Review Article
Volume 1 Issue 6 - 2017
Neurological Disorders and the Blood Brain Barrier: 1. Epilepsy
International Institute of Medicine and Science, California, USA
*Corresponding Author: Alain L Fymat. International Institute of Medicine and Science, California, USA.
Received: November 28, 2017; Published: December 04, 2017
Abstract
In the neurological literature, we find approximately 400 known neural disorders. A number of these disorders may be due to a disruption or failure of the blood brain barrier (BBB), including epilepsy. The convergence between BBB studies and clinical investigations of epilepsies has historically been limited to interactions between putative anti-epileptic drugs (AED) and the endothelium. These studies were based on the observation that many promising AEDs are excluded by the BBB. They are thus clinically unusable in spite of significant potency and selectivity. Separately, advances in neuroradiology have further enhanced our ability to image and study the human cerebrovasculature, and stimulated developments in the research of metabolic deficiencies linked to seizure disorders. Prior to 1986, BBB research in epilepsy focused on three main areas: (a) ultrastructural studies, (b) brain glucose availability and transport, and (c) clinical uses of AEDs. However, contrast-based imaging techniques (computed tomography, CT; fMRI: Functional magnetic imaging; PET: Positron emission tomography; nuclear scanning) and medical procedures such as BBB disruption provided a framework that demonstrated that the BBB could be reversibly disrupted by pathologic or iatrogenic manipulations. This concept of BBB breakdown for therapeutic purposes has also unveiled a previously unrecognized role for BBB failure as a possible etiologic mechanism in epileptogenesis. This led to important implications in terms of central nervous system (CNS) drug delivery to a “multiple drug resistant (MDR)” brain. More recently, it has become apparent that MDR is only one of the aspects in BBB research that may impact how we define, prevent and treat seizure disorders.
Here, I will briefly describe the brain protective barriers (BPB), particularly the BBB, discuss the link between the BBB and epilepsy, and dwell at length on drug delivery across the BBB, including the use of nanotechnology. I will also address multi-drug and anti-epileptic drug (AED) resistance. Finally, I will analyze the growing body of evidence that shows that inflammatory mechanisms may participate in the pathological changes observed in the epileptic brain, with increasing awareness that blood-borne cells or signals may participate in epileptogenesis by virtue of a leaky BBB.
Keywords: Anti-epileptic drugs; Anti-epileptic drug resistance; Blood brain barrier; Brain Inflammation; Epilepsy; Epileptogenesis; Nanotechnology drug delivery; Neurological disorders; Neuroradiology; Multi-drug resistance
Abbreviations: AD: Alzheimer's Disease; AED: Anti-Epileptic Drugs; BBB: Blood brain barrier; BBBD: BBB disruption; B(CSF)B; Brain CSF Barrier; B(iCSF)B: Brain-Inner CSF Barrier; B[oCSF]B: Brain-Outer CSF Barrier; BPB: Brain Protective Barriers; B[R]B): Brain Retinal Barrier; CNS: Central Nervous System; CSF: cerebrospinal fluid; CT: Computed Tomography; fMRI: Functional MRI; GABA: Gamma Amino Butyric Acid; GLUT1: Glucose Transporter-1; HDCA: HexaDecyl CyanoAcrylate; HIV: Human Immunodeficiency Virus; HIVAD: HIV-associated dementia; HIVE: HIV Encephalitis; 5-HT: 5-hydroxytryptamine (serotonin); LDLRP/Epic: Low Density Lipoprotein/Related Protein/Engineered Peptide Compound; M: Mast cell; MDR: Multiple Drug Resistance; MDR1P: MDR-1 Protein; MDRPF: Protein Family; MEN: Magneto-Electric Nanoparticles; MHC: Major Histocompatibiity Complex; MRI: Magnetic Resonance Imaging; ND: Nano Devices; NM: Nano Medicine; NP: Nano Particles; NS: Nuclear Scanning; NT: Nano Technology; PACA: PolyAlkylCyanoAcrylate; PLGA: PolyLacticCoGlycolic Acid; PMA: Peptidomimetic Monoclonal Antibodies; RLIP-76: RalA Binding Protein-1; RES: Reticulo-Endothelial System; RS: Radionuclide Scanning; T: T cell; TNF: Tumor Necrosis Factor
Disorders Mentioned: Alzheimer disease; Epilepsy; HIV; HIV-associated dementia; HIV encephalitis; Hypoxia; Meningitis; Multiple sclerosis; Parkinson's disease; Rasmussen syndrome
Drugs listed: Anti-epileptic drugs; Bradykinin; Carbamazepine; Dalagrin; Daunomycin; Doxorubicin; Mannitol; Phenytoin.
Introduction
In the neurological literature, we find approximately 400 known neural disorders some of which may perhaps be better classified as mental disorders. A number of these disorders may be due to a disruption or failure of the blood brain barrier (BBB) such as, for example: meningitis (an inflammation of the meninges or membranes surrounding the brain and spinal cord); epilepsy (chronic or acute seizures caused by inflammation); multiple sclerosis (MS, a disease of either the immune system or/and the breaking down of the BBB in a section of the brain or spinal cord); Alzheimer's disease (AD, a disease in which amyloid beta contained in blood plasma enters the brain and adheres to the surface of astrocytes); possibly prion and prion-like diseases such as Parkinson's disease (PD) and AD; HIV encephalitis (HIVE), a precursor of HIV-associated dementia (HIVAD) in which latent HIV can cross the BBB inside circulating monocytes in the blood stream; and systemic inflammation (sterile or infectious) that may lead to effects on the brain, cause sickness behavior and induce or/and accelerate brain diseases such as MS and PD. Table 1 summarizes for each disease the corresponding BBB effect [1].
| Disease | Bbb effect | Disease | BBB effect |
| Alzheimer | Disruption/breakdown | Multiple sclerosis (immune system deficiency) | Breakdown |
| Brain abscess | Unknown mechanism | Neuromyelitis optica (Devic's disease) | Breakdown |
| Cerebral edema | Opening (due to hypoxia) | Prion and prion-like diseases (Parkinson, Alzheimer) | Unknown penetration mechanism |
| De Vivo | Unknown mechanism | Progressive multi-focal leuko-encephalopathy | Disruption |
| Epilepsy | Disruption/Failure | Rabies | Increased permeability |
| HIV encephalitis (latent hiv crosses the BBB) |
Damage (inflammatory) | Systemic inflammation (sterile, infectious) | Disruption? |
| Meningitis | Disruption | Tripanosomasis (sleep thickness) | Disruption |
Source: Reference: (1) Fymat (2017).
Table 1: Some brain diseases and their corresponding effects on the BBB.
Table 1: Some brain diseases and their corresponding effects on the BBB.
Notwithstanding the indications in Table 1, the convergence between BBB studies and clinical investigations of epilepsies has historically been limited to interactions between putative anti-epileptic drugs (AED) and the endothelium [2]. These studies were based on the observation that many promising AEDs are excluded by the BBB. They are thus clinically unusable in spite of significant potency and selectivity, as revealed by in vitro screening or animal models. More recently, it has become apparent that multiple drug resistance (MDR) is only one of the aspects in BBB research that may impact how we define, prevent and treat seizure disorders. For a recent review of epilepsy, see [3].
Figure 1 [from (6)] illustrates the interest in research related to epilepsy and the BBB has been increasing steadily since 1975. Figure 1.A shows general trends in epilepsy research over the past three decades where “low” and “high” are arbitrary units based on Medline searches, textbook entries, etc. Figure 1.B quantifies these trends by plotting the number of articles published according to PubMed citations when one searches (“BBB or cerebral endothelia” and “seizures or epilepsy”). The grey (lower portion of the) bars represent the number of articles published in a given 5-year period while the darker grey (upper portions of the) bars represent the cumulative number of articles. Given the linear trend observed in the number of articles published in a given 5-year period, the striped area represent the projected number of articles to be published through 2005 (6).
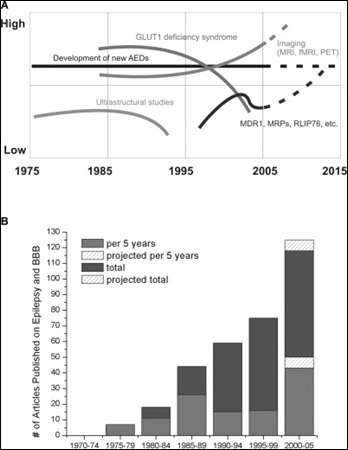
Source: Reference (6) Oby and Janigro (2006).
Figure 1: Increasing interest in research in epilepsy and the blood brain barrier.
Figure 1: Increasing interest in research in epilepsy and the blood brain barrier.
The Brain Protective Barriers
The brain has five protective barriers (BPB) that hinder the delivery of therapeutic drugs. They describe the five main interfaces between the central nervous system (CNS) and the periphery.
The brain has five protective barriers (BPB) that hinder the delivery of therapeutic drugs. They describe the five main interfaces between the central nervous system (CNS) and the periphery.
These include: The blood brain barrier (BBB) that extends down the spinal cord; the brain cerebrospinal fluid (CSF) barrier [B(CSF)B]; the brain-inner CSF barrier [B(iCSF)B]; the brain-outer CSF barrier [B(oCSF)B]; and the brain retinal barrier [B(R)B]. We shall mostly be concerned with the BBB and use the denomination BBB as if it applied (though erroneously) to all BPBs.
All interfaces are physical and metabolic barriers that serve to regulate and protect the microenvironment of the brain. Composed of a monolayer of brain capillary endothelial cells, they are formed by tight junctions. In the case of the BBB, the tight junctions are between the endothelial cells of the primary vasculature with primary manifestation being the impermeability of the capillary wall due to the presence of the junctions and a low endocytic activity. There is a relative paucity of fenestrae and pinocytotic vesicles that restrict brain uptake of circulating molecules. For B(CSF)B, the tight junctions are between the epithelial cells of the choroid plexus. In the case of the B(iCSF)B, the junctions are between the neuro-ependymal cells lining the ventricular surfaces. As for b(oCSF)B, the junctions are between the endothelial cells of the arachnoid vessels (the pia arachnoid).
Thus, the BBB limits access to the brain to small nonpolar molecules by passive diffusion or catalyzed transport of large and/or polar molecules [4]. It hinders the delivery of most pharmaceuticals (diagnostic, therapeutic agents) to the brain [3]. ABC efflux transporters at the BBB influence the brain uptake of a variety of therapeutic agents, including many AEDs [5].
Links between Epilepsy and the Blood Brain Barrier
Oby and Janigro [6] have proposed links between the BBB and epilepsy. Drug resistance, which affects ~ 30% of patients, and the possible role of the BBB, obviously remain an important focus of epilepsy research. Additionally, a compromised BBB has been associated with seizures in a number of disorders. Not only congenital defects, such as GLUT1 deficiency, but acquired deficiencies, like those resulting from brain tumors, head trauma, etc., often result in seizure disorders. More recently systemic and immune triggers have been implicated in a leaky BBB and neuroinflammation. Understanding the nature of the role of BBB in these disorders is imperative in the treatment of the disease, but the fundamental question of whether the compromised integrity of the BBB is a component of the etiology of epilepsy or a consequence of seizures remains unanswered.
Oby and Janigro [6] have proposed links between the BBB and epilepsy. Drug resistance, which affects ~ 30% of patients, and the possible role of the BBB, obviously remain an important focus of epilepsy research. Additionally, a compromised BBB has been associated with seizures in a number of disorders. Not only congenital defects, such as GLUT1 deficiency, but acquired deficiencies, like those resulting from brain tumors, head trauma, etc., often result in seizure disorders. More recently systemic and immune triggers have been implicated in a leaky BBB and neuroinflammation. Understanding the nature of the role of BBB in these disorders is imperative in the treatment of the disease, but the fundamental question of whether the compromised integrity of the BBB is a component of the etiology of epilepsy or a consequence of seizures remains unanswered.
Epilepsy is a complex network of causes and effects. Diagramed in Figure 2 are selected connections between epilepsy and the BBB, highlighting topics of interest here, including: 5-HT: 5-hydroxytryptamine (serotonin); T cell (T); Mast cell (M); tumor necrosis factor (TNF); multi-drug resistance-1 protein (MDR1); RalA Binding Protein 1 (RLIP76); multi-drug resistance protein family (MDRP); glucose transporter 1 (GLUT1); and BBB disruption (BBBD).
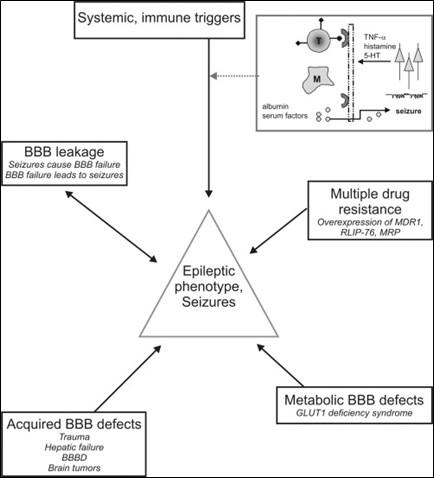
Source: Reference (6) Oby and Janigro (2006).
Figure 2: Increasing interest in research in epilepsy and the blood brain barrier.
Figure 2: Increasing interest in research in epilepsy and the blood brain barrier.
Involvement in refractory epilepsy
Permeability of the BBB is one of the factors determining the bioavailability of therapeutic drugs and resistance to chemically different AEDs. It becomes particularly relevant in drug resistant patients. There are two main theories describing drug resistance in epilepsy: (1) the target (or pharmacodynamic) hypothesis of pharmacokinetic resistance and (2) the transporter or pharmacokinetic hypothesis. The former hypothesis is based on a modification of the molecules targeted by the AED, thus reducing the efficacy of the drug. Changes in known AED targets include:
Permeability of the BBB is one of the factors determining the bioavailability of therapeutic drugs and resistance to chemically different AEDs. It becomes particularly relevant in drug resistant patients. There are two main theories describing drug resistance in epilepsy: (1) the target (or pharmacodynamic) hypothesis of pharmacokinetic resistance and (2) the transporter or pharmacokinetic hypothesis. The former hypothesis is based on a modification of the molecules targeted by the AED, thus reducing the efficacy of the drug. Changes in known AED targets include:
- Altered subunit expression in sodium channels [7-10];
- Expression of AED sensitive sodium channels in interneurons [11, 12];
- Increased expression of T-type calcium channels [13-14];
- Decrease of GABA-A receptor α1 subunits and increase of GABA-A receptor α4 subunits [15]. Recently, the possibility that GABA currents are kinetically altered in drug resistant epileptic brain has been proposed [16].
The latter hypothesis suggests that effective concentrations of the AED are not attained in the brain because of aberrant functioning of multidrug transporters. Changes in drug efflux transporters include:
- Overexpression of P-glycoprotein (MDR-1) [17-19];
- Overexpression of MRP-1 [20,21];
- Overexpression of MRP-2 [22]; and
- Overexpression of MVP [23].
Although the issue of the relative contributions of the two hypotheses remains unresolved, the transporter hypothesis emphasizes a predominant role of the BBB, and thus will be our primary focus here.
Failure and Etiology
BBB disruption after acute head trauma is a well-known pathologic finding in human (and also animal) studies and [humans (24-25)]. This disruption may persist for weeks to years after the injury and may be associated with abnormal EEG activity [24]. Whether this abnormal activity develops into epilepsy is currently unknown, but observations have suggested BBB disruption in conjunction with a slowing in EEG activity may be a precursor to seizures [26]. Others have observed persistent BBB disruption in the absence of any evidence of active epileptic foci [27].
BBB disruption after acute head trauma is a well-known pathologic finding in human (and also animal) studies and [humans (24-25)]. This disruption may persist for weeks to years after the injury and may be associated with abnormal EEG activity [24]. Whether this abnormal activity develops into epilepsy is currently unknown, but observations have suggested BBB disruption in conjunction with a slowing in EEG activity may be a precursor to seizures [26]. Others have observed persistent BBB disruption in the absence of any evidence of active epileptic foci [27].
It has been demonstrated that with relatively severe loss of BBB function there is extravasation of serum albumin into capillary endothelial cells, basal lamina and neuropil [28]. In human tissue resected from epileptogenic foci, actively spiking regions are characterized by more extravasation than less actively spiking areas [24]. Thus, the BBB integrity is closely correlated to the electrophysiological properties of the tissue as evaluated by intra-operative EEG.
Drug Delivery across the Blood Brain Barrier
Several approaches are available for drug delivery across the BBB:
Mannitol intracarotid infusion
The heightened interest in osmotic opening of the BBB as a viable mechanism of increased drug delivery to the brain provides an opportunity to explore the connection between BBB disruption and seizures in a more controlled environment [29-32]. Osmotic opening of the BBB by intracarotid infusion of a hypertonic mannitol solution is mediated by: (a) vasodilatation, (b) shrinkage of cerebrovascular endothelial cells, (c) modulation of the contractile state of the endothelial cytoskeleton, and (d) junction proteins by increased intracellular calcium, with widening of the inter-endothelial tight junctions to an estimated radius of 200 Å. The marked increase in apparent BBB permeability to intravascular substances (10-fold for small molecules) following the osmotic procedure is due to both increased diffusion and bulk fluid flow across the tight junctions. The permeability effect is largely reversed within minutes to hours.
The heightened interest in osmotic opening of the BBB as a viable mechanism of increased drug delivery to the brain provides an opportunity to explore the connection between BBB disruption and seizures in a more controlled environment [29-32]. Osmotic opening of the BBB by intracarotid infusion of a hypertonic mannitol solution is mediated by: (a) vasodilatation, (b) shrinkage of cerebrovascular endothelial cells, (c) modulation of the contractile state of the endothelial cytoskeleton, and (d) junction proteins by increased intracellular calcium, with widening of the inter-endothelial tight junctions to an estimated radius of 200 Å. The marked increase in apparent BBB permeability to intravascular substances (10-fold for small molecules) following the osmotic procedure is due to both increased diffusion and bulk fluid flow across the tight junctions. The permeability effect is largely reversed within minutes to hours.
Unfortunately, disruption procedures often result in seizures during or within 24 hours of the BBB modification [31,33]. In fact, seizures are a primary complication of osmotic BBB disruption and occur in a relatively large number of patients (13–55%) in spite of AED pretreatment. (Note: This may be due to the use of meglumine iothalamate, a known epileptogenic agent used as a computed tomography (CT) contrast agent. Although seizures continued to occur when BBB disruption was monitored by radionuclide scanning (RS) rather than CT, the frequency of occurrence is significantly reduced.)
The long-term effects of BBB disruption are unknown and may include hyperexcitability and the formation of an epileptogenic focus. As yet, there is no data available concerning if and to what extent BBB disruption precedes the development of acquired epilepsy in humans.
Other disrupting medical procedures or conditions
In addition to iatrogenic BBB disruption, other medical procedures or conditions implicating BBB failure and linked to seizure disorders exist. For example, between 6 and 36% of transplant patients experience seizures, commonly caused by drugs, metabolic derangements or hypoxic-ischemic injury [34-41]. Although the seizures are usually transient and easily treated, it has been hypothesized that the focal loss of BBB induced by immunosuppresants may play a significant role in the development of partial seizures in a subpopulation of transplant recipients.
In addition to iatrogenic BBB disruption, other medical procedures or conditions implicating BBB failure and linked to seizure disorders exist. For example, between 6 and 36% of transplant patients experience seizures, commonly caused by drugs, metabolic derangements or hypoxic-ischemic injury [34-41]. Although the seizures are usually transient and easily treated, it has been hypothesized that the focal loss of BBB induced by immunosuppresants may play a significant role in the development of partial seizures in a subpopulation of transplant recipients.
In conclusion, evidence exists that leakage of the BBB may result in the development of seizures, but a clear cut relationship and the exact nature of the offending mechanisms have remained elusive. This is likely due to the complexity of disease conditions associated with BBB leaks. These include: (a) concomitant hemodynamic disturbances (intracerebral hemorrhage or embolic stroke), (b) loss of autoregulation of cerebral blood flow (e.g., in traumatic brain injury), (c) changes in intracranial pressure due to edema, (d) inflammation, etc. Furthermore, the lack of EEG data may actually underestimate the true impact of BBB failure on the breakdown of neuronal control.
Physiological approaches
The development of new drugs against CNS disorders has not kept pace with progress in molecular neurosciences. Thus, new drugs discovered are unable to cross the BBB, the culprit being the lack of appropriate delivery systems. Further, localized and controlled delivery of the drugs at the desired sites is preferred because it reduces toxicity and increases treatment efficiency/efficacy. Here, LDLRP/Epic, a low density lipoprotein/related protein with engineered peptide compound, is a new effective therapeutics. It improves the transcytosis capacity of specific receptors expressed across the BBB.
The development of new drugs against CNS disorders has not kept pace with progress in molecular neurosciences. Thus, new drugs discovered are unable to cross the BBB, the culprit being the lack of appropriate delivery systems. Further, localized and controlled delivery of the drugs at the desired sites is preferred because it reduces toxicity and increases treatment efficiency/efficacy. Here, LDLRP/Epic, a low density lipoprotein/related protein with engineered peptide compound, is a new effective therapeutics. It improves the transcytosis capacity of specific receptors expressed across the BBB.
Chemical delivery systems
These delivery systems include:
These delivery systems include:
- Lipid-mediated transport;
- Pro-drug approach; and
- Lock-in systems.
Biological delivery
The pharmaceuticals are re-engineered to cross the BBB via specific endogenous transporters located within the brain capillary endothelium.
The pharmaceuticals are re-engineered to cross the BBB via specific endogenous transporters located within the brain capillary endothelium.
Other drug delivery systems
These include:
These include:
- Receptor-mediated transport systems that enable drug molecules to cross the BBB in vivo. Such systems exist for certain endogenous peptides (insulin, transferin);
- Solid lipids;
- Polymeric;
- Mesoporous silica; and
- Inorganic.
Use of Nanotechnology in Drug Delivery
One of the most promising applications of nanotechnology is in clinical neuroscience and multiple tasks can be carried in a pre-defined sequence [42-52].
One of the most promising applications of nanotechnology is in clinical neuroscience and multiple tasks can be carried in a pre-defined sequence [42-52].
The layered NP consists of three components: (a) a core vesicle with a double-layered membrane. It is filled with water and hydrophilic and/or hydrophobic drugs; (b) a multi-layered shell, and (c) an exterior shell that targets the NPs to cancer cells and prevents them from being detected by the immune system. The purposes of a multi-layered shell are: to stabilize the NPs; prevent drug leakage; target the NPs to the slightly acidic environment of the tumor; minimize the interactions of the NPs with non-cancerous cells; and pass unnoticed by the immune system. The multi-layered NP can also transport drugs that are not easily stored in the core (e.g., highly charged nucleic acids). These molecules can be separated from drugs in the core that could inactivate their therapeutic effects (e.g., plasma drugs).
Several nanocarriers have been developed for drug delivery at the right address. However, challenges still remain, including: How not to let the medicine(s) act before they reach the right place. Carriers usually encapsulate drugs through long-range electrostatic interactions wherein the carrier attracts oppositely-charged medicines. Other tools are available to trigger the release of drugs, e.g. magnetic fields, different pH-values, etc., but, in each case, the problem of efficiency of the drug release remains. Nonetheless, work is still needed to determine the most effective NTs for brain tumors.
Nanoparticles
The various NPs include:
The various NPs include:
- Microspheres;
- Bionanocapsules;
- Radiolabeled polyethylene glycol-coated: HexaDecylCyanoAcrylate; (HDCA); PolyAlkylCyanoAcrylate (PACA); PolyLacticCoGlycolic Acid (PLGA); Peptidomimetic Monoclonal Antibodies; (PMA);
- Magneto-Electric Nanoparticles (MENs) for targeted delivery and drug release across the BBB + wireless stimulation of cells deep in the brain;
- Bioavailability-improved nanoscale particles and molecules: Nanoscale particles and molecules can also be developed to improve drug bioavailability, i.e., the presence of drug molecules where they are needed in the brain and where they will do the most good. Drug delivery focuses on maximizing bioavailability both at specific places in the body and over a period of time. It can be achieved by molecular targeting by nano-engineered devices targeting the molecules and delivering drugs with cell precision. The basic process to use drug delivery involves at least three steps: (i) Encapsulation of the drugs; (ii) Successful delivery of said drugs to the targeted region of the brain; and (iii) Successful release of that drug there. Several NPs are employed, including: nutshells (that can be targeted to bond to cancerous cells by conjugated antibodies or peptides to anopheles' surface); platelet-coated NPs (that can deliver higher doses of medication drugs to targeted sites in the body, thus greatly enhancing their therapeutic effects); biocompatible and biodegradable gelatin NPs (that can deliver multiple drugs to the brain bypassing the blood brain barrier (BBB) to treat a variety of brain injuries and neurological diseases in stroke and other victims); and shape-shifting engineered NPs (that can be tailored to deliver drugs to specified tumors and nowhere else); and
- Nanogels: They were previously discarded because they stick together with their neighbors (lost colloidal stability) when trying to “upload” the drugs within them. This made delivery impossible or ineffective. However, a solution to the stickiness was developed by Prof. Potemkin at the University of Florida. Figure 3 is an illustration of multi-shell nanogels with responsive shell permeability.
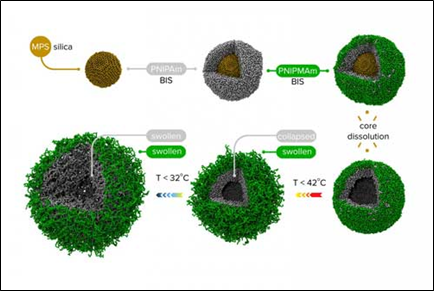
Source: Professor Igor Potemkin, Lomonosov Moscow State University.
Figure 3: Multi-shells nanogels with responsive shell permeability.
Figure 3: Multi-shells nanogels with responsive shell permeability.
Potemkin solved the nanogel problem by creating a carrier surrounded by 2 “membranes” (or shells) of different chemical structure around a silica core. At the end of the synthesis, the core will be chemically dissolved leaving only the “empty” cavity. The “outer” porous shell plays a protective (stabilizing) role and hinders aggregation of nano-capsules, while the pores of the “inner” shell can open and close depending on the temperature due to variable interactions between its monomeric units. At the time of filling, the pores of both shells are “open” and the nanogel absorbs the drug molecules as a sponge. Then, the temperature changes and the pores of the inner shell close locking-in the cavity and readying the drug for delivery. Subsequently, the pores will open again and the guest molecules will be released only in the places where the temperature allows. Nonetheless, work is still needed to demonstrate that nanogels are the ideal drug delivery carrier.
Other available technologies include:
- Liposomes;
- Peptides [52]: A peptide is a compound of 2 or more amino acids in which the alpha carboxyl group of one is united with the alpha amino group of the other with the elimination of a molecule of water, thus forming a peptide bond. A polypeptide is a peptide formed by the union of an infinitely (usually large) number of amino acids. Peptides are able to cross the BBB through various mechanisms providing new diagnostic and therapeutic avenues. Various mechanisms are under study. To help in this effort, the “brainpeps” database has been developed. It includes transport information, prioritization of peptide choices for evaluating different BBB responses, study of quantitative BBB behavior, etc. For example, casomorphion (a heptapeptide) is able to pass the BBB (16);
- HexaDecylCyanoAcrylate (HDCA): Although these have been used against sarcomas in rats, they are not yet ready for clinical trials because the NPs accumulate in the surrounding healthy tissue;
- PolyAlkylCyanoAcrylate (PACA);
- The more promising PolyLacticCoGlycolic Acid (PLGA) coated with polysorbate 80 or poloxamer 188. Loaded with Doxorubicin, it can be employed in the treatment of glioblastomas (phase I); and
- Magneto-Electric Nanoparticles (MENs).
It is clear that NP-based delivery enables sophisticated tactics to fight disease. With their small size and their intricate engineering design, NPs can improve control over drug release profiles, both spatially as well as temporally, and can reduce harmful side effects.
Engineered nanoscale devices
Nanoscale devices can be engineered to aid the delivery of life-saving drug treatments (including cancer) at the affected sites. Such minute devices have the potential to be engineered to efficiently and more safely deliver drug treatments directly to the location of diseased cells while helping avoid harm to healthy cells that fall victim to toxic drugs administered by conventional means. Engineered NDs include:
Nanoscale devices can be engineered to aid the delivery of life-saving drug treatments (including cancer) at the affected sites. Such minute devices have the potential to be engineered to efficiently and more safely deliver drug treatments directly to the location of diseased cells while helping avoid harm to healthy cells that fall victim to toxic drugs administered by conventional means. Engineered NDs include:
- Improved pharmacokinetic strategies of drug molecules (biodistribution, bioavailability, controlled and site-specific drug release);
- Decreased peripheral toxicity;
- Influenced manufacturing factors (type of polymers and surfactants, particle size and size distribution, drug molecules); and
- Limitations of drug amount delivered, and physiological factors [phagocytic activity of the reticulo-endothelial system (RES), protein opsonization].
Miniaturized drug delivery systems
Several "nano-carriages" for drug delivery to the right address have been created but many challenges remain, chief among them being how not to let the medicine act before it gets to the right place in the brain. The carriers usually encapsulate drugs through long-range electrostatic interactions wherein the carrier attracts oppositely charged medicine. Other tools are available to trigger the release of drugs, for example, an external magnetic field, different pH values, etc.Such systems, loaded with life-saving drugs, may revolutionize the way in which cancer is treated with chemotherapy, reducing the debilitating side effects of the therapy, making medications more effective, and all the while preserving the healthy living cells. These include: (a) Protein Cages (containing the anticancer drug daunomycin, a small amount of acid and set at a pH below neutral), which slightly open to let the drug jump inside the tumor, stay in until it came in contact with cancer cells. They can kill more than 70% without attacking healthy cells; (b) Microbubbles (microscopic balls of gas enclosed in an ultra-thin layer of fat which can be injected into the blood stream). Theoretically, upon reaching the unhealthy part of the brain, they are burst with ultrasound waves, releasing the drug exactly where it is needed. Because the entire blood stream is not being flooded with the drug, side-effects from chemotherapy can be greatly reduced; and (c) Multi-shell hollow nanogels with responsive shell permeability described above.
Several "nano-carriages" for drug delivery to the right address have been created but many challenges remain, chief among them being how not to let the medicine act before it gets to the right place in the brain. The carriers usually encapsulate drugs through long-range electrostatic interactions wherein the carrier attracts oppositely charged medicine. Other tools are available to trigger the release of drugs, for example, an external magnetic field, different pH values, etc.Such systems, loaded with life-saving drugs, may revolutionize the way in which cancer is treated with chemotherapy, reducing the debilitating side effects of the therapy, making medications more effective, and all the while preserving the healthy living cells. These include: (a) Protein Cages (containing the anticancer drug daunomycin, a small amount of acid and set at a pH below neutral), which slightly open to let the drug jump inside the tumor, stay in until it came in contact with cancer cells. They can kill more than 70% without attacking healthy cells; (b) Microbubbles (microscopic balls of gas enclosed in an ultra-thin layer of fat which can be injected into the blood stream). Theoretically, upon reaching the unhealthy part of the brain, they are burst with ultrasound waves, releasing the drug exactly where it is needed. Because the entire blood stream is not being flooded with the drug, side-effects from chemotherapy can be greatly reduced; and (c) Multi-shell hollow nanogels with responsive shell permeability described above.
There are several clinical advantages to these NPs. Specifically, they:
- Circulate throughout the bloodstream without being attacked by the immune system;
- Preferentially bind to cancerous areas allowing them to deliver and release their drug payloads specifically there;
- Are non-toxic as the platelet membranes are nanoparticle cores made of a biodegradable polymer that can be safely metabolized by the body; and
- Can be packed with many small drug molecules that diffuse out of the polymer core and through the platelet membrane onto their targets.
Nanocarriers May Carry New Hope for Brain Cancer Therapy
Glioblastoma multiform (GBM), a cancer of the brain also known as "octopus tumors" because of the manner in which the cancer cells extend their tendrils into the surrounding tissue, is virtually inoperable, resistant to therapies, and always fatal, usually within 15 months of onset. Each year, GBM kills approximately 15,000 people in the United States. One of the major obstacles to treatment is the blood brain barrier (BBB), the network of blood vessels that allows essential nutrients to enter the brain but blocks the passage of other substances. What is desperately needed is a means of effectively transporting therapeutic drugs through this barrier.
Glioblastoma multiform (GBM), a cancer of the brain also known as "octopus tumors" because of the manner in which the cancer cells extend their tendrils into the surrounding tissue, is virtually inoperable, resistant to therapies, and always fatal, usually within 15 months of onset. Each year, GBM kills approximately 15,000 people in the United States. One of the major obstacles to treatment is the blood brain barrier (BBB), the network of blood vessels that allows essential nutrients to enter the brain but blocks the passage of other substances. What is desperately needed is a means of effectively transporting therapeutic drugs through this barrier.
Dr. Ting Hu, a nanoscience expert at Lawrence Berkeley National Laboratory (Berkeley Lab) may have the solution in the form of a new family of nanocarriers formed from the self-assembly of amphiphilic peptides and polymers, called "3HM" for coiled-coil 3-helix micelles [53]. At only 20 nanometers in size and featuring a unique hierarchical structure, these new 3HM nanocarriers meet all the size and stability requirements for effectively delivering therapeutic drugs to GBM tumors. Amphiphiles are chemical compounds that feature both hydrophilic (water-loving) and lipophilic (fat-loving) properties. Micelles are spherical aggregates of amphiphiles. Using the radioactive form of copper (Cu-64) in combination with positron emission tomography (PET) and magnetic resonance imaging (MRI), Dr. Hu and his team demonstrated that 3HM can cross the BBB and accumulate inside GBM tumors at nearly double the concentration rate of current FDA-approved nanocarriers. (Figure 4).
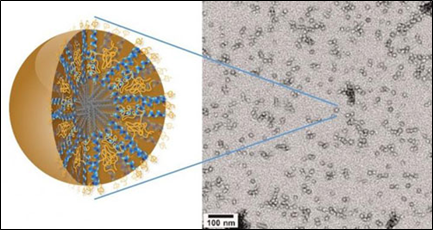
Source: Dr. Ting Hu, Lawrence Berkeley National Laboratory.
Figure 4: New 3HM nanocarriers for effectively delivery of therapeutic drugs to brain cancer tumors.
Figure 4: New 3HM nanocarriers for effectively delivery of therapeutic drugs to brain cancer tumors.
The 3 HM nanocarriers have shown very good attributes for the treatment of brain cancers in terms of long circulation, deep tumor penetration and low accumulation in off-target organs such as the liver and spleen. The fact that they are able to cross the BBB of GBM-bearing rats and selectively accumulate within tumor tissue, opens the possibility of treating GBM via intravenous drug administration rather than invasive measures. While there is still a lot to learn about why 3HM is able to do what it does, so far all the results have been very positive.
Glial cells provide physical and chemical support for neurons. Approximately 90-percent of all the cells in the brain are glial cells which, unlike neurons, undergo a cycle of birth, differentiation, and mitosis. Undergoing this cycle makes glial cells vulnerable to becoming cancerous. When they do, they can take on different shapes, which often makes detection difficult until the tumors are dangerously large. The multiple shapes of a cancerous glial cell also make it difficult to identify and locate all of the cell's tendrils. Removal or destruction of the main tumor mass while leaving these tendrils intact is ineffective therapy.
Although there are FDA-approved therapeutic drugs for the treatment of GBM, these treatments have had little impact on patient survival rate because the BBB has limited the accumulation of therapeutics within the brain. Typically, GBM therapeutics are ferried across the blood brain barrier in special liposomes that are approximately 110 nanometers in size. By contrast, the 3HM nanocarriers are only about 20 nanometers in size. Their smaller size and unique hierarchical structure afforded the 3HM nanocarriers much greater access to rat GBM tumors than 110-nanometer liposomes. Copper-64 is used to label both 3HM and liposome nanocarriers for systematic PET and MRI studies to find out how a nanocarrier's size might affect the pharmacokinetics and biodistribution in rats with GBM tumors. The results not only confirmed the effectiveness of 3HM as GBM delivery vessels, they also suggest that PET and MRI imaging of nanoparticle distribution and tumor kinetics can be used to improve the future design of nanoparticles for GBM treatment.
Multi-Drug and Anti-Epileptic Drug Resistance
The complex pattern of MDR-1 expression in epileptic patients does not directly support a significant pharmacokinetic role in human epilepsy [54-57]. While localization of the drug extrusion pump in the BBB is consistent with the pharmacokinetic explanation for drug resistance, it is still unclear if or how the presence of MDR-1 in the parenchyma affects drug delivery and distribution or whether it is involved in other cellular functions. In a recent study [58-60], brain: plasma AED ratios were determined intra-operatively during lobectomies performed to alleviate drug-resistant seizures. The brain: plasma ratio of carbamazepine was 1.48 when therapeutic serum levels (15–34 μM) were achieved. When concentrations of carbamazepine found in multiple drug resistant brain were directly applied to human cortical slices from drug resistant patients made hyperexcitable and hypersynchronous by Mg2+-free media, bursting frequency was not significantly affected, but overall excitability was reduced by 40%. Similar results were obtained for phenytoin. At higher AED concentrations (60–200 μM), a dose-dependent decrease of bursting frequency and amplitude was observed. These results support the hypothesis that multiple drug resistance to AEDs involves cerebrovascular changes that impede the achievement of appropriate drug levels in the central nervous system.
The complex pattern of MDR-1 expression in epileptic patients does not directly support a significant pharmacokinetic role in human epilepsy [54-57]. While localization of the drug extrusion pump in the BBB is consistent with the pharmacokinetic explanation for drug resistance, it is still unclear if or how the presence of MDR-1 in the parenchyma affects drug delivery and distribution or whether it is involved in other cellular functions. In a recent study [58-60], brain: plasma AED ratios were determined intra-operatively during lobectomies performed to alleviate drug-resistant seizures. The brain: plasma ratio of carbamazepine was 1.48 when therapeutic serum levels (15–34 μM) were achieved. When concentrations of carbamazepine found in multiple drug resistant brain were directly applied to human cortical slices from drug resistant patients made hyperexcitable and hypersynchronous by Mg2+-free media, bursting frequency was not significantly affected, but overall excitability was reduced by 40%. Similar results were obtained for phenytoin. At higher AED concentrations (60–200 μM), a dose-dependent decrease of bursting frequency and amplitude was observed. These results support the hypothesis that multiple drug resistance to AEDs involves cerebrovascular changes that impede the achievement of appropriate drug levels in the central nervous system.
Newly discovered drug transporters, often with properties similar to MDR-1, suggest that MDR-1 might not be the exclusive mechanism responsible for drug efflux. In particular, RLIP76 is important in transporting phenytoin and carbamazepine at the human BBB, highlighting a potentially significant function in determining drug-resistance in epilepsy. In fact, RLIP76 fulfills many of the predicted properties for a mediator of CNS pharmacoresistance [61,62], including:
- Presence at the anatomical interface between brain and blood;
- Transport of AEDs;
- Functional expression in brain microvascular endothelial cells but not in parenchymal glia or neurons; and
- Increased CNS accumulation of phenytoin in RLIP76−/−mice.
The role of RLIP76 in AED resistance is still in its infancy and more research is needed to fully evaluate its potential as a drug resistance candidate. Even with the increasing information regarding drug transporters, it is unclear if or how the distribution of MDR-1, MRPs, and RLIP76 is related to the pathology of epilepsy itself. It is possible that their over-expression is a response to a hostile environment or that they are regulated exclusively by chemotherapy.
A pervasive problem in studying drug resistance is the lack of brain tissue from patients with drug responsive epilepsy. So, while increased expression of drug transporters has been reported. in brain tissue of patients with refractory epilepsy, the lack of adequate controls makes comparison between drug resistant and drug respondent patients problematic. Thus, the question persists as to whether the increased drug transporter expression in patients with drug resistant epilepsy is a cause of pharmacoresistance, an effect of uncontrolled seizures, or an epiphenomenon occurring in epileptic brain tissue irrespective of drug responsiveness. Although sporadic information is available on the specific cellular biochemical changes that occur during the induction of seizures in animal models of epilepsy, such models may be able to provide some insight as to the question of cause and effect.
While seizures seem to be a factor in the overexpression of efflux drug transporters, AEDs themselves have also been shown to have an impact on the expression of MDR-1.
If the BBB is an impediment to CNS drug delivery, one is tempted to circumvent or disrupt the endothelial layer. This has been attempted for CNS tumors, and recent discoveries are supporting the approach by semi-invasive, chronic methodologies. One interesting question relates to the possibility that highly lipophilic drugs (e.g., AEDs) will poorly partition across a leaky BBB due to perivascular edema. If this were indeed the case, the design of more polar molecules may, paradoxically, be a solution.
In conclusion, the hypotheses of mechanisms of drug resistance are not mutually exclusive. In fact, the underlying mechanism of pharmacoresistant epilepsy is probably some combination of alterations of AED targets and transporters. An opening in the BBB would provide better access to the brain parenchyma for AEDs, perhaps in spite of an upregulation of multidrug transporters, but partition with perivascular edema may be a problem.
Glucose Transporter Altered Metabolism in Epilepsy
The BBB has developed a sophisticated mechanism to carry glucose efficiently into the brain. This sodium- and insulin-independent transport depends on endothelial expression of glucose transporter-1 (GLUT1), which mediates glucose transport across the BBB and is thus essential for brain energy metabolism [63,64]. First described in 1991, the GLUT-1 causes impaired transport of glucose across the BBB, interfering with cerebral energy metabolism and brain function, ultimately leading to seizures. While the resulting seizures do not generally respond to common AEDs, they can be controlled by strict adherence to a ketogenic diet [65-67]. Understanding the mechanism of action of the ketogenic diet may perhaps provide insight into how other types of seizures can be controlled.
The BBB has developed a sophisticated mechanism to carry glucose efficiently into the brain. This sodium- and insulin-independent transport depends on endothelial expression of glucose transporter-1 (GLUT1), which mediates glucose transport across the BBB and is thus essential for brain energy metabolism [63,64]. First described in 1991, the GLUT-1 causes impaired transport of glucose across the BBB, interfering with cerebral energy metabolism and brain function, ultimately leading to seizures. While the resulting seizures do not generally respond to common AEDs, they can be controlled by strict adherence to a ketogenic diet [65-67]. Understanding the mechanism of action of the ketogenic diet may perhaps provide insight into how other types of seizures can be controlled.
The ketogenic diet changes biochemical parameters of the blood, significantly altering the level of ketone bodies. Ketone bodies are the principal alternative energy source for the brain at times of glucose shortage. They exert an anticonvulsant effect that is maintained as long as the blood ketone bodies are elevated. Other possibilities exist including that cerebral ketone body metabolism reduces neuronal excitability by increasing cerebral energy reserves. Regardless of how a ketogenic diet may prevent seizures in GLUT1-deficient children, it is clear that BBB dysfunction is a major etiological event in this subtype of seizure disorders.
Inflammation, Epilepsy and Bbb
Neuroinflammation as a branch of immunology
Traditionally, neuroinflammation has been seen as a CNS-specific branch of immunology. Thus, a great deal of effort has been made to find immunocompetent or inflammatory cells in the brain (or spinal cord) parenchyma. A schematic representation of the cells and molecules involved in cerebral inflammation is shown in Figure 5, which provides a summary of events that may link intravascular inflammatory events to pro-epileptogenic events in the brain parenchyma.
Traditionally, neuroinflammation has been seen as a CNS-specific branch of immunology. Thus, a great deal of effort has been made to find immunocompetent or inflammatory cells in the brain (or spinal cord) parenchyma. A schematic representation of the cells and molecules involved in cerebral inflammation is shown in Figure 5, which provides a summary of events that may link intravascular inflammatory events to pro-epileptogenic events in the brain parenchyma.
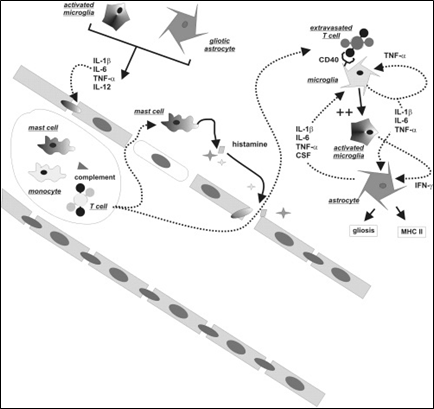
Source: Reference (6) Oby and Janigro (2006).
Figure 5: Summary of events linking intravascular inflammatory events to pro-epileptogenic events in the brain parenchyma.
Figure 5: Summary of events linking intravascular inflammatory events to pro-epileptogenic events in the brain parenchyma.
(IL = Interleukin (IL); TNF = Tumor necrosis factor; IFN = Interferon; CSF = Colony-stimulating factor; MHC = Major histocompatibility complex)
The schematic representation in Figure 4 does not implicate the presence of a particular pathogen, and may actually occur under sterile inflammatory conditions. Under normal conditions, an intact BBB separates the immune system from the CNS parenchyma. Trafficking of white blood cells is restricted to specific regions of the vasculature, namely the Virchow-Robin space above the leptomeningeal fusion, and the subarachnoid space. When the BBB is breached, both molecular (e.g., complement) and cellular players may extravasate. The mechanism of this BBB attack by intravascular agents implicates metalloproteinases and other molecules released by activated blood cells. The abnormal permeation across the barrier results in further, and perhaps distal, disruption of tight junctions, this time mediated by release of inflammatory mediators by both extravasated blood cells and activated microglia. Frank cellular immunoagression occurs if and when histocompatibility mechanisms are activated and antibody-mediated reactions occur.
It is now clear that virtually every class of brain cells has some potential or propensity to replicate immunological or inflammatory processes.
Not all brain blood vessels constitute a barrier
Not all the blood vessels in the brain constitute a BBB: only capillary vessels are endowed with a full-blown BBB phenotype. Vessels of increasing diameter have comparably increasing levels of leakiness and thus superficial vessels of large diameter are the leakiest, while penetrating pial vessels and descending penetrating vessels tend to have an intermediate barrier function. Since most animals, including vertebrates, have some form of barrier separating their blood circulation from the brain or the central nervous system, it has been speculated that profound evolutionary pressure existed to create such a complex organ. The CNS of vertebrates lacks lymphatic drainage; thus, passage of molecules or ions across the capillary wall will result in a net gain of water into the brain compartment, soon leading to an increase in intracranial pressure. This is a most damaging situation since the brain is contained within a rigid skull. Thus, the combination of a restricted volume and the lack of effective drainage for solutes leaving the blood for the brain parenchyma is probably one of the leading implications for the necessity of a tight barrier between the blood and the brain. The relationship between various BBB compartments (Virchow-Robin space; venules; penetrating pial vessels) has been reviewed in detail elsewhere [68]).
Not all the blood vessels in the brain constitute a BBB: only capillary vessels are endowed with a full-blown BBB phenotype. Vessels of increasing diameter have comparably increasing levels of leakiness and thus superficial vessels of large diameter are the leakiest, while penetrating pial vessels and descending penetrating vessels tend to have an intermediate barrier function. Since most animals, including vertebrates, have some form of barrier separating their blood circulation from the brain or the central nervous system, it has been speculated that profound evolutionary pressure existed to create such a complex organ. The CNS of vertebrates lacks lymphatic drainage; thus, passage of molecules or ions across the capillary wall will result in a net gain of water into the brain compartment, soon leading to an increase in intracranial pressure. This is a most damaging situation since the brain is contained within a rigid skull. Thus, the combination of a restricted volume and the lack of effective drainage for solutes leaving the blood for the brain parenchyma is probably one of the leading implications for the necessity of a tight barrier between the blood and the brain. The relationship between various BBB compartments (Virchow-Robin space; venules; penetrating pial vessels) has been reviewed in detail elsewhere [68]).
Cellular mechanisms that initiate changes in the blood brain barrier
Our understanding of the cellular mechanisms that initiate changes in BBB permeability is limited. Several vasoactive or inflammatory compounds, which include bradykinin, complement 3a, ATP, histamine and serotonin from mast cells, interleukins, arachidonic acid and its metabolites, interferon alpha and beta, prostaglandins, and tumor necrosis factor, have all been shown to alter BBB permeability [69-73]. A subsequent rise in intracellular calcium may stimulate cyclic nucleotide production, which in turn leads to pinocytosis and vesicular transport. It has been proposed that the rise in intracellular calcium also triggers a contraction of the endothelial cells, which increases permeability by deforming or opening the intercellular tight junctions. The role of vasoactive agents in the control of BBB permeability, edema formation, and leukocyte infiltration is a key field of study. Given the prominent role of BBB integrity in the control of brain homeostasis and neuronal excitability, it is simple to predict that inflammatory changes affecting BBB integrity may have a profound impact on brain function.
Our understanding of the cellular mechanisms that initiate changes in BBB permeability is limited. Several vasoactive or inflammatory compounds, which include bradykinin, complement 3a, ATP, histamine and serotonin from mast cells, interleukins, arachidonic acid and its metabolites, interferon alpha and beta, prostaglandins, and tumor necrosis factor, have all been shown to alter BBB permeability [69-73]. A subsequent rise in intracellular calcium may stimulate cyclic nucleotide production, which in turn leads to pinocytosis and vesicular transport. It has been proposed that the rise in intracellular calcium also triggers a contraction of the endothelial cells, which increases permeability by deforming or opening the intercellular tight junctions. The role of vasoactive agents in the control of BBB permeability, edema formation, and leukocyte infiltration is a key field of study. Given the prominent role of BBB integrity in the control of brain homeostasis and neuronal excitability, it is simple to predict that inflammatory changes affecting BBB integrity may have a profound impact on brain function.
The tightness of the BBB is a serious hindrance to the entry of both immunocompetent cells and specific antibodies, which are necessary if the immune system is to attack infectious agents or abnormal autologous cells undergoing uncontrolled proliferation in the brain. The highly specialized tight endothelium isolates the brain from immune surveillance and allows only a few mononuclear cells (activated T cells) to migrate into the CNS [74]. Therefore, the low expression of major histocompatibility complex (MHC) antigens, the low number of antigen-presenting cells, and the fact that the CNS is not drained by a fully developed lymphatic vasculature, makes the brain an “immunoprivileged” site. However, when inflammation does occur, there is extensive leukocyte migration into the brain. Both brain endothelial cells and astrocytes can act as antigen presenting cells in order to facilitate the entry of T-lymphocytes and antibodies. The BBB itself plays an active role in the mediation of this neuroimmune response, either by the production of inflammatory mediators or by expression of adhesion molecules.
In addition to an intrinsic inflammatory mechanism, it is increasingly clear that the peripheral immune system may, under certain circumstances, provoke havoc in the CNS. This is a rare occurrence, however, thanks in part to a BBB mechanism that (1) impedes or hampers cell migration across the endothelial cell monolayer; (2) prevents or reduces chemoattraction of potentially harmful macrophages. The flipside of this is that a CNS-specific antigen may be considered as nonself and thus lead to autoimmunity. Again, even when this happens, the BBB minimizes the risks associated with the presence of offending effector cells in the peripheral circulation.
Immune system influence by seizures
Clinical evidences link inflammation of the CNS to the development of seizures. In Rasmussen's syndrome, a very rare form of brain malfunction which may occur at any time in childhood, it is known that brain cells usually in only one hemisphere are inflamed. Rasmussen's encephalitis was originally thought to be a chronic form of viral encephalitis but is now considered to be an autoimmune disease of the brain and is more properly termed Rasmussen's syndrome. Starting in one area of one side of the brain, the disease appears to gradually and progressively involve that side of the brain causing progressive and intractable focal seizures, a hemiparesis, and expressive aphasia when the left hemisphere is involved. Immune therapy with steroids, immunoglobulins, or plasmaphoresis provides only temporary relief from seizures.
Clinical evidences link inflammation of the CNS to the development of seizures. In Rasmussen's syndrome, a very rare form of brain malfunction which may occur at any time in childhood, it is known that brain cells usually in only one hemisphere are inflamed. Rasmussen's encephalitis was originally thought to be a chronic form of viral encephalitis but is now considered to be an autoimmune disease of the brain and is more properly termed Rasmussen's syndrome. Starting in one area of one side of the brain, the disease appears to gradually and progressively involve that side of the brain causing progressive and intractable focal seizures, a hemiparesis, and expressive aphasia when the left hemisphere is involved. Immune therapy with steroids, immunoglobulins, or plasmaphoresis provides only temporary relief from seizures.
How Rasmussen's relates to other epilepsies in terms of etiology and pathology, relationship to seizure focus, and origin of offending cells and mediators has yet to be fully elucidated. However, it is remarkable that the animal studies led to the hypothesis that brain-derived inflammatory mediators and cells are “activated” or released, while it is clear that in Rasmussen's the origin is systemic. It is thus possible that seizures influence the immune system of humans in a fashion that is not replicable in animal models. An excellent recent review by Vezzani and Granata addressed most of the issues linking inflammation to the BBB and epilepsy [75].
Conclusion
The BBB is intimately interconnected with the cause, effect and treatment of seizures. These relationships continue to move toward the forefront of epilepsy research and offer a distinctive opportunity to further our understanding of the disease. With the constant refinement of existing technologies and development of new technologies (e.g., nanotechnology) and, our ability to image, manipulate and explore the BBB will only improve, thereby enabling the next generation of advances.
References
- Fymat AL. “Nanoneurology: Drug delivery across the brain protective barriers”, Journal of Nanomedicine Research 5.1 (2017): 1-4. 00105. DOI: 10:5406/jnmr/2017.05.00105.
- Fymat AL. “Epilepsy: A review”. Journal Current Opinions on Neurological Science 1.5 (2017): 240-254.
- Cornford EM and Oldendorf WH. “Epilepsy and the blood-brain barrier”. Advances in Neurology 44 (1986): 787–812.
- Pardridge WM. “Blood-brain barrier biology and methodology”. Journal of Neurovirology 5.6 (1999): 556-569.
- Golden PL and Pollack GM. “Blood-brain barrier efflux transport”. Journal of Pharmaceutical Science 92.9 (2003): 1739–1753.
- Oby E and Janigro A. “The Blood–Brain Barrier and Epilepsy”, Epilepsia 26 October 2006 , DOI: 10.1111/j.1528-1167.2006.00817.x
- Ellerkmann RK., et al. “Molecular and functional changes in voltage-dependent Na (+) channels following pilocarpine-induced status epilepticus in rat dentate granule cells”. Neuroscience 119.2 (2003): 323-333.
- Whitaker WR., et al. “Changes in the mRNAs encoding voltage-gated sodium channel types II and III in human epileptic hippocampus”. Neuroscience 106 (2001): 275-285.
- Aronica E., et al. “Induction of neonatal sodium channel II and III alpha-isoform mRNAs in neurons and microglia after status epilepticus in the rat hippocampus”. European Journal of Neuroscience 13.6 (2001):1261–1266.
- Bartolomei F., et al. “Changes in the mRNAs encoding subtypes I, II and III sodium channel alpha subunits following kainate-induced seizures in rat brain”. Journal of Neurocytology 26.10 (1997): 667-678.
- Remy S., et al. “Anticonvulsant pharmacology of voltage-gated Na+channels in hippocampal neurons of control and chronically epileptic rats”. European Journal of Neuroscience 17.12 (2003): 2648-2258.
- Remy S and Beck H. “Molecular and cellular mechanisms of pharmacoresistance in epilepsy”. Brain 129 (pt 1) (2006): 18-35.
- Su H., et al. “Upregulation of a T-type Ca2+channel causes a long-lasting modification of neuronal firing mode after status epilepticus”. Journal of Neuroscience 22 (2002): 3645-3655.
- Huguenard JR. “Block of T -Type Ca (2+) channels is an important action of succinimide antiabsence drugs”. Epilepsy Currents 2 (2002): 49-52.
- Brooks-Kayal AR., et al. “Selective changes in single cell GABA(A) receptor subunit expression and function in temporal lobe epilepsy”. Natural Medicine 4.10 (1998): 1166-1172.
- Brooks-Kayal AR., et al. “Selective changes in single cell GABA(A) receptor subunit expression and function in temporal lobe epilepsy”. Nature Medicine 4.10 (1998): 1166-1172.
- Marchi N., et al. “Significance of MDR-1 and multiple drug resistance in refractory human epileptic brain”. BMC Medicine 2 (2004): 37.
- Aronica E., et al. “Expression and cellular distribution of multidrug transporter proteins in two major causes of medically intractable epilepsy: focal cortical dysplasia and glioneuronal tumors”. Neuroscience 118 (2003): 417-429.
- Tishler DM., et al. “MDR-1 gene expression in brain of patients with medically intractable epilepsy”. Epilepsia 36 (1995b): 1-6.
- Sisodiya SM., et al. “Drug resistance in epilepsy: expression of drug resistance proteins in common causes of refractory epilepsy”. Brain 125(pt 1) (2002): 22-31.
- Sisodiya SM., et al. “Multidrug-resistance protein 1 in focal cortical dysplasia”. Lancet 357 (2001): 42–43.
- Dombrowski SM., et al. “Overexpression of multiple drug resistance genes in endothelial cells from patients with refractory epilepsy”. Epilepsia 42 (2001):1501-1506.
- Van Vliet EA., et al. “Expression and cellular distribution of major vault protein: a putative marker for pharmacoresistance in a rat model for temporal lobe epilepsy”. Epilepsia 45 (2004): 1506-1516.
- Korn A., et al. “Focal cortical dysfunction and blood-brain barrier disruption in patients with postconcussion syndrome”. Journal of Clinical Neurophysiology 22 (2005):1-9.
- Baskaya MK., et al. “The biphasic opening of the blood-brain barrier in the cortex and hippocampus after traumatic brain injury in rats”. Neuroscience Letters 226.1 (1997): 33-36.
- Pavlovsky L., et al. “Persistent BBB disruption may underlie alpha interferon-induced seizures”. Journal of Neurology 252.1 (2005): 42-46.
- Tomkins O., et al. “Frequent blood-brain barrier disruption in the human cerebral cortex”. Cellular and Molecular Neurobiology 21.6 (2001): 675-691.
- Cornford EM., et al. “The human brain GLUT1 glucose transporter: ultrastructural localization to the blood-brain barrier endothelia”. Journal of Cerebral Blood Flow & Metabolism: SAGE Journals 14.1 (1994): 106-112.
- Siegal T., et al. “In vivo assessment of the window of barrier opening after osmotic blood-brain barrier disruption in humans”. Journal of Neurosurgery 92.4 (2000): 599-605.
- Kroll RA and Neuwelt EA. “Outwitting the blood-brain barrier for therapeutic purposes: osmotic opening and other means”. Neurosurgery 42.5 (1998): 1083-1099.
- Neuwelt EA., et al. “Osmotic blood-brain barrier modification: clinical documentation by enhanced CT scanning and/or radionuclide brain scanning”. American Journal of Roentgenology 141.4 (1983): 829-835.
- Neuwelt EA., et al. “Therapeutic efficacy of multiagent chemotherapy with drug delivery enhancement by blood-brain barrier modification in glioblastoma”. Neurosurgery 19 (1986): 573–582.
- Roman Goldstein S., et al. “Osmotic blood-brain barrier disruption: CT and radionuclide imaging”. American Journal of Neuroradiology 15.3 (1994): 581-590.
- Patchell RA. “Neurological complications of organ transplantation”. Annals of Neurology 36 (1994): 688-703.
- Gilmore RL. “Seizures and antiepileptic drug use in transplant patients”. Neurologic Clinics 6.2 (1988): 279-296.
- Greig NH. “Drug delivery to the brain by blood–brain barrier circumvention and drug modification. Implications of the Blood–Brain Barrier and its Manipulation”, in Newwelt EA, Plenum Press, New York, (1989): 311-367.
- Smith QR. “Drug delivery to the brain and the role of carrier mediated transport”. Advances in Experimental Medicine and Biology 331 (1993): 83-93.
- Rapoport SI., et al. “Regional cerebrovascular permeability to [14C] sucrose after osmotic opening of the blood-brain barrier”. Brain Research 150.3 (1978): 653-657.
- Sanovich E., et al. “Pathway across blood-brain barrier opened by the bradykinin agonist, RMP-7”. Brain Research 705.1–2 (1995): 125-135.
- Fromm MF. “P-glycoprotein: A defense mechanism limiting oral bioavailability and CNS accumulation of drugs”. International Journal of Clinical Pharmacology and Therapeutics38.2 (2000): 69-74.
- Golden PL and Pardridge WM. “Brain microvascular P-glycoprotein and a revised model of multidrug resistance in brain”. Cellular and Molecular Neurobiology 20 (2000): 165-181.
- Kreuter J. “Nanoparticles and microparticles for drug and vaccine delivery”. Journal of Anatomy 189.3 (1996): 503-505.
- Kreuter J. “Nanoparticles in Encyclopedia of Pharmaceutical Technology, Editors: Swarbick J and Boylan JC, Marcel Dekker, New York (1994): 165–90.
- Kreuter J. “Large scale production problems and manufacturing of nanoparticles” in Specialized Drug Delivery Systems, Editor: Tyle P, Marcel Dekker, New York (1990): 257-266.
- Kreuter J. “Nanoparticulate systems for brain delivery of drugs”. Advanced Drug Delivery Reviews 47.1 (2001): 65-81.
- Marty JJ., et al. “Nanoparticles–A new colloidal drug delivery system”. Pharmaceutica Acta Helvetiae53 (1978): 17-23.
- Muller RH. et al. “Solid lipid nanoparticles—An alternative colloidal carrier for controlled drug delivery”. European Journal of Pharmaceutics and Biopharmaceutics50.1 (1995): 162-177.
- Schwarz C., et al. “Solid lipid nanoparticles for controlled drug delivery: Production, characterization and sterilization”. Journal of Controlled Release30 (1994): 83-96.
- Schroder U., et al. “Nanoparticle technology for delivery of drugs across the blood-brain barrier”. Journal of Pharmaceutical Sciences87.11 (1998): 1305-1307.
- TakadaY., et al. “Rapid high affinity transport of a chemotherapeutic amino acid across the blood-brain barrier”. Cancer Research 52.8 (1992): 2191-2196.
- Schroder U and Sabel BA. “Nanoparticles, a drug carrier system to pass the blood-brain barrier, permit central analgesic effects of i.v. dalagrin injections”. Brain Research710 (1996): 121-124.
- Kreuter J., et al. “Passage of peptides through the blood–brain barrier with colloidal particles (nanoparticles)”. Brain Research 674.1 ((1995): 171-174.
- Birrenbach G and Speiser PP. “Polymerized micelles and their use as adjuvants in immunology”. Journal of Pharmaceutical Sciences65 (1976): 1763-1766.
- Gelprina SE., et al. “Chemotherapy of brain tumors using doxorubicin bound to polysorbate-80 coated nanoparticles” in Proceedings of the 3rd World Meeting APV/APGI, Berlin,April 3-6, (2000): 441–2.
- Gulyaev AE., et al. “Significant transport of doxorubicin into the brain with polysorbate-80 coated nanoparticles”. Pharmaceutical Research 16 (1999): 1564-1569.
- Gupta PK and Hung CT. “Targeted delivery of low dose doxorubicin hydrochloride administered via magnetic albumin microspheres in rats”. Journal of Microencapsulation7 (1990): 85-94.
- Gupta PK., et al. “Quantitation of the release of doxorubicin from colloidal drug forms using dynamic dialysis”. International Journal of Pharmaceutics 76.2 (1986): 141-145.
- Marroni M., et al. “Vascular and parenchymal mechanisms in multiple drug resistance: a lesson from human epilepsy”. Current Drug Targets 4 (2003): 297-304.
- Abbott NJ., et al. “Drug resistance in epilepsy: the role of the blood-brain barrier”. Novartis Foundation Symposia 243 (2002): 38-47.
- Oby E., et al. “In vitro responsiveness of human drug resistant tissue to antiepileptic drugs: insights into the mechanisms of pharmacoresistance”. Brain Research 1186 (2006):201-213.
- Awasthi S., et al. “RLIP76, a non-ABC transporter, and drug resistance in epilepsy”. BMC Neuroscience 6 (2005): 61.
- Czeisler B and Janigro D. “Reading and writing the Blood Brain Barrier: relevance to therapeutics”. Recent Patents on CNS Drug Discovery 1.2 (2006): 57–73.
- Cornford EM., et al. “High expression of the Glut1 glucose transporter in human brain hemangioblastoma endothelium”. Journal of Neuropathology & Experimental Neurology 54 (1995): 842-851.
- De Vivo DC., et al. “Defective glucose transport across the blood-brain barrier as a cause of persistent hypoglycorrhachia, seizures, and developmental delay”. The New England Journal of Medicine 325 (1991): 703-709.
- Withrow CD. “The ketogenic diet: mechanism of anticonvulsant action”. Advances in Neurology 27 (1980): 635-642.
- Morris AA. “Cerebral ketone body metabolism”. Journal of Inherited Metabolic Disease 28 (2005): 109-121.
- DeVivo DC., et al. “Chronic ketosis and cerebral metabolism”. Annals of Neurology 3 (1978): 331-337.
- Grant GA and Janigro D. “The blood-brain barrier”, in WinnHR, eds. Youmans neurological surgery. Saunders, Philadelphia (2004): 153–74.
- Sinclair CJ., et al. “Stimulation of nucleoside efflux and inhibition of adenosine kinase by A1 adenosine receptor activation”. Biochemical Pharmacology 59: 477-483.
- Hogue DL and Ling V. “A human nucleobase transporter-like cDNA (SLC23A1): member of a transporter family conserved from bacteria to mammals”. Genomics 59 (1999):18-23.
- Cass CE., et al. “Recent advances in the molecular biology of nucleoside transporters of mammalian cells”. Biochemistry and Cell Biology 76.5 (1998): 761-770.
- Paul DL. “New functions for gap junctions”. Current Opinion in Cell Biology 7.5 (1995): 665-672.
- Davies A., et al. “Cloning of a human nucleoside transporter implicated in the cellular uptake of adenosine and chemotherapeutic drugs”. Nature Medicine 3 (1977): 89-93.
- Silverman A., et al. “Mast Cells migrate from blood to brain”. Journal of Neuroscience 20 (2000): 401-408.
- Vezzani A and Granata T. “Brain inflammation in epilepsy: experimental and clinical evidence”. Epilepsia 46 (2005): 1724-1743.
Citation:
Alain L Fymat. “Neurological Disorders and the Blood Brain Barrier: 1. Epilepsy”. Current Opinions in Neurological Science
1.6 (2017): 277-293.
Copyright: © 2017 Alain L Fymat. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.






























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.