Research Article
Volume 2 Issue 2 - 2018
Study of Genetics Mutations ZIC1, ZIC2 and ZIC4 Genes in Dandy-Walker Syndrome Human 2018
Molecular Genetics-IRAN-TABRIZ, Director of the Division of Medical Genetics and Molecular Research
*Corresponding Author: Shahin Asadi, Molecular Genetics-IRAN-TABRIZ, Director of the Division of Medical Genetics and Molecular
Research.
Received: January 01, 2018; Published: March 27, 2018
Abstract
In this research we have analyzed 30 people. 10 patients Dandy-Walker syndrome and 20 persons control group. The genes ZIC1 and ZIC4, analyzed in terms of genetic mutations made. In this research, people who have genetic mutations were targeted, with nervous disorders Dandy-Walker syndrome. In fact, of all people with Dandy-Walker syndrome. 10 patients Dandy-Walker syndrome had a genetic mutations in the genes ZIC1 and ZIC4 Dandy-Walker syndrome. Any genetic mutations in the target genes control group, did not show.
Keywords: Genetic study; Dandy-Walker syndrome; Mutations the gene ZIC1; ZIC4, RT-PCR; Real Time-PCR
Introduction
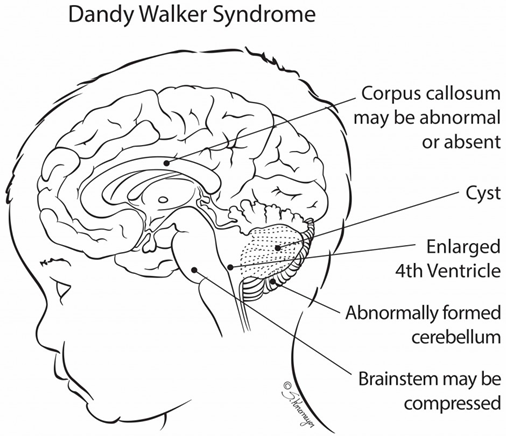
Dandy-Walker syndrome is a rare congenital disorder of the human brain that occurs during the development of the embryo from cerebellum to ventricle 4. The cerebellum is a region of the brain that helps to coordinate the movement and coordinate behavior on a regular basis. Brain or Brachial Ventricle System is a collection of four interconnected structures in the brain that are filled from the cerebrospinal fluid to the central duct of the spinal cord. The lining and ventricular coating consists of an epithelium membrane called epindimocyte.
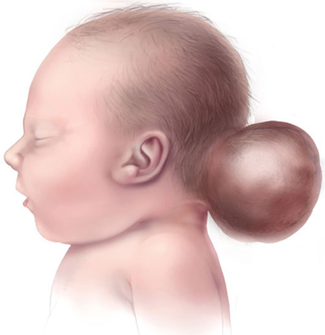
As people age, the brain's larger ventricles and neurons disappear. Dandy-Walker's syndrome reveals an abnormal condition of head and head size, lack of development of the cerebellar midline, cystic enlargement of the fourth ventricle of the brain, and enlargement of the skull base (posterior cavity). Dandy-Walker syndrome, sometimes (20-80%), is associated with hydrocephalus, in which the flow of natural fluid in the spinal cord leads to the accumulation of excessive amounts of fluid in and around the brain. This can cause abnormal high pressure within the skull and head swelling, which can lead to nerve damage. The degree of inability to control muscle coordination is different in the Dandy-Walker syndrome, but is usually life-long [1-8].
Signs and symptoms of Dandy-Walker syndrome Signs of Dandy-Walker syndrome usually include: delayed growth, muscle weakness (hypotonia), muscle cramps (spasm), poor coordination in balance (imbalance), and enlargement of the circumference of the head and increased internal pressure of the skull due to hydrocephalus in the head. Subjective retardation occurs in less than half of the people with Dandy-Walker syndrome. This condition is often seen in those who have severe hydrocephalus, or chromosomal abnormalities, and other birth defects [9-11].
Seizures occur in 15-30% of those affected by Dandy-Walker's syndrome. In addition, respiratory control centers in the brain stem of people with Dandy-Walker syndrome are sometimes damaged, which can lead to respiratory failure in these individuals. The enlargement of the ventricular cysts 4 or enlargement of the posterior cavity of the brain is one of the most prominent features of the Dandy-Walker syndrome [12-15].
Materials and Methods
In this Research, 10 patients with Dandy-Walker syndrome and 20 persons control group were researched. Peripheral blood samples from patients and parents with written permission control was prepared. After separation of serum, using Real Time-PCR technique of tRNA molecules were collected. To isolate Neuroglia cells erythrocytes were precipitated from hydroxyethyl starch (HES) was used. At this stage, HES solution in ratio of 1to5with the peripheral blood of patients and controls were mixed. After 70 minutes of incubation at room temperature, the supernatant was removed and centrifuged for 11 min at 540 Gera.
The cells sediment with PBS (phosphate buffered saline), pipetazh and slowly soluble carbohydrate ratio of 1 to 2 on ficole (Ficol) was poured in the 680G was centrifuged for 31 minutes. Mono nuclear Neuroglia cells also are included, has a lower density than ficole and soon which they are based. The remaining erythrocytes has a molecular weight greater than Ficole and deposited in test tubes. The supernatant, which contained the mono nuclear cells was removed, and the 600 Gera was centrifuged for 17 minutes. Finally, the sediment cell, the antibody and Neuroglia cells was added after 31 minutes incubation at 5°C, the cell mixture was passed from pillar LSMACS. Then the cells were washed with PBS and attached to the column LSMACSS pam Stem cell culture medium containing the transcription genes ZIC1, ZIC4, and were kept.
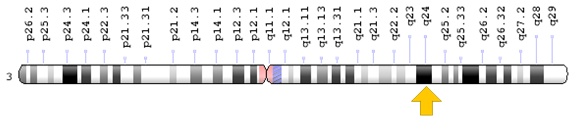
Figure 4: Schematic view of the long arm of chromosome number 3, where ZIC1 and ZIC4 genes located in 3q24.3 region remove or mutate the long arm of the chromosome due to the removal of the corresponding region, leading to Dandy-Walker syndrome be.
To determine the purity of Neuroglia cells are extracted, flow cytometry was used. For this purpose, approximately 6-7 × 103 Neuroglial cells were transfer red to1.5 ml Eppendorf tube and then was centrifuged at 2000 rpm for 11 minutes at time. Remove the supernatant culture medium and there maining sediment, 100μl of PBS buffer was added. After adding 5-10 μl PE monoclonal anti body to the cell suspension for 70 min at 5°C, incubated and readimme diately by flow cytometry. For example, rather than control anti body Neuroglia cells PE, IgG1 negative control solution was used.
Total mRNA extraction proceed unre includes:
- 1 ml solution spilled Qiazolon cells, and slowly and carefully mixed and incubated at room temperature for 7 minutes. Then 250 μl chloroform solution to target mix, then transfer the micro tubes were added, and the shaker well was mixed for 15 seconds. The present mix for 5 minutes at room temperature and then incubated for 26 min at 3°C on was centrifuged at 14200 rpm era. Remove the upper phase product were transfer reductase new microtube and to the one times the volume of cold ethanol was added. The resulting mixture for 24 hours at -20°C were incubated.
- Then for 48 min at 4°C on was centrifuged at 14000 rpm era. Remove the super natantand the white precipitate, 1ml of cold 75% ethanol was added to separate the sediment from micro tubes were vortex well. The resulting mixture for 20 min at 5°C on by the time we were centrifuged 14000 rpm. Ethanol and the sediment was removed and placed at room temperature until completely dry deposition. The precipitate was dissolved in 20 μl sterile water and at a later stage, the concentration of extracted mRNA was determined.
To assessment the quality of mi-RNAs, the RT-PCR technique was used. The cDNA synthesis inreverse transcription reaction (RT) kit (Fermentas K1622) and 1 μl oligoprimers 21 (dT) was performed. Following the PCR reaction 2 μM dNTP, 1 μg cDNA, Fermentas PCR buffer 1X, 0/75μM MgCl2, 1.25 U/μL Tag DNA at 95°C for 4 min, 95°C for 30s, annealing temperature 58°C for 30s, and 72°C for 45 seconds, 35 cycles were performed. Then 1.5% agarose gel, the PCR product was dumped in wells after electrophorese is with ethidium bromide staining and color were evaluated.
Results
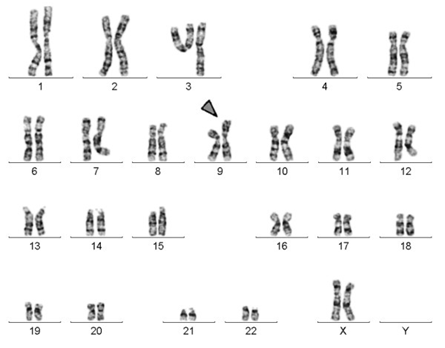
Figure 5: Schematic view of the karyotype of human chromosomes, which has a short arm of one of the chromosomes 9 doubled.
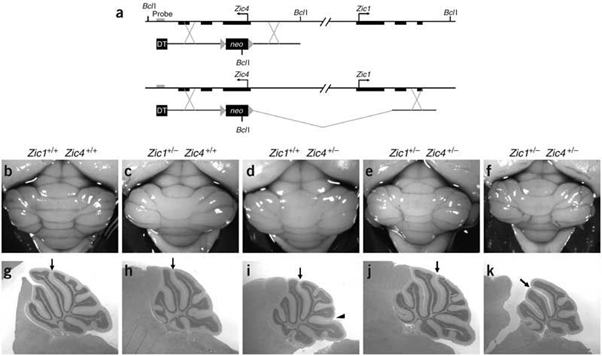
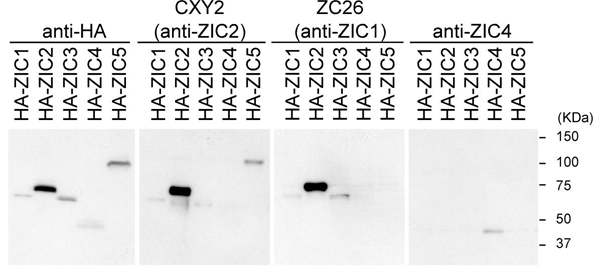
Figure 6: Microscopic image of the specific differentiation of neuronal cells by expressing ZIC1 and ZIC4 genes in the human brain.
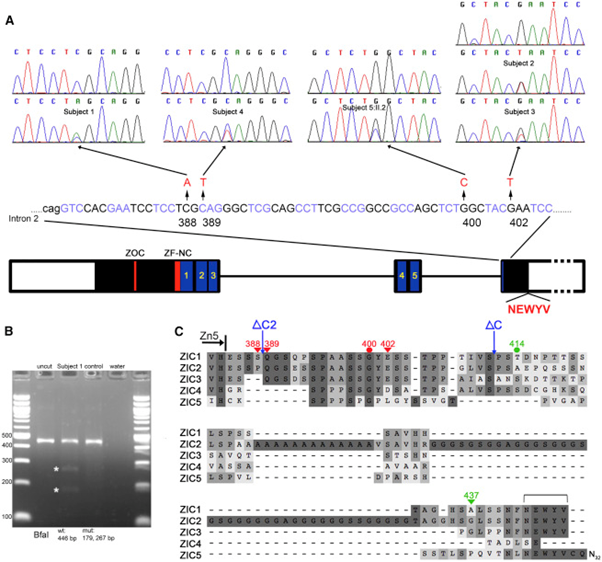
Figure 7: Schematic of the formation of the band and expression of the mutated genes ZIC1 and ZIC4 in the human brain.
Figure 8: Schematic diagram of the cumulative fragmentation diagram of mutated genes ZIC1 and ZIC4 in the brain of Dandy-Walker's syndrome.
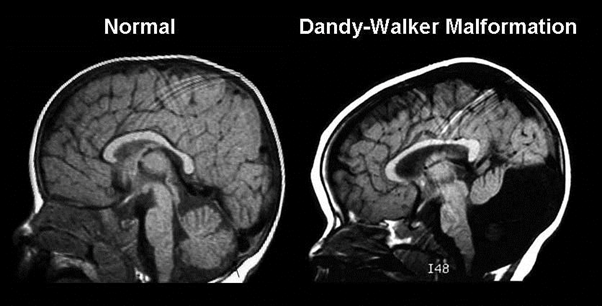
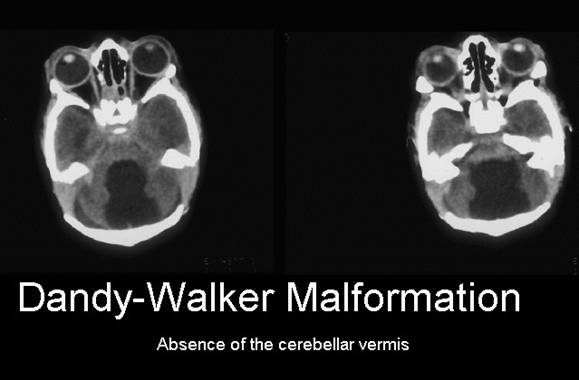
Figure 10: MRI radiography image of the head and skull of a person with Dandy-Walker syndrome compared to normal human.
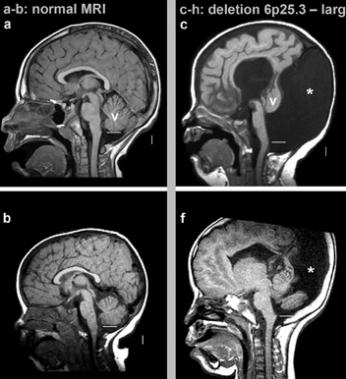
Figure 11: MRI radiography image of tomorrow with Dandy-Walker
syndrome, with the removal of the short arm of chromosome 6, as
6p25.3 compared to normal person.
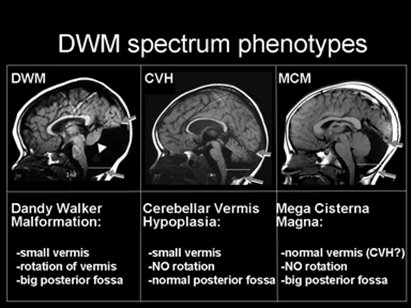
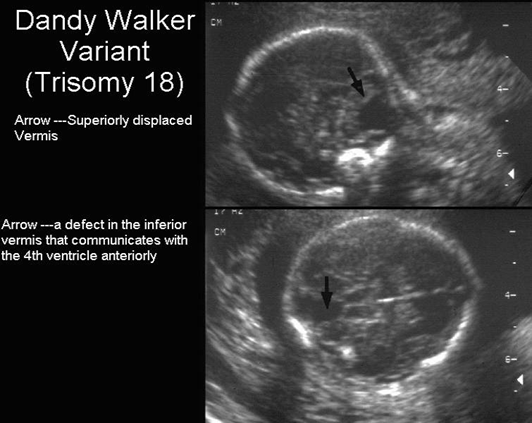
Figure 13: MRI radiologic image of the person with Dandy-Walker
syndrome (DWM) compared with those with cerebellar viremia
hypoplasia (CVH) and cerebellar mega cysterma (MCM).
Discussion and Conclusion
According to the study results of sequencing the genome of patients with Dandy-Walker syndrome, and the genetic mutations
ZIC1, ZIC4 genes found that about 100% of patients with Dandy-Walker syndrome, they have this genetic mutations. Patients with
Dandy-Walker syndrome, unusual and frightening images in the process of Dandy-Walker syndrome, experience. Lot epigenetic factors
involved in Dandy-Walker syndrome. But the most prominent factor to induce Dandy-Walker syndrome, mutations is ZIC1, ZIC4 genes.
This genes can induce the birth and can also be induced in the adulthood. Our study suggests that epigenetic factors, such as contaminated
ecosystems and unhealthy diets, inappropriate diets, and the ability of humans to induce this syndrome can be helpful.
References
- National Institute of Neurological Disorders and Stroke. “NINDS Dandy–Walker Syndrome Information Page”.
- Guibaud L., et al. “Prenatal diagnosis of 'isolated' Dandy-Walker malformation: imaging findings and prenatal counselling”. Prenatal Diagnosis 32.2 (2012): 185-193.
- Romero R., et al. “Prenatal diagnosis of congenital anomalies”. Appleton & Lange, Norwalk (1988): 30-33.
- Kaplan LC. “Congenital Dandy Walker malformation associated with first trimester warfarin: A case report and literature review”. Teratology 32.3 (1985): 333–337.
- Badano Jose L., et al. “The Ciliopathies: An Emerging Class of Human Genetic Disorders”. Annual Review of Genomics and Human Genetics 7 (2006): 125-148.
- Grinberg I., et al. “Heterozygous deletion of the linked genes ZIC1 and ZIC4 is involved in Dandy–Walker malformation”. Nature Genetics 36.10 (2004): 1053–1055.
- Barkovich A., et al. “Revised classification of posterior fossa cysts and cystlike malformations based on the results of multiplanar MR imaging”. American Journal of Roentgenology 10 (1989): 977-988.
- Bordarier C and Aicardi J. “Dandy-Walker syndrome and agenesis of the cerebellar vermis: diagnostic problems and genetic counseling”. Developmental Medicine & Child Neurology 32.4 (1990): 285-294.
- Ecker J Shipp T., et al. “The sonographic diagnosis of Dandy-Walker and Dandy-Walker variant: associated findings and outcomes”. Prenatal Diagnosis 20.4(2000): 328-332.
- Grinberg I., et al. “Heterozygous deletion of the linked genes ZIC1 and ZIC4 is involved in Dandy-Walker malformation”. Nature Genetics 36 (2004): 1053-1055.
- Kalidasan V., et al. “The Dandy-Walker syndrome-a 10 year experience of its management and outcome”. Journal of Pediatric Surgery 31 (1996): 998-999.
- Kolble N., et al. “Dandy-Walker malformation: prenatal diagnosis and outcome”. Prenatal Diagnosis 20.4 (2000): 318-327.
- Metry DW., et al. “The many faces of PHACE syndrome”. The Journal of Pediatrics 139.1 (2001): 117–123.
- Yüceer N., et al. “Surgical treatment of 13 pediatric patients with Dandy–Walker syndrome”. Pediatric Neurosurgery 43.5 (2007): 358–363.
- Osenbach RK and Menezes AH. “Diagnosis and management of the Dandy–Walker malformation: 30 years of experience”. Pediatric Neurosurgery 18.4 (1992): 179–189.
Citation:
Shahin Asadi., et al. “Study of Genetics Mutations ZIC1, ZIC2 and ZIC4 Genes in Dandy-Walker Syndrome Human 2018 ”. Current
Opinions in Neurological Science 2.2 (2018): 445-455.
Copyright: © 2018 Shahin Asadi., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.