Opinion Article
Volume 2 Issue 6 - 2018
Neuroanatomical and Neurocognitive Functions of the Structure of the Mind: Clinical and Teaching Implications
1University of California – Davis Medical Center
2American University of Antigua College of Medicine
3University of North Texas and Private Practice
2American University of Antigua College of Medicine
3University of North Texas and Private Practice
*Corresponding Author: Amir Ramezani, PhD, 4860 Y St., Department of Physical Medicine and Rehabilitation, University of California, Davis, 916-734-3420.
Received: August 29, 2018; Published: September 26, 2018
Abstract
The neural underpinning of psychoanalytic concepts is an important endeavor to help advance integrative clinical, education, and research practices. The paper presents neuroscience correlates of structure of the mind theory (e.g., Id, Ego, and Superego), and their neuroanatomical and neurocognitive representations. A general framework is used to examine the research literature, which consists of understanding the principles of the structure of the mind concepts (e.g., global concept) and understanding associated neuroanatomical areas and neurocognitive functions (e.g., subsets).
This is the first qualitative study that highlights the similarity between the structure of the mind concepts, brain anatomy, and cognitive functions in one coherent framework. Literature review of the neurodevelopment, hierarchy of cerebral function, and cognitive functions that parallel the concepts of the Id, Ego and Superego is conducted. To assist with learning and potential applications in education, a table and 4 figures represent general conceptual framework as well as Freud’s original conception of neurosignal processing of the concept of repression.
These findings assist clinicians, educators, and research when studying the relationship between basic psychoanalytic concepts and brain function. The findings can help clinicians conceptualize patients with neurological deficits. These findings can also help provide a conceptual framework for educators and researchers to further teach and study the cross-section of psychoanalytic concept of the structure of the mind and brain anatomy and function. Educators can teach both psychoanalysis concepts and brain anatomy and cognition simultaneously utilizing this frame.
Keywords: Neuro anatomy; Cognition; Psychoanalysis; Psychodynamic; Psychotherapy; Education
Introduction
Psychoanalytic concepts of the structure of the mind (e.g., Id, Ego, and Superego) represent an important conceptual framework for clinicians, educators, and researchers to better understand psychiatric conditions and further develop psychoanalytic treatment modalities. The ideas of the Id, Ego, and Superego are not only foundational concepts but also prevalent ideas in psychoanalytic practice. Despite these importances, the relationships between the neural underpinnings and the structure of the mind theory have been largely under examined. The merging and understanding of the neural underpinnings of the structure of the mind concept can have major implications on clinical practice, education, and research theory.
Understanding the neuroscience correlates of psychoanalytic concepts is an important endeavor in the clinical psychoanalytic practice. With the advent of neuroscience methodology, it is increasingly easier to identify neuroanatomical and neurocognitive functioning underlying mental processes. Understanding the neuroanatomical and neurocognitive underpinnings of the structure of the mind theory helps clinicians to conceptualize patients and develop neuropsychoanalytically-informed treatment plans. This is particularly true when conducting psychoanalytically-informed therapy with patients suffering from neurological deficits such as frontal lobe pathology or traumatic brain injury (TBI). Furthermore, validating psychoanalytic theories through neuroscience findings helps to strengthen the field as a science.
Origins of the Neurological Basis of Psychoanalytic Concepts
Freud had attempted to quantify psychoanalytic concepts by using machine models for his theories. At the heart of his quantification approach was the neural path of repression, whereby a neuron redirects information to avoid a signal from being processed at a conscious level. Freud’s sketch of the neuronal representation of repression is shown in Figure 1. This sketch demonstrates that Freud had a fundamental grasp on how to translate macro concepts to micro neurobiological prepossess.
Freud had attempted to quantify psychoanalytic concepts by using machine models for his theories. At the heart of his quantification approach was the neural path of repression, whereby a neuron redirects information to avoid a signal from being processed at a conscious level. Freud’s sketch of the neuronal representation of repression is shown in Figure 1. This sketch demonstrates that Freud had a fundamental grasp on how to translate macro concepts to micro neurobiological prepossess.
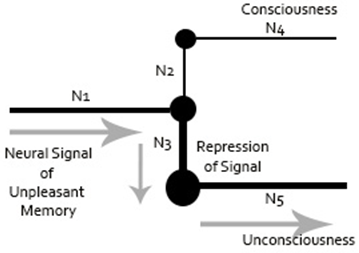
Figure 1: Representation of Freud’s Neuronal Mechanism Sketch (Freud, 1915a; Freud, 1895) drawn by Dr. Amir Ramezani.
Note for Figure 1: The figure represents an unpleasant neural signal coming to a juncture (N1), which gets repressed into unconsciousness (N5) by another neuron (N3). The signal is presented from reaching consciousness (N2 and N4). The size of each node represents level of activation.
Freud referred to his attempt to neurologically quantify psychoanalytic mental process as the Project, which was eventually titled The Project for a Scientific Psychology (Freud, 1895). He had illustrated hierarchy of consciousness, starting from the Id’s unconscious processes to the ego’s pre-conscious and conscious processes, to ultimately perceptual awareness. It is lower-level unconscious (e.g., Id) that would give rise to higher-level consciousness (e.g., Ego, Superego). However, Freud abandoned his project because he recognized the limitations of technology during this time period. He was unable to provide insight into the neuroanatomical and cognitive basis of the structure of the mind concepts (Solms, 1998).
Background of Integrating Neuroscience and Psychoanalysis
Recent advances in developmental neuroscience and neuroimaging has led to the discovery of multiple neural networks that function similarly to psychoanalytic concepts. Noble prize recipient, Eric Kandel (1998), who has extensively studied the biological basis of memory has indicted that the theory and practice of psychoanalysis is based on the interaction and exchange between neurobiology and psychoanalytic theory. Other neuroscientists report that psychoanalysis is currently the most coherent and intellectually satisfying view of the mind (Solms, 2004). Thus, a new perspective needs to become integrated in psychoanalytic clinical, educational, and research practice where the focus is on the neurologic basis of psychoanalytical concepts.
Recent advances in developmental neuroscience and neuroimaging has led to the discovery of multiple neural networks that function similarly to psychoanalytic concepts. Noble prize recipient, Eric Kandel (1998), who has extensively studied the biological basis of memory has indicted that the theory and practice of psychoanalysis is based on the interaction and exchange between neurobiology and psychoanalytic theory. Other neuroscientists report that psychoanalysis is currently the most coherent and intellectually satisfying view of the mind (Solms, 2004). Thus, a new perspective needs to become integrated in psychoanalytic clinical, educational, and research practice where the focus is on the neurologic basis of psychoanalytical concepts.
According to Panksepp (2011), neuropsychoanalysis, an integrative field of neuroscience and psychoanalysis, takes into account the deep evolutionary roots of the human mind and emotional disorders, and favors a more articulate understanding of primary-process brain affective networks. Additionally, Luciani., et al. (2014) states that the psychoanalytic approach on mental functioning can direct neuroscientific research toward a deeper understanding of basic psychodynamic concepts, such as the Ego, defense mechanisms, dream functions, and the projection of mental states. With these important trends in mind, major efforts have been undertaken to conceptually identify neurosubstrates of psychoanalytic concept to further clinical, educational, and research practice. These efforts have addressed diverse perspective in psychoanalytic thinking. These include identification of neurosubstrates of defense mechanisms, unconscious, consciousness, and transference.
For example, with regard to defense mechanisms, Vaillant (2011) hypothesized that defense mechanisms may also rise as a result of reduced frontal lobe connectivity. In other words, a lack of high-order inhibition maybe due to certain activation of psychological defenses, which arise from lower-order networks, thus implying that the frontal lobe is the source that inhibits defenses. Northoff, Bermpohl, Schoeneich, and Boeker (2007) noted that the defense mechanism of catatonic regression or sensorimotor regression is associated with impairments in specific regions of the frontal lobe, namely the orbitofrontal, mesial frontal, and premotor regions. Costa and Oliveira (2015) highlighted that the defense mechanisms might be related to testosterone changes while Wilner and Aubé (2014) argue that the defense mechanism of regression maybe related to lack of NMDA receptors. Furthermore, on the topic of regression, in reviewing clinical cases with hemispheric lesions, Kaplan-Solms and Solms (2000) noted that regression might be a result of right hemisphere damage (pp 258, 261).
Another example of researchers advancing psychoanalytic concepts through neuroscience discoveries is the topic of the unconscious. Woody and Phillips (1995) described how consciousness depends heavily on the collective exchange between non-conscious neural processing. Based on Freud’s early work and current neuroscience findings, they also formulated three distinct levels of unconsciousness to be studied. This includes the neural unconscious, which is a lower-level signal processing stage; the cognitive unconscious, which is a set of rule driven processes such as the workings of implicit memory and repression (e.g., repressing an unacceptable wish or traumatic memory); the psychodynamic unconscious, which is the meaning the individual assigns to the experience prior to reaching the consciousness. Regarding the cognitive unconsciousness, Pugh (2002) linked the implicit memory, an unconscious account of actions, motor abilities, habit formations, and emotional memory to basal ganglion and amygdala circuitry.
Preliminary case studies showed that both unconscious and conscious processes might share common neuroanatomical regions. Solms (2013) discussed multiple patients whose insular cortex were completely obliterated by herpes simplex encephalitis or had no formation of the cerebral cortex due to in utero, infarctions of the anterior cerebral artery. In these cases, patients were able to show or communicate an awareness of themselves or a sense of their existence. Given the widely accepted notion that consciousness is of cortical origin, this unique finding demonstrates that lower-order brain stem and limbic functions attributed to the Id can also have a conscious element, similar to higher-order cortex functions that were attributed to the Ego and Superego.
Another psychoanalytic area that is emerging in the neuroscience literature is the topic of transference. Westen and Gabbard (2002) implicated that transference is an accumulation of activation of procedural memory nodes, episodic memory nodes, and affective-motivational cortical and subcortical systems. Tiberga (2014) noted that transference of false memory (e.g., patient having believed that they knew their therapist) occurred with orbitofrontal damage. Researchers suggest that in the presence of lack of inhibition (e.g., dysfunction in orbitofrontal), transference can rise and operate on the pleasure principle, rather than the reality principle.
Levin (1997) noted that transference derives from multiple neuroanatomical and neurocognitive systems, which include working memory systems (example of a frontal lobe function) and cortical association areas related to sensory processing, inter-hemisphere processing, vestibular-cerebellar system, and limbic regions (e.g., hippocampus, mammillary body, and amygdala). Table 1 summarizes the psychoanalytic concepts and inferences drawn by researchers about their corresponding brain anatomy and neurocognitive functions.
| Research Literature | Psychoanalytic Concept | Neuroanatomical and Neurocognitive Inference |
| Vaillant (2011) | Activation of defense mechanism | Decrease in frontal lobe connectivity and increase activity in lower-order subcortical sites |
| Northoff., et al. (2007) | Catatonic regressive defense | Orbitofrontal, mesial frontal, and premotor |
| Woody and Phillips (1995) | Neural unconscious | Signal processing stage |
| Cognitive unconscious | Rules of implicit memory and repression | |
| Psychodynamic unconscious | Meaning of experience before information reaches the consciousness | |
| Solms (2013) | Consciousness Across Superego, Ego and Id | Lower-order brain stem and limbic functions also have a conscious element, similar to higher-order cortex functions |
| Westen and Gabbard (2002) | Transference | Transference is an accumulation of activation of procedural memory nodes, episodic memory nodes, and affective-motivational cortical and subcortical systems |
| Tiberga (2014) | Transference | Working memory and frontal lobe function, and cortical association areas related to sensory processing, inter-hemisphere processing, vestibular-cerebellar system, and limbic regions concerning hippocampus, mammillary body, and amygdala |
| Pugh (2002) | Unconscious account of actions and emotional memory | Implicit memory, motor abilities, habit formations, and emotional memory to basal ganglion and amygdala circuitry |
Table 1: Summary of Psychoanalytic Concepts, Brain Anatomy and Function.
Identifying neurosubstrates of defense mechanisms, the unconscious, and transference helps clinicians to better conceptualize patients with brain related changes. These efforts help to advance the neuroscience basis of psychoanalytic concepts and how they can be applied in the clinical conceptualization of patients and the mind as a whole. However, with the exception of Solms (2013), there still remains a gap in the neural conceptualization of the structure of the mind theory of the Id, Ego, and Superego. Despite the structure of the mind theory being a foundational and highly prevalent concept in psychoanalytic thinking, there is little research that describes the neurological basis of such concepts.
Aims
This paper aims to highlight the common relationship between the structure of the mind concepts (e.g., Id, Ego, and Superego) and related neuroanatomical structures and neurocognitive functioning. These aims will be achieved through the literature review of the neurodevelopment, hierarchy of cerebral function and the neurological processes that parallel the concepts of the Id, Ego, and Superego. The summary of each concept of the structure of the mind is in a three-tier representation. The first tier represents global concept such as the psychoanalytic concept (e.g., Id). The second tier represents the subset such as the neuroanatomical association of the psychoanalytic concepts (e.g., brain stem). The third tier represents the subset of neurocognitive association of the psychoanalytic concepts (e.g., implicit memory). The summary of the three-tier representations is depicted through a figure at the end of each subsection to further assist with conceptualization.
This paper aims to highlight the common relationship between the structure of the mind concepts (e.g., Id, Ego, and Superego) and related neuroanatomical structures and neurocognitive functioning. These aims will be achieved through the literature review of the neurodevelopment, hierarchy of cerebral function and the neurological processes that parallel the concepts of the Id, Ego, and Superego. The summary of each concept of the structure of the mind is in a three-tier representation. The first tier represents global concept such as the psychoanalytic concept (e.g., Id). The second tier represents the subset such as the neuroanatomical association of the psychoanalytic concepts (e.g., brain stem). The third tier represents the subset of neurocognitive association of the psychoanalytic concepts (e.g., implicit memory). The summary of the three-tier representations is depicted through a figure at the end of each subsection to further assist with conceptualization.
These neuroscience findings provide an opportunity for clinicians, educators, and researchers to deepen their understanding of the relationship between the dynamic process that occur within the structures of the mind (Id, Ego, and Superego) and their neurological functions. Identifying neuroanatomical regions that function similar to the concepts of the Id, Ego, and Superego can further help clinicians conceptualize patients with neurological deficits. This will also help provide a conceptual framework for educators and researchers to further teach and study the cross-section of psychoanalytic concept of the structure of the mind and the overlap of brain anatomy and cognitive function.
Neuroanatomical and Neurocognitive Aspects of the Id
The Id’s concepts of inborn-primitive drive, lower-order hierarchical structure, pleasure principle, and internal body sensory perception appear to correlate with intrinsic neural-networks, brain stem regions, and limbic-cortical areas. The following section will review neurodevelopmental, cerebral hierarchy, neural reward circuitry, and the relationship between frontal lobe structures and limbic structures.
The Id’s concepts of inborn-primitive drive, lower-order hierarchical structure, pleasure principle, and internal body sensory perception appear to correlate with intrinsic neural-networks, brain stem regions, and limbic-cortical areas. The following section will review neurodevelopmental, cerebral hierarchy, neural reward circuitry, and the relationship between frontal lobe structures and limbic structures.
The Id and Neurodevelopment
A neurodevelopmental appreciation can help clinicians and educators to better understand innate neural formation and pre-wired functions that accumulates to form the basis of the Id. Psychoanalytic theory indicates that humans are born with the Id. That is, humans are born with a primitive instinct that helps us adapt and survive. Davis (2002) suggests that early in life, while neurons are developing, a selection process takes place in which neurons are assigned a relative value. The selection process of neuron depends on stimulation from external or internal sources. Experience can selectively strengthen or weaken neuronal connectivity.
A neurodevelopmental appreciation can help clinicians and educators to better understand innate neural formation and pre-wired functions that accumulates to form the basis of the Id. Psychoanalytic theory indicates that humans are born with the Id. That is, humans are born with a primitive instinct that helps us adapt and survive. Davis (2002) suggests that early in life, while neurons are developing, a selection process takes place in which neurons are assigned a relative value. The selection process of neuron depends on stimulation from external or internal sources. Experience can selectively strengthen or weaken neuronal connectivity.
The selection process is mediated by a value system and through neurotransmitters such as dopamine, norepinephrine, and serotonin. At birth some neurons are hardwire in order to develop into certain functions. For instance, infants are pre-wired with homeostatic values or functions such as hunger, lightness/darkness responses, and safety/danger detection. These innate hard-wirings evolved to optimize adaptation and survival by allowing the organism to respond appropriately to reward and aversive stimuli.
Additionally, Greenough., et al. (1987) stated that although cortical patterning begins in the embryonic period it remains malleable and impressionable for an extended period of time. He tested this theory using two constructs known as “experience expectant” and “experience dependent” learning. Both constructs suggest that throughout development, experience plays an essential role in establishing and refining neural organization in a manner that allows an organism to adapt to the eventualities of the world in which it lives.
According to psychoanalytic theory, humans are born with primitive biological mechanisms that function to govern basic survival behaviors and impulses. The Id contains these innate impulses and drives (Freud, 1915a; Kahn, 2002). The Id can be viewed as an inborn neural-network that contains a repertoire of adaptive and survival behaviors. These lower-order/subcortical adaptive inborn neural-network, or the Id, can be inhibited or enhanced by environmental learning, which usually takes place in the higher-order processing networks of the cerebral cortex. Later we discuss how these selective inhibition or enhancements of the neural-network can be related to the environmental learning of the Ego and the Superego.
The Id and Hierarchy of Cerebral Function
A thorough review of the hierarchy of cerebral function offers insight into the development of the Id and its exchange with higher-order structures. The Id is conceptualized to be the substructure of the Ego, and the Superego (Freud, 1915a, 1915b, 1923). The Id is innate; however, as the child learns from the environment it develops physiological structures, which conceptually functions as the Ego and Superego. These structures emerge to compete with the Id and inhibit the Id’s urges and drives (Freud, 1923).
A thorough review of the hierarchy of cerebral function offers insight into the development of the Id and its exchange with higher-order structures. The Id is conceptualized to be the substructure of the Ego, and the Superego (Freud, 1915a, 1915b, 1923). The Id is innate; however, as the child learns from the environment it develops physiological structures, which conceptually functions as the Ego and Superego. These structures emerge to compete with the Id and inhibit the Id’s urges and drives (Freud, 1923).
Researchers identified specific parts of the brain involved in the decisions of delayed gratification. The research demonstrated that the hippocampus, associated with memory, and the nucleus accumbens, associated with pleasure, work together in making decisions of this type (Abela, 2015). The development of these new physiological structures is adaptive in that they learn to defer immediate gratification. Due to the structural development of the brain, the Ego and the Superego overlay the Id. In other words, the Id can be viewed as the base out of which two other structures emerge, similar to how a blossoming tree above ground comes from the roots below ground.
The thalamus plays a major role in mediating connections between the Id’s lower-order function to the Ego and Superego’s higher-order function. Understanding the role of thalamic nuclei and their influence on cortical functioning can shed light onto the functions of the Id and its exchange between Ego and Superego. The following are examples of thalamic nuclei mediated cerebral functions: the frontal cortex has direct connections with the dorsal medial nuclei of thalamus, which is the emotional response center. In experimental animals, stimulation of this area produces rage and aggression (White, 2008).
The primary motor cortex has direct connections to the ventrolateral nuclei of the thalamus, which receives motor input from the basal ganglia and cerebellum; the primary sensory cortex has direct connections with the ventral, posterior-laterailis nuclei of the thalamus, which receives pain and temperature sensory input from the spinothalamic tract and dorsal columns; the parietal cortex has direct connections to the lateral posterior nuclei of the thalamus; the temporal lobe has direct connections with medial geniculate body of thalamus, which receives auditory input from the superior olive and inferior colliculus of tectum; the visual cortex has direct connections to the lateral geniculate body, which received visual input from the optic nerve (White, 2008).
These physiological findings suggest that the primitive areas of the brain, such as the thalamus, are the basis on which our higher brain functions are built, such as the functions of the Ego and Superego. Sensory information requires the thalamocortical track to give rise to conciseness. Similar to how the Id is needed to give rise to the Ego and Superego.
The Id and Limbic-Frontal Mediation
According to Freud, the Id is governed by the pleasure-principle (Freud, 1915a). The pleasure-principle indicates that the Id is concerned with internal instincts and urges. The Id is pre-programmed to engage in reward seeking behavior and recognize rewarding stimuli while avoiding punishing stimuli (Freud, 1923). Additionally, the Id is responsible for primitive functions such as sexual behavior, aggression, eating, and hunger. The Id is also responsible for emotional states such as happiness, sadness, excitement, anxiety, anger, guilt, and rage (Freud, 1915b; Freud, 1923). These primitive emotions are experienced in the body as the Id subsumes automatic bodily functions and sensory processing. Ultimately, the Id represents a wide range of functions that are vital to human survival (Kahn, 2002).
According to Freud, the Id is governed by the pleasure-principle (Freud, 1915a). The pleasure-principle indicates that the Id is concerned with internal instincts and urges. The Id is pre-programmed to engage in reward seeking behavior and recognize rewarding stimuli while avoiding punishing stimuli (Freud, 1923). Additionally, the Id is responsible for primitive functions such as sexual behavior, aggression, eating, and hunger. The Id is also responsible for emotional states such as happiness, sadness, excitement, anxiety, anger, guilt, and rage (Freud, 1915b; Freud, 1923). These primitive emotions are experienced in the body as the Id subsumes automatic bodily functions and sensory processing. Ultimately, the Id represents a wide range of functions that are vital to human survival (Kahn, 2002).
The concept of the Id parallels certain functions of the limbic system and the brain stem. In psychoanalytic theory, the Id impulses strive for expression, while the Superego inhibits these impulses. At the same time, the Ego functions as a mediator between the Id and the Superego in order to produce a coherent expression of thought, emotions or behavior that is appropriate in society, yet satisfies internal impulses while regulating lower-order, subcortical impulses (Prochaska and Norcross, 2003). Higher-order cerebral functions, such as the frontal lobe modulate the expression of emotions and inhibition of impulses. With this in mind, conceptually the Id is being mediated by the Ego and Superego.
Regarding neurocognitive function, Pugh (2002) noted that the operations of implicit memory (e.g., a non-conscious emotional memory and motor memory) to be corresponding to the Id’s concepts of unconscious memories. The neural circuitry of implicit memory has been implicated to be limbic structures such as the basal ganglion and amygdala circuitry as well as cerebellar regions (Reber, 2013; Pugh, 2002).
The Pleasure Principle and Reward Circuitry
The neural circuitry associated with the Id’s pleasure principle can be better understood by examining basic neuroimaging activation paradigms in limbic structures. The Id’s pleasure principle drives an individual to seek pleasure and to avoid pain. If we predict the Id to be localized within the limbic system and lower/ mid brain regions, then the reward areas must be activated during sexual activity, food seeking, money / power, and other behaviors initiated by the Id.
The neural circuitry associated with the Id’s pleasure principle can be better understood by examining basic neuroimaging activation paradigms in limbic structures. The Id’s pleasure principle drives an individual to seek pleasure and to avoid pain. If we predict the Id to be localized within the limbic system and lower/ mid brain regions, then the reward areas must be activated during sexual activity, food seeking, money / power, and other behaviors initiated by the Id.
The reward centers that have been found in the brain are the nucleus accumbency, medial forebrain bundle, and ventral tegmental area. Neuroimaging studies have found reward centers becoming activated by both primary (e.g., sex and food) and secondary (e.g., money) reward stimuli. Berns, McClure, Pagnoni, Montague (2001) found certain areas of the brain to be responsible for reward. Twenty- five normal participants underwent fMRI (functional Magnetic Resonance Imaging) scans while receiving a reward, such as juice or water.
This study showed that the nucleus accumbency responds to juice and water as a source of reward. Additionally, the research questioned if the certainty of the reward influenced the reaction to its administration. FMRI showed significant stimulation in nucleus accumbency and medial orbital frontal cortex when juice or water was administered unpredictably. Thus, when the frontal area of the brain becomes aware of stimulus, then the reward may not be as intense as an unpredicted stimulus (Berns., et al. 2001). In order for a predictor to take place, the feedback of stimuli must be given to higher-level conscious. Therefore, once the stimulus becomes conscious, or once Ego and Superego can predict reward stimulus, then it can properly habituate or inhibit reward areas. Therefore, it is likely that in order for the Id to circumvent the Superego, the stimulus must remain unconscious to avoid conflicts with the Superego.
The Id’s associated limbic structures not only become activated by primary reward stimuli, but also become activated by secondary reward. Thut, Schultz, Roelcke (1997) found that the secondary reinforcers, such as money or financial gain, stimulate rewards centers. They measured regional cerebral blood flow in 10 right-handed healthy subjects performing a delayed go-no go task in two different reinforcement conditions. During the experiment, the subjects with correct responses were either rewarded by money or a simple “ok” reinforcer. Behaviors rewarded by money, as compared with the “ok” reinforcement, were most significantly associated with activation of dorsolateral and orbital frontal cortex and also involved the midbrain structures and thalamus. Although arousal effects cannot be completely excluded, these results may reflect the processing of reward information through the interaction of the thalamus with the frontal lobe. From a psychoanalytical perspective, the Id interacts with the Superego to express reward through the filter, namely the ego, into a “more acceptable” version of the primitive desire. Therefore, this study may suggest a modulation effect between the Id and Superego, via lower and higher-order neuroanatomical regions using neurotransmitters such as dopamine and serotonin.
The aforementioned pleasure/reward neuroanatomical regions that Knutson, et al. (2000), Thut., et al. (1997), and Berns., et al. (2001) identified parallel the pleasure principle of the Id, which is described as purely seeking reward in order to avoid pain and suffering. Therefore, the neural anatomical areas involved in reward seeking behavior of the Id may be warehoused in the insula, caudate, putamen, striatal, and mesial forebrain.
The Id and the Internal Body-Sensory Perception
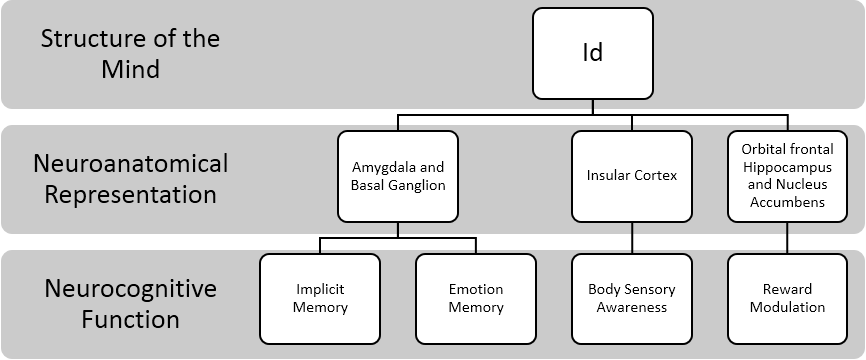
As indicated above, Freud likened the Id to the internal body that is cut off from the external world and has a world of perception of its own (Solms, 2013). Additionally, Freud indicated that “the Id detects with extraordinary acuteness certain changes in its interior, especially oscillations in the tension of its instinctual needs (Freud, 1940). This maybe one reason why the Id plays a major role in unconscious processes such as transference and countertransference, two mental processes that allows the clinician to become aware of certain mental projections and defense. This would be highly important for clinicians as they may wish to become aware of their own body’s sensory cues to better detect transference and separate out their own countertransference from that of the patient. The summary of the aforementioned concepts of the Id is depicted in the figure 2.
As indicated above, Freud likened the Id to the internal body that is cut off from the external world and has a world of perception of its own (Solms, 2013). Additionally, Freud indicated that “the Id detects with extraordinary acuteness certain changes in its interior, especially oscillations in the tension of its instinctual needs (Freud, 1940). This maybe one reason why the Id plays a major role in unconscious processes such as transference and countertransference, two mental processes that allows the clinician to become aware of certain mental projections and defense. This would be highly important for clinicians as they may wish to become aware of their own body’s sensory cues to better detect transference and separate out their own countertransference from that of the patient. The summary of the aforementioned concepts of the Id is depicted in the figure 2.
Neuroanatomical and Neurocognitive Aspects of the Ego
The Ego and Neurodevelopment
According to Freudian theory, a child is born with an innate adaptive system he termed the Ego (Freud, 1915a). As mentioned before, neurons are preprogrammed with values and drives, and the limbic system regions are associated with in-born instincts (Davis, 2002). However, for a child to be adaptive in society, he/she must surrender to the rules and structure of society. The child must forgo the "pleasure principle" of this natural state and accept the "reality principle" of society (Freud, 1923). The “reality principle” allows the mind to be realistic in its desires about the external world, thus setting the stage for a battle between immediate gratification and the deferral of that gratification (Prochaska and Norcross 2003).
The Ego and Neurodevelopment
According to Freudian theory, a child is born with an innate adaptive system he termed the Ego (Freud, 1915a). As mentioned before, neurons are preprogrammed with values and drives, and the limbic system regions are associated with in-born instincts (Davis, 2002). However, for a child to be adaptive in society, he/she must surrender to the rules and structure of society. The child must forgo the "pleasure principle" of this natural state and accept the "reality principle" of society (Freud, 1923). The “reality principle” allows the mind to be realistic in its desires about the external world, thus setting the stage for a battle between immediate gratification and the deferral of that gratification (Prochaska and Norcross 2003).
The pleasure-principle drives one to seek pleasure and to avoid pain. As a child matures, he/she begins to learn to endure pain and to defer gratification in an effort to better meet the demands and obstacles of his environment. Thus, the Ego develops as the child aligns his needs with social norms (Freud, 1915a, 1914b, 1923). The Ego is not governed by the pleasure principle, but obeys the reality principle, which aims to integrate the environment, people, social norms, and personal needs.
The Ego does so by taking reality into account and defending against the unbending rules of the Superego (Prochaska and Norcross 2003). The Superego is the faculty that seeks to police what it deems unacceptable in society. The Superego achieves this through feelings of guilt and shame, therefore it can be said that the Superego represents all moral restrictions and is striving towards perfection (Kahn, 2002). Therefore, the Ego plays a central role in decision making between not only the Id’s instincts but also the Superego’s unbending social rules and morals.
Regarding brain and cognitive development, from birth to 18 months, implicit memory housed in subcortical regions and lower-order networks (e.g., basal ganglion and amygdala, Pugh, 2002) are the dominant encoding systems (Seigel, 1999). However, after 18 months, the dentate gyrus of the hippocampus develops, which forms explicit memory system, a conscious representation of memory for factual and situational information that is related to oneself, namely autobiographical memory (Mishkin and Appenzeller, 1987; Seigel, 1999). The idea of the formation of explicit memory of a self is further reinforced by the earlier development research on mirrors and self-recognition.
Children can’t explicitly recognize themselves prior to 18 months (Amsterdam, 1972). Around 18-24 months, the child begins to discriminate themselves seen in a mirror from other people (Nielsen, Dissanayake, Kashima, 2003). This suggests that the child has started construct a sense of self as a result of brain development. Self-recognition can also be related to explicit memory and hippocampal/temporal lobe structures that have formed at this age. Freud also noted that the development of the Ego around the same age and functions similarly to develop a sense of self (Freud, 1923).
Ego and Default Mode Network
The Ego is conceptualized to construct the self (Freud, 1923). Yet, a few questions remain unanswered when conceptualizing the correlates of the Ego and brain function: How does the Ego construct a self at a neurological level? What neuroanatomical regions and neurocognitive processes are responsible for the construction of a self? The answer may lie in the different functions of explicit memory systems and default modes network of information processing.
The Ego is conceptualized to construct the self (Freud, 1923). Yet, a few questions remain unanswered when conceptualizing the correlates of the Ego and brain function: How does the Ego construct a self at a neurological level? What neuroanatomical regions and neurocognitive processes are responsible for the construction of a self? The answer may lie in the different functions of explicit memory systems and default modes network of information processing.
Tulving (1993) noted that part of explicit memory contains the cognitive faculty of mental time travel, which is the ability to search and sort through past and future memories. This ability helps to integrate different time experience to construct a meaningful sense of self. Klein (2012) argued that there are distinct neurological networks that process a sense of self in the Ego.
Recent advance in neuroimaging has noted a similar concept, namely the default mode network, which is also responsible for processing past and future to construct a sense of self. The default network involves medial frontal lobe, posterior cingulate gyrus, lateral parietal lobe, and medial temporal lobe structures (McCormicl., et al. 2014). Interesting enough, explicit memory networks also become activated in medial temporal lobe, hippocampal regions (Rugg and Vilberg, 2013), and frontal-temporal network (Wagner., et al. 1998), parietal lobe (Cabeza, 2008), and posterior cingulate gyrus (Schott., et al. 2005). McCormicl and colleagues (2014) also showed that there is notable cross talk between the posterior parts of the default mode network and medial temporal lobe structures. Lesion and neurodegenerative studies show that the medial temporal lobe is responsible for explicit memory functioning (Koenig., et al. 2008).
The Ego and Executive Functioning
Modern theorists often liken the Ego to executive functioning, which is the mental capacity to manage and control cognition as well as organize information into tasks such as engaging attention, decision-making, planning, and sequencing skills. Goldman-Rakic (1996) suggests that the prefrontal cortex performs functions of both the Ego and the Superego. For example, some aspects of working memory are stored in the prefrontal association cortex. In order to recall specific information, the prefrontal association cortex performs executive functions to produce the information from working memory.
Modern theorists often liken the Ego to executive functioning, which is the mental capacity to manage and control cognition as well as organize information into tasks such as engaging attention, decision-making, planning, and sequencing skills. Goldman-Rakic (1996) suggests that the prefrontal cortex performs functions of both the Ego and the Superego. For example, some aspects of working memory are stored in the prefrontal association cortex. In order to recall specific information, the prefrontal association cortex performs executive functions to produce the information from working memory.
A classic example of this phenomenon is seen in the case of Phineas T. Gauge. In 1848, Phineas was involved in a work related accident while laying railroad tracks. Due to an explosion, a metal rod passed through his head injuring much of his prefrontal cortex. While Phineas survived physically, his social capabilities were dramatically altered. Phineas underwent a major personality change and became irresponsible, profane, disregarded social conventions, and showed a deficit in social decision-making (Sanfey, Hastie, Colvin and Grafman, 2003).
In a sense, Phineas experienced Ego depletion which lifted the Superego’s strong inhibition over the Id and was able to act out his primitive impulses with no regard to societal norms. According to Braumeister, Bratslavsky, Muraven and Tice (1998), Ego depletion is the idea that willpower or self-control depends on a limited supply of mental resources that when depleted, will reduce self-control. Clinically, this is important because it explains why patients with severe prefrontal cortex damage or low frontal lobe activity seem to have disorganized behavior and an inability for delayed gratification, a hallmark trait of the Ego.
It is important to remember that each level of Freud’s personality theory, Id, Ego and Superego, contains both conscious and unconscious processes. However, the Ego is thought to be a mostly conscious apparatus that mediates the Id and the Superego. In connecting this idea with neuroscience experimental findings, Tononi and Edelman (1998) indicate that conscious experience involves the activation or deactivation of widely distributed brain areas. Tononi and Edelman reported that unconsciousness is associated with a significant decrease in neural activity in both the cerebral cortex and thalamus in subjects who are comatose or deeply anesthetized. This finding suggests that thalamocortical neural tracts play a significant role in consciousness.
Tononi and Edelman conclude the following: “Classical lesion and stimulation studies suggest that many brain structures outside the thalamocortical system have no direct influence on conscious experience” (p.1849). Clinically, patients with thalamic stroke or lesions in the thalamus may have deficits in consciousness or lack of Ego modulation of instinctual drives (aka Ego depletion). Additionally, other theories of consciousness have been linked to thalamocortical rhythm oscillations in thalamocortical-corticothalamic (TC-CT) pathway activity. Ward (2011) proposed that primary conscious awareness ascends from synchronized activity in dendrites of neurons in dorsal thalamic nuclei, facilitated particularly by inhibitory interactions with thalamic reticular neurons. Ward (2011) referred to this idea as the dynamic core theory of conscious experience.
The orbitofrontal cortex (OFC) in humans is known to process information regarding rewards and punishment. Similar to the Superego that evaluates reward and desires, these processes are described to be prerequisites to complex emotional and social behavioral regulation (Kringelbach and Rolls, 2004). The orbitofrontal cortex is responsible for the relative reward value of primary reinforcers (unlearned reinforcers, e.g., food), secondary reinforcers (learned reinforcers, e.g., money), and the reversal of learned associations between primary and secondary reinforcers. These types of reward learning situations are involved in emotion.
The orbitofrontal cortex receives projections from the magnocellular, medial, nucleus of the mediodorsal thalamus and receives inputs from all of the sensory modalities in order to determine the pleasantness or unpleasantness of sensory information from all modalities (Kringelbach and Rolls, 2004), including lower-order regions of the Id. The function of the OFC would be analogous to Dietrich, Fodor, Zucker, Bruckner, (2009, p 59) idea of the Ego that sub-serve to synthesize and modulate rewards, instincts, pleasantness, and sensations.
Many of the orbitofrontal cortex’s projections, especially those from the basil nucleus and the amygdala are topographically organized and reciprocal. McDonald and Mott (2016) suggest that the amygdala nuclear complex and hippocampal/parahippocampal regions are key areas in the limbic system that plays an important role in emotional learning and memory. Despite established evidence that glutamatergic pyramidal cells in the hippocampus and amygdala mediate interconnections between these areas, recent research indicates that long-range GABAergic projection neurons are also involved (McDonald, Mott, 2016). While the amygdala is seen as playing a major role in emotional learning, the PFC has the cognitive function of using that emotional information.
Recent studies have attempted to uncover the interactions between brain regions involving emotional information and decision-making. “Evidence from studies of rodents, humans and non-human primates indicates that interactions between the basolateral complex and the amygdala and the orbitofrontal cortex are crucial for generating and using reinforce expectancies that guide goal directed behavior” (Holland, Gallagher, 2004). While the interaction is important in establishing expectancies of incentive of future behavior, the OFC is much more crucial in guiding behavior based on the outcome expectancies. The OFC’s influence is even greater in cases where the suppression of a response is based on competing expectancy outcomes (Holland, Gallagher, 2004).
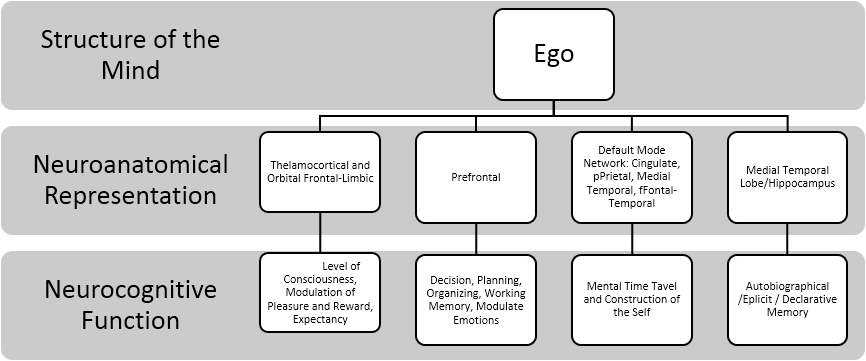
While the amygdala helps to learn these contingencies, it is the orbitofrontal cortex that uses these expectancies to regulate and suppress behavior. Thus placing the functions of the Ego-ideal within the PFC. Additionally, Minamoto, Osaka, Yaoi and Osaka (2014) demonstrated that ego-blocking situations (e.g., scenario where frustrater disappoints a frustratee, which theoretically blocks the frustratee's desire) activated bilateral ventrolateral PFC. This may suggest that ventrolateral PFC again modulates lower order limbic structures that give rise to emotions such as anger and rage. The summary of the aforementioned concepts of the Ego is illustrated in the Figure 3.
Neuroanatomical and Neurocognitive Aspects of the Superego
The Superego and Neurodevelopment
Psychoanalytic theory postulates that the Superego develops around 3-5 years of age during the phallic stage of psychosocial development (Freud, 1924). According to psychoanalytical theory, the Superego neurodevelopment in a child is a steady process that is completed once the father’s norm (or normative behaviors) is internalized and the sexual urges toward the mother are repressed, thus leading to the dissolution of the Oedipus complex (Freud, 1924).
The Superego and Neurodevelopment
Psychoanalytic theory postulates that the Superego develops around 3-5 years of age during the phallic stage of psychosocial development (Freud, 1924). According to psychoanalytical theory, the Superego neurodevelopment in a child is a steady process that is completed once the father’s norm (or normative behaviors) is internalized and the sexual urges toward the mother are repressed, thus leading to the dissolution of the Oedipus complex (Freud, 1924).
The Oedipus complex is a controversial idea of Freud that explains the internal conflict a young boy experiences as a result of sexual desires for his mother, to which, the young boy wants to possess his mother and be rid of his father. During the phallic stages, the boy becomes fixated on his penis and therefore develops castration anxiety. In order to resolve this anxiety, the boy adopts values and behaviors that are masculine and fatherly in nature through a process known as identification. As a consequence, the boy takes on the male gender role and subsequently adopts the same sex parents’ behaviors, ideals and values, which become the Superego (Freud, 1905).
Dietrich and colleagues (2009, p 60) identified three higher-order cerebral functions of the Superego: management of social rules and demands; Ego-ideals or ideal images of the self; and restrictions or impulse control. A neuroanatomical account of these functions may help to shed light onto patients who are over or under restricted as a result of a neurological deficits.
The Superego, Rules, Risk Taking, Ventromedial PFC (vmPFC) and Insular Cortex Damage
As mentioned earlier, the Superego develops gradually and serves as a regulating agent of primitive impulses. According to Payne (1927), the Superego achieves this regulation through fear of punishment and anxiety, and the anticipation of guilt. These intense emotions function to uphold certain rules oriented beliefs. The Superego incorporates the rules and morals that are learned by one’s parents, society, and environment (i.e. religious upbringing).
As mentioned earlier, the Superego develops gradually and serves as a regulating agent of primitive impulses. According to Payne (1927), the Superego achieves this regulation through fear of punishment and anxiety, and the anticipation of guilt. These intense emotions function to uphold certain rules oriented beliefs. The Superego incorporates the rules and morals that are learned by one’s parents, society, and environment (i.e. religious upbringing).
One particular higher-order, executive functioning task is rule learning. One needs an intact PFC in order to learn rules and the learning of those rules influence social behaviors. Several studies have attributed poor social judgment and decision making abilities to damage of the vmPFC. While these brain injured patients perform adequately on tests of cognitive abilities, they are described as being ‘socially incompetent’, ‘decides against his best interest’, and ‘doesn’t learn from his mistakes’ (Sanfey, Hastie, Colvin and Grafman 2003).
Monitor and forethought about risk taking tends to be a major part of the Superego’s frontal systems. A study by Clark, Bechara, Damasio, Aitken, Sahakian and Robbins (2008) examined the outcomes in risky decision-making in healthy subjects and subjects with either vmPFC damage, insular cortex damage, or damage to the Dorsolateral Prefrontal Cortex (DLFC). The four subject groups were compared on the Cambridge Gamble Task, a well-established measure of risky decision-making, especially when outcome probabilities are ambiguous.
Results showed that normal and frontal brain injured patients without damage to vmPFC, initially made risky bets because of the high potential for payoff. However, when the probability of losing increased, both healthy and frontal lesion patients appropriately reduced their wager. Subjects with vmPFC and insular lesions failed to adjust their bets despite the odds of winning, thus maintaining their risky behavior and not being able to abstract rules and calculate risk from their own behaviors (Clark, Bechara, Damasio, Aitken, Sahakian, and Robbins, 2008).
Pujara, Wolf, Baskaya, and Koenigs (2015) administered a gambling task to measure risk taking in patients with bilateral vmPFC lesions, patients with lesions non-vmPFC, and healthy adults. Patients with vmPFC lesions were found to increase gambling in loss conditions and decrease gambling in win conditions, suggesting that lesions to the vmPFC reduce risky decision-making.
Other researchers using similar lesion methodology and gambling tests to assess risk taking have further supported the role of the vmPFC and insular cortex in risk calculation and decision making (Ishii, Tsutsui, Iijima, 2013; Adida., et al. 2011). This further implicates that in the absence of the Superego’s frontal systems (e.g., vmPFC and insular cortex) the Id’s risk taking and impulsive decision-making increase.
The Superego, Moral Emotions, and Frontotemporal Structures
According to the psychoanalytic theory, the Superego inhibits the demands of the Ego and Id by calculating the amount of guilt and shame to ultimately favor societal norms over sexual and aggressive impulses and a sense of self (Payne, 1927). Guilt and shame are examples of sociomoral emotions such as fear of punishment, indignation, and embarrassment (Tangney, Stuewig, and Mashek, 2007). Reviewing the neural underpinnings of moral convention and sociomoral emotions can help understand the Superegos construct and brain correlates.
According to the psychoanalytic theory, the Superego inhibits the demands of the Ego and Id by calculating the amount of guilt and shame to ultimately favor societal norms over sexual and aggressive impulses and a sense of self (Payne, 1927). Guilt and shame are examples of sociomoral emotions such as fear of punishment, indignation, and embarrassment (Tangney, Stuewig, and Mashek, 2007). Reviewing the neural underpinnings of moral convention and sociomoral emotions can help understand the Superegos construct and brain correlates.
There are two interesting cases of damage to the orbitofrontal cortex in which the damage was acquired early in life. These patients showed behavioral problems throughout their lives that were resistant to corrective behavioral interventions. In addition, the patients did not have knowledge of moral and societal conventions (Kringelbach, 2004). This finding is supported by evidence that the prefrontal cortex develops remarkably late, and full myelination does not occur until early adulthood in many cases (Sapolsky 2004).
Conversely, subjects with orbitofrontal lesions acquired later in life retain their knowledge of societal conventions although they often failed to use the knowledge in mitigating their behaviors, as seen with Phineas T. Gage. Thus, the implication is that the orbitofrontal cortex is a necessary component in the development of personal morality that is based on reward/punishment learning (Kringelbach, 2004).
Research by Moll, Olivera-Souza, Eslinger, Bramati, Mourao-Miranda, Andreiuolo and Pessoa (2002) suggests that the orbital and medial section of the prefrontal cortex and the superior temporal sulcus region play a major role in moral appraisal. Moral emotions are those involving the interests of society or of another person. Moral emotions are evoked rather rapidly by a perception of a moral violation, and they involve a rapid unconscious appraisal of the situation.
For example, the unconscious primitive urges of the Id are held in check by the reality principle of the Ego, which also seeks pleasure but in a socially acceptable manner. Freud (1923) stated that the Ego is “like a man on horseback, who has to hold in check the superior strength of the horse.” Moreover, if the Ego fails in its task to mitigate the urges of the headstrong Id, then anxiety is experienced along with feelings of guilt and shame brought about by the moral Superego.
Moral evaluation is said to be an automatic process and in Freudian terms it would be considered entirely unconscious. In attempting to understand the automaticity of the prefrontal cortex’s moral evaluative response, Mendez (2009) stated that many researchers and scientists have viewed morality as the sixth human sense, supporting the notion that humans possess an intrinsic morality network. Mendez (2009) went on to state that if a ‘moral sense” indeed existed, then there should be specific brain mechanisms for morality as well as associated pathology for those mechanisms.
In efforts to understand the neuroanatomy of moral behavior, investigators have used fMRI in normal and abnormal subjects undertaking tasks or dilemmas of moral reasoning and judgment (Green, Sommerville, Nystrom, Darley and Cohen, 2001). In other research, subjects were shown images aimed at evoking moral emotions and fMRI imaging during this study showed increased activation of the amygdala, thalamus and the upper midbrain with recruitment of the orbital and medical PFC. This evidence indicates that the orbital and medical areas of the PFC as well as the superior temporal sulcus, play an important role in moral appraisals (Mott., et al. 2002).
The vmPFC, specifically on the right, is central to this neuroanatomical moral network. Tasks requiring explicit moral judgments, viewing of morally stimulating photos and the concept of fairness, guilt and charity have all caused the vmPFC area of the brain to become aroused on imaging studies (Mendez, 2009). Additionally, Greene., et al. (2001) discusses findings that show enhanced vmPFC activation on presenting moral dilemmas on a personal level versus a moral dilemma on an impersonal level in which the subject’s actions would not cause serious harm to someone else.
These findings highlight the critical role the vmPFC and Superego plays in negotiating through moral dilemmas. Clinically, this is significant because advances in neuroimaging have revealed a great deal of information about the study of morality and its associated pathology. Subsequent studies have shown that patients with damage to the PFC are able to verbalize what is appropriate in specific situations, but are unable to act appropriately when the time comes and instead chose to pursue behaviors aimed to attain immediate gratification. Understanding this relationship clinically will help clinicians and researchers better anticipate diagnoses and treatment outcomes of their patients.
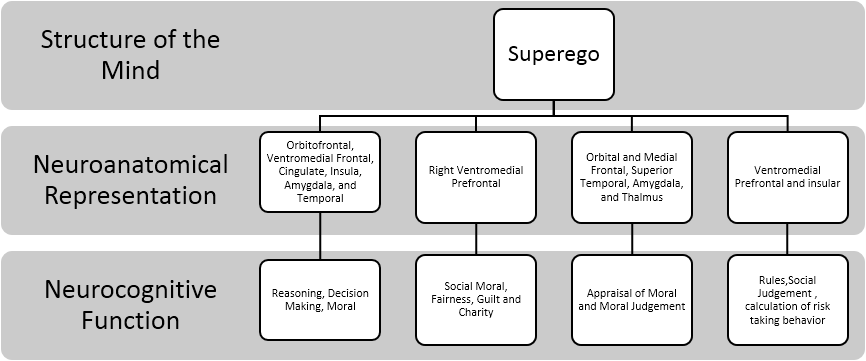
Studies also support a wide range of structures outside of the vmPFC and ventrolateral PFC to be involved in moral. Boccia and colleagues (2016) conducted a meta-analysis of fMRI of moral reasoning. They found multiple higher-order and lower-order regions to be associated with moral reasoning task. These include orbitofrontal cortex, ventromedial PFC, cingulate cortex, insula, amygdala, and temporal lobe. Researchers noted that moral reasoning has pre-wired functional specialization that activates higher-order and lower-order neural circuitry. The summary of the aforementioned concepts of the Superego is depicted in Figure 4.
Conclusions
Summary
Traditionally, the areas of the psychoanalytic concepts of the structure of the mind and neuroscience have been studied as separate entities. This paper aimed to further explore the overlapping relationships between the two in detail and provide a framework to understand both psychoanalytic concepts of the structure of the mind (e.g., Id, Ego, and Superego) and brain function. Overall, each structure of the mind concept had unique brain areas and functions.
Traditionally, the areas of the psychoanalytic concepts of the structure of the mind and neuroscience have been studied as separate entities. This paper aimed to further explore the overlapping relationships between the two in detail and provide a framework to understand both psychoanalytic concepts of the structure of the mind (e.g., Id, Ego, and Superego) and brain function. Overall, each structure of the mind concept had unique brain areas and functions.
In our literature review, we found that the concept of the Id appeared to be localized in subcorticolimbic and lower-order (e.g., mammalian-reptilian) systems. This was consistent with Solom (2013) and his inference on limbic structures and brain stem structures housing the majority of the functions of the Id. Specifically, we found that the Id’s neuroanatomical representations are retained in the amygdala, basal ganglion, insular cortex, hippocampus, and nucleus accumbens. These areas were largely responsible for the Id’s unconscious processing/implicit memory, emotional memory, and body awareness. We also noted that the cross-talk between the orbital frontal lobe and the limbic structures may assist in reward modulation or functioning as the reward principle.
We also found that the Ego concept appeared to have a wide distribution of neuroanatomical and neurocognitive functions, ranging from lower-primitive cortical sites to higher-cortical sites. The Ego’s neuroanatomical representations were noted in the default mode network, orbital frontal lobe, prefrontal lobe, medial frontotemporal lobes and medial temporal lobe and thalamocortical tracks. Functionally, these areas reflect modulation of level of consciousness, pleasure/reward expectancy, decision making, planning, organization, working memory, and modulation of intense emotions such as anger and rage. These anatomical areas also reflected the ability to sort and organize past and future mental activities (e.g., mental time travel) and the construction of the self through the retrieval of autobiographical memory. This occurs mainly via the declarative/explicit long-term memory system.
Lastly, the literature showed that the Superego concept appeared to be localized in the PFC, orbitofrontal, ventromedial frontal, cingulate, amygdala, temporal lobe, and insular cortex. Regarding functioning, these areas were responsible for moral judgment, reasoning/risk calculation when decision-making. Other functions include socially mediated emotions and moral experiences such as feelings of guilt and shame as well as ideas of charity and fairness. Interestingly, some areas of the limbic system were noted to play a role in the concept of the Superego (e.g., amygdala). This suggests that the Superego’s lower-order brain areas share similar neural circuitry as the Id. This would be consistent with the idea that the Superego largely functions at an unconscious level, similar to the Id.
Implications
The aforementioned provide a conceptual framework for the overlapping areas of psychoanalytic concept of the structure of the mind and brain function. There are wide range application for this integrative framework in clinical, educating, and research practice. Regarding clinical practice, understanding the different personality or structure of the mind elements will foster advanced integrative thinking and further the understanding of clinical conceptualization of neurological deficits in a psychotherapeutic and psychoanalytic context. Understanding the Id, Ego, and Superego’s neurological underpinnings can help professionals to conceptualize patients with neurological deficits with focused interventions.
The aforementioned provide a conceptual framework for the overlapping areas of psychoanalytic concept of the structure of the mind and brain function. There are wide range application for this integrative framework in clinical, educating, and research practice. Regarding clinical practice, understanding the different personality or structure of the mind elements will foster advanced integrative thinking and further the understanding of clinical conceptualization of neurological deficits in a psychotherapeutic and psychoanalytic context. Understanding the Id, Ego, and Superego’s neurological underpinnings can help professionals to conceptualize patients with neurological deficits with focused interventions.
Much like how cognition is inferred from anatomical brain regions (e.g., explicit verbal memory is housed in the medial temporal lobe/hippocampus), psychoanalytic conceptualization can be inferred from anatomic brain regions. For example, thelamocortical tract impairment or default mode network dysregulation may lead to conceptual difficulties with Ego integration, problems with construction of a sense of self, or decision making between primitive impulse and sociomoral demands. This sheds light and opens the door for a larger scale case conceptualization with a neuroanatomical and neurocognitive backing. Research in this area would need to be further conducted to fully see the applicability.
Regarding education, educators can teach both psychoanalysis and brain function simultaneously. In this framework, the psychoanalytic concept is the global theory and brain anatomy and cognitive functions are the subsets. Therefore, the educator can easily implement a curriculum that teaches both the psychoanalytic concepts and the anatomical and cognitive functional aspects of the brain in a hierarchical manner. Utilizing the outline and the table and figures presented earlier in this article can also facilitate learning.
Teaching such topics simultaneously would require a stepwise fashion. This includes, first, introducing psychoanalytic concepts (e.g., global concept). Next, introducing names of anatomical areas (e.g., subsets). Finally, linking the cognitive functions of the anatomical regions back to the psychoanalytic concept will complete the three-tier understanding. This review is not exhaustive and there are many challenges with this framework that can be addressed in future investigations. Researchers may wish to conduct a full review of only activation paradigms utilizing functional MRI to further tease out the parallels between the Id, Ego, and Superego concepts and brain function. Also, to our knowledge, this is the first study that highlights the similarity between the structure of the mind concepts, brain anatomy, and cognitive functions in one coherent view. Nevertheless, how this translates to clinical and education needs further empirical investigation. Future researchers and educators may wish to see how trainees are able to cognitively absorb this multifaceted overlay of concepts and examine how multi-subject learning is accelerated.
References
- Abela AR., et al. “Hippocampal interplay with the nucleus accumbens is critical for decisions about time”. European Journal of Neuroscience 42.5 (2015): 2224–2233.
- Adida M., et al. Decision-making and schizophrenia. Encephale37.2(2011): 110-116.
- Amsterdam B. “Mirror self-image reactions before age two”. Developmental Psychobiology 5.4 (1972): 297-305.
- Baumeister RF., et al. "Ego depletion: Is the active self a limited resource?" Journal of Personality and Social Psychology 74.5 (1998):1252–1265.
- Berns GS., et al. “Predictability Modulates Human Brain Response to Reward”. The Journal of Neuroscience21.8 (2001): 2793–2798.
- Boccia M., et al. “Neural foundation of human moral reasoning: an ALE meta-analysis about the role of personal perspective”. Brain Imaging and Behavior 11.1 (2016): 1-15.
- Cabeza R. “Role of parietal regions in episodic memory retrieval: The dual attentional processes hypothesis”. Neuropsychologia 46.7 (2008): 1813–1827.
- Clark L., et al. “Differential effects of Insular and Ventromedical Prefrontal Cortex Lesions on Risky Decision-Making”. Brain: A Journal of Neurology131.5(2008): 1311-1322.
- Costa RM and Oliveira RF. “Maladaptive defense mechanisms are associated with decoupling of testosterone from sexual desire in women of reproductive age”. Neuropsychoanalysis 17.2(2015):121-134.
- Corvin A and Fitzgerald M., “Evidence-based medicine: Psychoanalysis and psychotherapy”. Psychoanalytic psychotherapy14.2(2000): 143-151.
- Cunningham WA., et al. “Neural components of social evaluation”. Journal of Personality and Social Psychology 85.4 (2003):639-694
- Davis SM. “The Relevance of Gerald Edelman's Theory of Neuronal Group Selection and Nonlinear Dynamic Systems for Psychoanalysis”. Psychoanalytic Inquiry 22.5 (2002): 814-840.
- Dietrich D., et al. “Simulating the Mind: A Technical Neuropsychoanalytical Approach”. Springer, New York: NY. (2009).
- Delville Y., et al. “Neural Connections of the Anterior Hypothalamus and Agonistic Behavior in Golden Hamsters”. Brain Behavior and Evolution 55 (2000): 53-76.
- Ferris CF., et al. “Vasopressin/Serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters”. Journal of Neurosciences17.11 (1997): 4331-4340.
- Freud S. “Project for a scientific psychology”. Standard Edition 1 (1895): 281–397.
- Freud S. (1905). Three essays on the theory of sexuality. In J. Strachey (ed. and trans.), The standard edition of the complete psychological works of Sigmund Freud, 7: 123-243 London, Hogarth Press, 1958 (Original work published in 1905).
- Freud S. “The unconscious”. Standard Edition 14 (1915a): 166–204.
- Freud S. “Instincts and their vicissitudes”. Standard Edition 14 (1915b): 117–140.
- Freud S. “The Ego and the Id”. Standard Edition 19 (1923): 12–59.
- Freud S. “An Outline of Psychoanalysis”. Standard Edition 23 (1940): 144-207.
- Green JD., et al. “An fMRI investigation of emotional engagement in moral judgment”. Science 293.5537 (2001):2105-2518.
- Greenough WT., et al. “Experience and brain development”. Child Development 58.3 (1987): 539–559.
- Goldman-Rakic P. “Regional and cellular fractionation of working memory”. Proceedings of the National Academy of Sciences of the United States of America 93.24 (1996): 13473-13480.
- Guyton AC and Hall JE. “Medical Physiology”. W.B. Saunders Company, PV: Philadelphia (2000).
- Hergenhahn BR. An Introduction to the History of Psychology. 4th edition: Wadsworth Thomason learning, CA: Belmont (2001).
- Holland P C and Gallagher M. “Amygdala-frontal interactions and reward expectancy”. Current Opinion in Neurobiology14.2 (2004): 148-155
- Ishii, H., et al. “Risk taking and the insular cortex”. Brain Nerve65.8 (2013): 965-972.
- Kaplan-Solms K and Solms M. “Clinical Studies in Neuro-Psychoanalysis”. London: Karnac (2000).
- Kahn, M. Basic Freud: psychoanalytic thought for the 21st century. Basic Books, NY: New York (2002).
- Klein S. “The self and its brain”. Social Cognition 30 (2012): 474-516.
- Kendel ER. “A new intellectual framework for psychoanalysis”. American Journal of psychiatry155.4(1998): 457-469.
- Knutson B., et al. “FMRI visualization of brain activity during a Monetary Incentive Delay Task”. NeuroImage12.1 (2000): 20–27.
- Koenig, P., et al. “Medial Temporal Lobe Involvement in an Implicit Memory Task: Evidence of Collaborating Implicit and Explicit Memory Systems from fMRI and Alzheimer’s disease”. Cerebral Cortex 18.12 (2008): 2831–2843.
- Kringelbach ML and Rolls ET. “The functional Neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology”. Progress in Neurobiology72.5(2003): 341-372.
- Luciani M., et al. “Neural correlate of the projection of mental states on the not-structured visual stimuli”. Neuroscience Letters 573 (2014): 24–29.
- Levin FM. “Integrating some mind and brain views of transference: the phenomena”. Journal of the AmericanPsychoanalytic Association45.4 (1997)1121-1151.
- Mansvelder HD and McGehee D. “Long-Term Potentiation of Excitatory Inputs to Brain Reward Areas by Nicotine”. Neuron27.2 (2000): 349–357.
- Mishkin M and Appenzeller T. “The anatomy of memory”. Scientific American 256.6 (1987): 80–89.
- Minamoto T., et al. “Extrapunitive and Intropunitive Individuals Activate Different Parts of the Prefrontal Cortex under an Ego-Blocking Frustration”. PLoS One 9.1 (2014): e86036.
- McCormick C., et al. “Linking DMN connectivity to episodic memory capacity: what can we learn from patients with medial temporal lobe damage?” NeuroImage: Clinical 16 (2014): 188-196.
- McDonald AJ and Mott D. “Functional Neuroanatomy of Amygdalohippocampal Interconnections and their Role in Learning and Memory”. The Journal of Neuroscience Research 95.3 (2017): 797-820.
- Moll J., et al. “The neural correlates of moral sensitivity: A functional magmentic resonance imaging investigation of basic and moral emotions”. The Journal of Neuroscience22.7(2002): 2730-2736.
- Nielsen M., et al. “A longitudinal investigation of self-other discrimination and the emergence of mirror self-recognition”. Infant Behavior and Development 26.2 (2003): 213–226.
- Noam G., et al. “Ego Development and Psychopathology: A Study of Hospitalized Adolescents.” Child Development 55.1 (1984): 189-194.
- Northoff G., et al. “How does our brain constitute defense mechanisms? First-person neuroscience and psychoanalysis”. Psychotherapy and Psychosomatics76.3 (2007): 141-153.
- Panksepp J. “Cross-species affective neuroscience decoding of the primal affective experiences of humans and related animals”. PLoS One 6.9 (2011): e21236.
- Payne SM. “Observations on the formation and function of the super-ego in normal and abnormal psychological states”. British Journal of Medical Psychology 7(1927): 73–87.
- Pribram C. “A century of progress”. Annals of the New York Academy of Sciences 843 (1998): 1-10.
- Prochaska JO and Norcross JC. Systems of Psychotherapy: A Transtheoretical Analysis, 5th ed. Thomason Brooks Cole, CA: Pacific Grove. (2003).
- Pujara MS., et al. “Ventromedial prefrontal cortex damage alters relative risk tolerance for prospective gains and losses”. Neuropsychologia 79 (2015): 10-15.
- Pugh G. “Freud's 'problem': cognitive neuroscience and psychoanalysis working together on memory”. International Journal of Psychoanalysis 83.6(2002): 1375-1394.
- Reber PJ. “The neural basis of implicit learning and memory: A review of neuropsychological and neuroimaging research”. Neuropsychologia 51.10 (2013): 2026-2042.
- Rugg MD and Vilberg KL. “Brain networks underlying episodic memory retrieval”. Current Opinion in Neurobiology 23.2 (2013): 255–260.
- Sanfey AG., et al. “Phineas gage: Decision-making and the human prefrontal cortex”. Neuropsychologia41(2003): 1218-1229.
- Sapolsky RM., “The frontal cortex and the criminal justice system”: One contribution of the 16 to a theme Issue ‘Law and the brain’: Philosophical transactions Biological Sciences 359 (2004): 1787-1796.
- Schott BH., et al. “Redefining implicit and explicit memory: The functional neuroanatomy of priming, remembering, and control of retrieval”. Proceedings of the National Academy of Sciences of the United States of America 102.4 (2005): 1257–1262.
- Siegel D J. “The developing mind: Toward a neurobiology of interpersonal experience”. New York, NY: Guilford (1999).
- Solms, M. “Freud Returns”. Scientific American 290.5(2004): 57-62.
- Solms M. “Before and after Freud’s Project”. Annals of the New York Academy of Sciences 843 (1998): 1-10.
- Solms M. “The Conscious Id”. Neuropsychoanalysis 15.1 (2013): 5-19.
- Snowden R. “Teach Yourself Freud”. McGraw-Hill (2006): 105-107.
- Tangney JP., et al. “Moral emotions and moral behavior”. Annual Review of Psychology58(2007): 345-372.
- Temur A and Askerov F. “Morphological Changes in the Some Centers of Hypothalamus during Food Deprivation”. Journal of Neurological Sciences 22.1(2005): 35-42.
- Thut G., et al. “Activation of the human brain by monetary reward”. NeuroReport 8.5 (1997): 1225–1228.
- Tiberga K. “Confabulating in the transference”. Neuropsychoanalysis 16.1 (2014): 57-67.
- Tononi G and Edelman GM. “Consciousness and complexity”. Science 282. 5395(1998): 1846-1851.
- Tulving E. “What is episodic memory?” Current Directions in Psychological Science 2.3 (1993): 67-70.
- Vaillant GE. “Involuntary coping mechanisms: a psychodynamic perspective”. Dialogues in Clinical Neuroscience13.3 (2011): 366-370.
- Volkow ND., et al. Brain DA D2 receptors predict reinforcing effects of stimulants in humans: Replication study. Synapse 46.2 (2002): 79–82.
- Wagner AD., et al. “Material-specific lateralization of prefrontal activation during episodic encoding and retrieval”. NeuroReport 9 (1998): 3711–3717.
- Ward LM. “The thalamic dynamic core theory of conscious experience”. Consciousness and Cognition20.2 (2011): 464–486.
- Westen D. “The scientific status of unconscious processes: Is Freud really dead?” Journal of the American Psychoanalytic Association47.4(1999): 1061-1106.
- Westen D and Gabbard GO. “Developments in cognitive neuroscience: II. Implications for theories of transference”. Journal of the American Psychoanalytic Association 50.1 (2002):99-134.
- White J. Road Map Neuroscience, Second Edition. McGraw-Hill 66 (2008) 158-172, 168-170.
- Wilner A and Aubé M. “A convergent neurological and psychoanalytic view of the concept of regression and mental structure in a case of NMDA receptor encephalitis”. Neuropsychoanalysis16.2 (2014): 97-113.
- Woody JM and Phillips JL. “Freud's project for a scientific psychology after 100 years: The unconscious mind in the era of cognitive neuroscience”. Philosophy, Psychiatry, and Psychology 2.2(1995):123-134.
Citation:
Amir Ramezani., et al. “Neuroanatomical and Neurocognitive Functions of the Structure of the Mind: Clinical and Teaching
Implications”. Current Opinions in Neurological Science 2.6 (2018): 567-584.
Copyright: © 2018 Amir Ramezani., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.






























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.