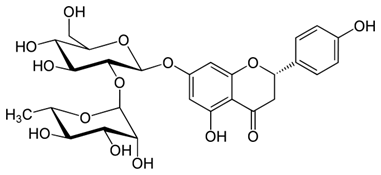
Naringin (figure 4) treatment reduced OS, mitochondrial dysfunction and adverse behavioral alterations in rats with Huntington like symptoms (Kumar & Kumar, 2010). There are indications that the drugs may function via an NO mechanism (Kovacic & Somanathan, 2013b).
Mithramycin A (figure 5) is known to exert a neuroprotective effect (Osada, Kosuge, Ishige, & Ito, 2013). It acts to protect cortical neurons against OS-induced cell death and prolongs survival of HD mice.
Hesperidin (figure 6), a phenol and phenolic ether, treatment reduced OS, mitochondrial dysfunction and adverse behavioral alterations in rats with Huntington like symptoms (Kumar & Kumar, 2010).
Neurodegenerative diseases, such as HD, share similar bio reactions that lead to neurodegeneration. Curcumin (figure 7), a phenol and phenolic ether, from turmeric, possesses various biochemical properties that include attenuation of OS from mitochondrial dysfunction and from inflammatory responses (Kim, Kim, & Han 2012). Other properties include lessoning of ROS and prevention of heavy metal poisoning related to ROS-OS.
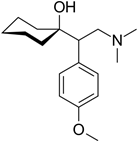
Clinical drugs offer only symptomatic relief in HD (Gill, Jamwal, Kumar, & Deshmukh, 2017). Studies indicate that antidepressants, such as venlafaxine (figure 8), a phenolic ether, improve motor skills and neurobehavioral in Huntington like symptoms. AO properties, signaling, and anti-inflammatory (Kovacic & Somanathan, 2014) properties may be involved. Phenolic ethers can undergo dealkylation to AO phenols (Kovacic & Weston, 2017a).
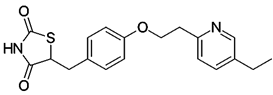
Pioglitazone (figure 9), a phenolic ether, protected against OS and mitochondrial dysfunction in a model of HD (Napolitano,. et al. 2011). Apparently, the drug interferes with signaling in a beneficial manner in HD pathogenesis. Nuclear translocation and histone modification appear to be novel approaches for treating HD.
Other Drugs
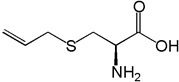
Garlic, which contains the AO S-Allyl cysteine (figure 10) as a major sulfur ingredient, is used as a medicine in various cultures (Qu., et al. 2016). Neuroprotective effects are exerted in HD, AD, and PD. Mechanisms may involve signaling associated with OS and neuroinflammation. Allyl sulfides undergo reductive cleaving to the thiols and allyl radicals (Koval, 1994).
Garlic, which contains the AO S-Allyl cysteine (figure 10) as a major sulfur ingredient, is used as a medicine in various cultures (Qu., et al. 2016). Neuroprotective effects are exerted in HD, AD, and PD. Mechanisms may involve signaling associated with OS and neuroinflammation. Allyl sulfides undergo reductive cleaving to the thiols and allyl radicals (Koval, 1994).
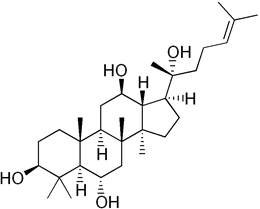
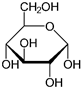
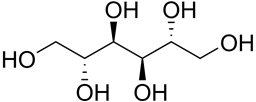
Protopanaxatriol (PPT) (figure 11), a polyol, is extracted from ginseng and was studied in alleviation of HD model (Gao., et al. 2015). PPT administration decreases ROS generation, scavenges free radicals, restores AO enzyme activity and protects against OS. The action is associated with the AO property. Sugar polyols, e.g. glucopyranose (figure 12) possess significant AO capacity. Most of the alcohol groups are secondary making for similarity to (figure 11: Protopanaxatriol). Mannitol (figure 13) is a potent antioxidant, and large amounts might be expected to quench H2O2, and thus cripple H2O2-mediated defenses (Williamson, Jennings, Guo, & Pharr, 2002) Hence, PPT is a class analog of sugar polyols with similar properties.
Another antidepressant, sertraline (figure 14) improves motor skills and neurobehavioral in Huntington like symptoms (Gill, Jamwal, Kumar, & Deshmukh, 2017). This secondary amine is related to N, N-diphenyl-p-phenylenediamine, which displays various AO properties (Halliwell & Gutteridge, 1999b)
3-Nitropropionic acid is a toxic model of HD involving mitochondrial inhibition and free radical generation diseases including HD. Astrocytes are cells that maintain neural health and homeostasis (Ramachandran & Thangarajan, 2016). Astrocyte dysfunction is involved in the pathogenesis of many neurodegenerative diseases, such as HD, AD, and PD. Compounds were identified by screening that protect against OS, which is associated with these diseases. In addition, there was activation of AO responses in some cases.
Herbal drugs
In a study with herbal formula B302, there was alleviation of heart failure in HD by suppression of OS, apoptosis, and inflammation (Lin., et al. 2015). The herbal formula B401 was found to protect against neurodegeration in HD by attenuating OS apoptosis in HD (Wang., et al, 2015). The treatment reduces ROS levels and increases expression of SOD.
In a study with herbal formula B302, there was alleviation of heart failure in HD by suppression of OS, apoptosis, and inflammation (Lin., et al. 2015). The herbal formula B401 was found to protect against neurodegeration in HD by attenuating OS apoptosis in HD (Wang., et al, 2015). The treatment reduces ROS levels and increases expression of SOD.
The extract of the plant, convolvulus pluricaulis (CPE), is used to combat neurotoxicity (Malik, Choudhary, & Kumar, 2015). It should be further explored for treatment of HD. Calendula officinalis linn flower extract displays potent AO, anti-inflammatory, and neuroprotective activities (Shivasharan., et al. 2013). OS and NO mechanisms have been proposed for induced neurotoxicity. CPE treatment attenuated oxidative damage, behavioral alteration, and neuronal loss.
Findings indicate that Withania somnifera root extract bestows neuroprotective actions on a model of HD via AO activity (Kumar & Kumar, 2009). In addition, the adverse biochemical alterations were restored by the extract. The harmful effects included increased lipid peroxidation, depleted AO enzymes (SOD and catalase), and inhibition of mitochondrial activity. There is a prior similar treatment of herbal drugs (Halliwell & Gutteridge, 1999a).
Structure-activity relationship (SAR) is addressed in a prior article (Kovacic & Weston, 2017d). Note the discussion on effect of number of phenolic AO groups. Applications can also be made to alcohol AO groups.
Conclusion
Various drugs have proven effective in treatment of Huntington’s disease. The major ones are phenolic, similar to AD and PD. There are a variety of herbal formulas and extracts that appear to display neuroprotective and anti-inflammatory properties. SAR is applicable, as for AD and PD (Kovacic & Weston, 2017d). More work is needed on the important aspects of prevention, cure, and treatment of the outward expression of the symptoms of HD. This review is the latest in a series on brain illnesses, with the prior ones comprising Alzheimer’s disease (Kovacic & Weston, 2017a), Parkinson’s disease (Kovacic & Weston, 2017b), Schizophrenia (Kovacic & Weston, 2017c), Multiple Sclerosis (Kovacic & Weston, 2018a), Dementia (Kovacic & Weston, 2018b), and Depression (Kovacic & Weston, 2018c).
References
- Barker A., et al. “Huntington's disease. In C. Noggle The encyclopedia of neuropsychological disorders”. Springer Publishing Company (2011).
- Bhatt R., et al. “Development, characterization and nasal delivery of rosmarinic acid-loaded solid lipid nanoparticles for the effective management of Huntington's disease”. Drug Delivery 22.7 (2015): 931-939.
- Bhullar KS and Rupasinghe HP. “Polyphenols: multipotent therapeutic agents in neurodegenerative diseases”. Oxidative Medicine and Cellular Longevity(2013): 891748.
- Gao Y., et al. “Protopanaxtriol protects against 3-nitropropionic acid-induced oxidative stress in a rat model of Huntington's disease”. Acta Pharmacologica Sinica36.3 (2015): 311-322.
- Gill JS., et al. “Sertraline and venlafaxine improves motor performance and neurobehavioral deficit in quinolinic acid induced Huntington's like symptoms in rats: Possible neurotransmitters modulation”. Pharmacological Reports69.2 (2017): 306-313.
- Halliwell B and Gutteridge J. “Free Radicals in Biology and Medicine (3rd ed., Oxford science publications)”. Oxford: New York: Clarendon Press; Oxford University Press. (1999): 192-194.
- Halliwell B & Gutteridge J. “Free Radicals in Biology and Medicine (3rd ed., Oxford science publications)”. Oxford: New York: Clarendon Press; Oxford University Press. (1999): 825.
- Kim DS., et al. “Curcuminoids in neurodegenerative diseases”. Recent Patents on CNS Drug Discovery 7.3 (2012): 184-204.
- Klivényi P and Vécsei L. “Neurodegeneration: aging and dementia Etiopathogenic role of electron transport disorders Therapeutic possibilities”. Orvosi Hetilap138.6 (1997): 331-335.
- Kovacic P and Jacintho JD. “Reproductive toxins. Pervasive theme of oxidative stress and electron transfer”. Current Medicinal Chemistry 8 (2001): 863-892.
- Kovacic P and Osuna JA. “Mechanisms of anticancer agents: Emphasis on oxidative stress and electron transfer”. Current Pharmaceutical Design 6 (2000): 277-309.
- Kovacic P., et al. “Benzodiazepines: electron affinity, receptors and cell signaling - a multifaceted approach”. Journal of Receptors and Signal Transduction 33.6 (2013): 338-343.
- Kovacic P., et al. “Unifying electrostatic mechanism for receptor-ligand activity”. Journal of Receptors and Signal Transduction 27.5 (2007): 411-431.
- Kovacic P., et al. “Mechanism of mitochondrial uncouples, inhibitors, and toxins: Focus on electron transfer, free radicals, and structure-activity relationships”. Current Medicinal Chemistry 12.22 (2005): 2601-2623.
- Kovacic P and Somanathan R. “Ototoxicity and noise trauma: Electron transfer, reactive oxygen species, cell signaling, electrical effects, and protection by antioxidants”. Practical medical aspects Medical Hypotheses 70 (2008): 914-923.
- Kovacic P and Somanathan R. “Cell signaling, receptors, electrical effects and therapy in circadian rhythm”. Journal of Receptors and Signal Transduction 33 (2013): 267-275.
- Kovacic P and Somanathan R. “Multifaceted approach to circadian rhythm: Redox, oxidative stress, melatonin, antioxidants, nitric oxide, hypoxia, anesthetics, cortisol and cocaine”. Current Chemical Biology 7 (2013): 271-281.
- Kovacic P and Somanathan R. “Inflammation and Anti-Inflammatory Agents – Reactive Oxygen Species and Toxicity, in: I. Laher Systems Biology of Free Radicals and Antioxidants”. Springer-Verlag Berlin Heidelberg (2014): 3197-3216.
- Kovacic P and Thurn LA. “Cardiovascular toxicity from the perspective of oxidative stress, electron transfer, and prevention by antioxidants”. Current Vascular Pharmacology 3 (2005): 107-117.
- Kovacic P and Weston W. “Phenolic antioxidants as drugs for Alzheimer’s disease: oxidative stress and selectivity”. Novel Approaches in Drug Designing and Development (NAPDD) 3.20 (2017): 555606.
- Kovacic P and Weston W. “Treatment of Parkinson’s disease with phenolic antioxidant drugs: Oxidative stress, reactive oxygen species and selectivity”. Chronicles of Pharmaceutical Science 1.4 (2017): 193-198.
- Kovacic P and Weston W. “Cause and treatment of schizophrenia: Electron transfer, reactive oxygen species, oxidative stress, antioxidants, and unifying mechanism”. Chronicles of Pharmaceutical Science 1.6 (2017): 332-340.
- Kovacic P and Weston W. “Novel structure-activity relationship and quantitative data for phenolic drugs involving Alzheimer’s and Parkinson’s disease: Antioxidants, oxidative stress, and selectivity”. Chronicles of Pharmaceutical Science 1.5 (2017): 299-306.
- Kovacic P and Weston W. “Unifying Mechanism for Multiple Sclerosis and Amyotrophic Lateral Sclerosis: Reactive oxygen species, oxidative stress, and antioxidants”. Journal of Bio pharmaceutics and Therapeutic Challenges1.1 (2018): 1-8.
- Kovacic P and Weston W. “Dementia – unifying mechanism involving antioxidant therapy: reactive oxygen species, oxidative stress, and phenolics”. Chronicles of Pharmaceutical Science 2.6 (2018): 710-717.
- Kovacic P and Weston W. “Brain Depression – unifying mechanism involving antioxidant therapy: Reactive oxygen species, oxidative stress, and phenolics”. Chronicles of Pharmaceutical Science2.3 (2018): 545-553.
- Koval IV. “Sulfides: synthesis and properties”. Russian Chemical Reviews 63.4 (1994): 323-344.
- Kumar P and Kumar A. “Possible neuroprotective effect of Withania somnifera root extract against 3-nitropropionic acid-induced behavioral, biochemical, and mitochondrial dysfunction in an animal model of Huntington's disease”. Journal of Medicinal Food3 (2009): 591-600.
- Kumar P and Kumar A. “Protective effect of hesperidin and naringin against 3-nitropropionic acid induced Huntington's like symptoms in rats: possible role of nitric oxide”. Behavioural Brain Research 206.1 (2010): 38-46.
- Lin CL., et al. “Oral treatment with herbal formula B307 alleviates cardiac failure in aging R6/2 mice with Huntington's disease via suppressing oxidative stress, inflammation, and apoptosis”. Clinical Interventions in Aging 10 (2015): 1173-1187.
- Napolitano M., et al. “Protective effect of pioglitazone, a PPARγ ligand, in a 3 nitropropionic acid model of Huntington's disease”. Brain Research Bulletin 85.3 (2011): 231-237.
- Osada N., et al. “Mithramycin, an agent for developing new therapeutic drugs for neurodegenerative diseases”. Journal of Pharmacological Sciences 122.4 (2013): 251-256.
- Peñalver P., et al. “Alkylated resveratrol prodrugs and metabolites as potential therapeutics for neurodegenerative diseases”. European Journal of Medicinal Chemistry 146 (2018): 123-138.
- Pérez-Severiano F., et al. “Study of oxidative damage and antioxidant systems in two Huntington's disease rodent models”. Methods in Molecular Biology 10.10 (2013): 177-200.
- Peyser CE., et al. “Trial of d-alpha-tocopherol in Huntington's disease”. American Journal of Psychiatry 152.12 (1995): 1771-1775.
- Qu Z., et al. “Protective effects of AGE and its components on neuroinflammation and neurodegeneration”. NeuroMolecular Medicine18.3 (2016): 474-482.
- Ramachandran S and Thangarajan S. “A novel therapeutic application of solid lipid nanoparticles encapsulated thymoquinone (TQ-SLNs) on 3-nitroproponic acid induced Huntington's disease-like symptoms in wistar rats”. Chemico-Biological Interactions 256 (2016): 25-36.
- Rapoza K. (2017). Huntington's disease (chorea). In Harvard Medical School (Ed.), Health reference series: “Harvard Medical School health topics A-Z.” Harvard Health Publications.
- Ryu H and Ferrante RJ. “Emerging chemotherapeutic strategies for Huntington's disease”. Expert Opinion on Emerging Drugs10.2 (2005): 345-363.
- Shivasharan B.D., et al. “Protective effect of Calendula officinalis Linn. Flowers against 3-nitropropionic acid induced experimental Huntington's disease in rats”. Drug and Chemical Toxicology 36.4 (2013): 466-473.
- Stack EC and Ferrante RJ. “Huntington's disease: progress and potential in the field”. Expert Opinion on Investigational Drugs 16.12 (2007): 1933-1953.
- Walton JC., et al. “Neuronal nitric oxide synthase and NADPH oxidase interact to affect cognitive, affective, and social behaviors in mice”. Behavioural Brain Research 213 (2013): 320-326.
- Wang SE., et al. “Oral treatment with the herbal formula B401 protects against aging-dependent neurodegeneration by attenuating oxidative stress and apoptosis in the brain of R6/2 mice”. Clinical Interventions in Aging10 (2015): 1825-1837.
- Williamson JD., et al. “Sugar Alcohols, Salt Stress, and Fungal Resistance: Polyols—Multifunctional Plant Protection?”. Journal of the American Society for Horticultural Science 127.4 (2002): 467-473.
Citation:
Peter Kovacic and Wil Weston. “Huntington ’s disease – Unifying Mechanism Involving Antioxidant Therapy: Reactive Oxygen
Species, Oxidative Stress, and Phenolics”. Clinical Biotechnology and Microbiology 2.5 (2018): 485-494.
Copyright: © 2018 Peter Kovacic and Wil Weston. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.






























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.