Research Article
Volume 1 Issue 6 - 2017
Cause and Treatment of Schizophrenia: Electron Transfer, Reactive Oxygen Species, Oxidative Stress, Antioxidants, and Unifying Mechanism.
1Peter Kovacic, Department of Chemistry and Biochemistry, San Diego State University, San Diego, CA, USA
2Wil Weston, Library and Information Access, San Diego State University, San Diego, CA, USA
2Wil Weston, Library and Information Access, San Diego State University, San Diego, CA, USA
*Corresponding Author: Peter Kovacic, Department of Chemistry and Biochemistry, San Diego State University, San Diego CA 92182 USA.
Received: December 02, 2017; Published: December 06, 2017
Abstract
Reactive oxygen species (ROS) and oxidative stress (OS) play roles in schizophrenia (SCZ), as also in Alzheimer’s (AD) and Parkinson’s (PD) disease. Various sources, including oxidases, serve as generators of ROS-OS, such as mitochondria, NADPH, cytochromes P450, monoamines, ET metal complexes, G72 gene, and microglia. Diverse types of antioxidants (AOs) exert a positive influence on the harmful effects. However, there are fewer drugs in the phenols and phenolic ethers category, in comparison with AD and PD. A unifying mechanism based on ET-ROS-OS-AO is involved. Other possible influential aspects are discussed in a multifaceted approach.
Keywords: Schizophrenia; Radicals; Oxidative stress; Reactive oxygen species; Antioxidants
Abbreviations: ET: Electron transfer; ROS: Reactive oxygen species; OS: Oxidative stress; AO: Antioxidant; SCZ: Schizophrenia
Introduction
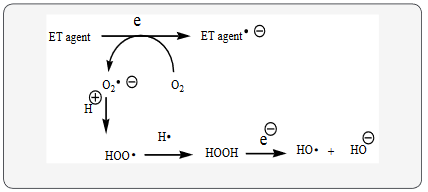
Schizophrenia (SCZ) fits into the unifying mechanism which has been widely applied previously as set forth in Kovacic and Somanathan’s (2017) article involving electron transfer (ET), reactive oxygen species (ROS) and oxidative stress (OS). This unifying mechanism argues that the preponderance of bioactive substances, usually as the metabolites, incorporate ET functionalities. We believe these ET-metabolites play an important role in physiological responses. The main group include quinones (or phenolic precursors), metal complexes (or complexors), aromatic nitro compounds (or reduced hydroxylamine and nitroso derviatives), and conjugated imines (or iminium species). Resultant redox cycling is illustrated in Scheme 1. In vivo redox cycling with oxygen can occur, giving rise to oxidative stress(OS) through generation of reactive oxygen species (ROS), such as hydrogen peroxide, hydroperoxides, alkyl peroxides, and diverse radicals (hydroxyl, alkoxyl, hydroperoxyl, and superoxide) (Scheme 1). Cellular and mitochrondrial enzymes can also perform cataytically in the reduction of O2.
In some cases ET results in involvement with normal electrical effects (e.g. neurochemistry). Generally, active entities possessing ET groups display reduction potentials in the physiologically responsive range (Kovacic & Somanathan, 2017). Hence, ET in vivo can occur resulting in production of ROS which can be beneficial in cell signaling at low concentrations, but produce toxic results at high levels. Electron donors consist of phenols, N-heterocycles or disulfides in proteins which produce relatively stable radical cations. ET, ROS and OS have been increasingly implicated in the mode of action of drugs and toxins, e.g. anticance drugs (Kovacic & Osuna, 2000), carcinogens (Kovacic & Jacintho, 2001), cardiovascular toxins (Kovacic & Thurn, 2005), toxins (Kovacic, Pozos, Somanathan, Shangari, & O’Brian, 2005), ototoxins (Kovacic & Somanathan, 2008) and various other catagories (Halliwell & Gutteridge, 1999).
In addition to the above, there is a plethora of experimental evidence supporting the theoretical framework. This evidence includes generation of the common ROS, lipid peroxidation, degradation products of oxidation, depletion of AOs, effect of exogenous AOs, and DNA oxidation and cleavage products, as well as electrochemical data (Kovacic & Somanathan, 2017). This comprehensive, unifying mechanism is consistent with the frequent observation that many ET substances display a variety of activities (e.g. multiple-drug properties), as well as toxic effects.
It is important to recognize that mode of action in the bio domain is often involved with many physiological actions and is multifaceted. In addition to the ET-ROS-OS in relation to mechanism, much attention in the literature is paid to AO action entailing physiological action.
Two sources will serve to describe the characteristics common to SCZ. It is a serious psychiatric illness whose main symptoms are hallucinations and delusions (Bentall, 2006). Other features are withdrawal, apathy, cognitive impairment, loosening of associations, emotional ambivalence, autism, world of fantasy, structural and neuro-developmental abnormalities. Various causes have been proposed such as genetic influences, pollution from industrialization, traumatic episodes, and abnormal personality.
Treatment has mostly involved antipsychotic drugs. These medicines appear to block dopamine receptors leading to the theory that SCZ is caused by abnormality in the dopamine system. Therapy based on family or cognitive behavior may be useful; however the present report deals with the properties of therapeutic drugs. Rather sparse treatment of sources for ROS-OS exists in the SCZ literature, as is also for the case for PD (Kovacic & Weston, 2017a) and AD (Kovacic & Weston, 2017b). However, searches reveal appreciable discussion of the generators of ROS.
Useful information is available from another source, the Harvard Medical School Health Topics A-Z (Lewine, 2016). SCZ patient have difficulty recognizing reality, behaving naturally and thinking logically. There is difficulty with thoughts and perception, and withdrawal from social contacts. Other symptoms are disorganized speech, diminished emotional expression, inability to engage in productive activity, deterioration in logical thinking, and unusual motor activity. The combination of limited speech, social skills, and behavior plus restrictive emotions suggest to some that the disease may be a combination of several illnesses. Some cases involve substance abuse and metabolic syndrome involving dysfunction in various organs where several genes are known to play a role.
Treatment can consist of a combination of medication, psychosocial, counseling, and support by family and community with medications which comprise older and more recent drugs. The primary side effect of these antipsychotic medications is sedation.
NADPH oxidase is an important producer of ROS which is involved in physiological responses in various organs, including the brain. A study by Markovic., et al. (2017) dealt with the effect of maternal deprivation (MD) on NADPH oxidase expression. MD causes behavioral changes which resemble SCZ. The AO GSH was reduced in quantity along with changes in OS parameters. Results point to extensive changes in redox aspects.
G72 is a SCZ susceptible gene which encodes a polypeptide. Activation of D-amino acid oxidase has been suggested as a means of reducing the neurotransmission of N-methyl-D-aspartate receptors (Wang., et al. 2015). G72 effectively increased ROS generation in cells. Evidence indicates participation of OS. The ROS result can be reversed by tempol which is a scavenger of ROS. Psychiatric symptoms were enhanced by increase in G72 levels in SCZ patients. ROS enhancement in mental illness may be a result of G72 overexpression in brain cells.
An earlier report (Drews, Otte, & Zimmer, 2012) also revealed G72 in SCZ. Results point to G72 as a participant in reduction of mitochondrial and synoptic defects, a pathomechanism of SZC. Many of the other findings (Drews, Otte, & Zimmer, 2012) were similar to those of Wang., et al. (2015). The ROS-OS aspects of mitochondrial action are treated elsewhere in this report (Drews, Otte, & Zimmer, 2012). A more recent article by Akyol., et al. (2017) on G72 protein levels also supports the suggestion that G72 is an activator of D-amino acid oxidase and may play a role in the pathogenesis of SCZ.
ROS generated by NO synthase have been implicated in an array of harmful behaviors which play a role in SCZ (Walton., et al. 2013). Evidence demonstrates that the enzymes produce redox signaling cascades which interact in the brain with resultant influences on social behavior and cognitive function.
Mitochondria provide another source of ROS-OS which has been claimed as a contributor to aging (Kovacic & Somanathan, 2017a; Kovacic., et al. 2005). Leakage of electrons occurs in the ET chain which react with oxygen to produce superoxide, a precursor of other ROS. Various neurotransmitters can undergo autoxidation of which dopamine is an example (Kovacic & Weston, 2017a). The molecule incorporates a catechol entity which metabolizes to an ET o-quinone resulting in toxic ROS-OS. Other examples are cytochromes P450, ET metal complexes of protein (e.g. iron), monoamine oxidases, and microglia that are microphages of the nervous system capable of generating superoxide and H2O2 (Halliwell & Gutteridge, 1999). A frequent operator in generation of ROS-OS is ET which may play an important function.
The present status of SCZ consists of symptom alleviation without a cure. Since ROS-OS appears to be the root of the illness, more research should be devoted to inhibition of the fundamental causes in order to achieve the desired cure and prevention. This approach may also be pertinent to other brain diseases, such as PD (Kovacic & Weston, 2017a) and AD (Kovacic & Weston, 2017b).
It is necessary to recognize the importance of the multifaceted nature of physiological action. In addition to ET-ROS-OS-AO, other factors are at play in SCZ as indicated in this discussion: cell signaling, mitochondria, receptor binding and enzyme inhibition (Walton., et al. 2013). The literature addresses the basic aspects of these items: cell signaling (Kovacic & Somanathan, 2012), mitochondria (Kovacic., et al. 2005), and receptor binding (Kovacic, Pozos, & Draskovich, 2007). The related articles on PD and AD deal with the role of AOs in decreasing ROS-OS. However, the specific source of the harmful species was not addressed. Information is available on generation of ROS-OS in the brain.
Discussion
Mechanisms involved in CNS diseases almost always involve OS. For this reason, AOs have been the object of much research, including clinical trials; yet, all of these clinical trials have failed (Sorce, Krause, & Jaquet, 2012). A promising option involves targeting of specific sources of ROS in the CNS, such as NOX enzymes. These enzymes are importantly involved in a variety of CNS disorders, including SCZ, AD, and PD. The review places focus on therapeutics aimed at NADPH oxides of the NOX class. These drugs could solve the present problem involving incurable CNS pathologies. The majority of the drugs are not phenolic.
Phenothiazines (PTZ) are important drugs used in SCZ treatment. A prior report (Cruz., et al. 2010) dealt with AO activity of thioridazine. Evidence indicates that PTZ enters the mitochondrial membrane and generates radicals which interact with thiol groups resulting in mitochondrial permeability transition associated with the release of cytochrome c.
Much evidence indicated that ROS are involved with psychiatric disorders, including SCZ. Findings show that the AO vitamin C reduced ROS production in rat blood resulting from certain drugs (Heiser., et al. 2010).
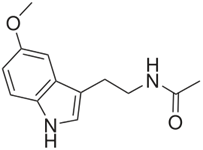
The neurohormone melatonin (Figure 1) is linked to the pathogenesis of SCZ. The hormone attenuates behavioral deficits and reduces OS in the brain in a SCZ model (Onaolapop, Aina, & Onaolapo, 2017). Evidence indicates that melatonin may be useful in SCZ therapy. The literature provides evidence for involvement as an AO agent by various means, including demethylation to the phenolic serotonin (Kovacic & Weston, 2017a, Kovacic & Weston, 2017b, Halliwell & Gutteridge, 1999). In a related report (da Silva Araujo., et al. 2017), melatonin reverses all SCZ-like symptoms associated with ROS-OS.
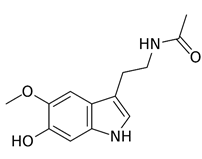
A 2014 article provides useful information on AO properties of melatonin (Kovacic & Somanathan, 2014). The principal product of metabolism is the 6-hydroxy derivative (Figure 2). Melatonin demethylation yield the corresponding phenol. Both phenolics are expected to exhibit AO properties. Representative examples are provided from the literature dealing with AO action of melatonin.
Environmental insults increase OS in the adolescent brain (Khan., et al. 2017). Redox dysregulation is an important factor in the development of SCZ and other neuropsychiatric disorders. Adolescent exposure to a dopamine transporter inhibitor produces elevation in indicators of OS. Evidence indicates that oxidative and immune-inflammatory pathways play a role in SCZ (da Silva Araujo., et al. 2017). Abundant literature deals with inflammation as well (Kovacic & Somanathan, 2014). The condition accompanies numerous illnesses, including aging. Many reports deal with a link between those conditions and the presence of ROS-OS.
Adjunctive treatment with AOs, such as vitamin E, rice bran oil, and curcumin, in the early stages may prevent injury by ROS-OS, as well as other deficits (Shireen, 2016). This 2016 review explains the role of OS with the aim of providing improved therapeutic drugs.
SCZ is a complex pathology. Neurochemical theories have been the most studied, leading to the neurodevelopmental theory. Findings indicate that oxidative and nitrosative stress are importantly involved (Mhillaj., et al. 2015). These findings may lead to novel biomarkers of pathology, as well as improved therapy. Similarly, the aim of a 2013 study by Tsai et al. was to investigate activities of OS markers in SCZ patients. The markers are superoxide dismutase (SOD), glutathione (GSH), and glutathione peroxidase (GPx). Results indicate that these markers may be indicators of SCZ severity. Antipsychotic drugs might influence GPx activity. In SCZ, the status and interrelationship of OS, NO, and thyroid hormones were determined. OS index was calculated as the percent ratio of peroxides and AO potential.
In the Dietrich-Muszalska, Kontek, & Rabe-Jablonska’s 2011a study, various drugs were investigated for their effect on lipid peroxidation. Quetiapine (QUE) and olanziapine did not produce OS. In addition, QUE demonstrates AO properties. OS in SCZ may result in part from treatment with certain antipsychotics; β-d-Glucan, a polysaccharide from yeast, exerts protection against harmful effects of lipid peroxidation (Dietrich-Muszalska., et al. 2011b). β-d-Glucan proved to be a more effective AO than resveratrol.
Treatment for millions worldwide is limited to a small number of SCZ victims. Despite drug treatment, the efficacy is suboptimal. A possible alternative may be AO therapy, based on involvement of OS. Use of AOs has been more fully investigated. Evidence suggests that AOs, such as N-acetyl cysteine, may provide distinct benefits (Reddy & Reddy, 2011). Additionally, vitamin E may offer a salutary effect; however, more clinical research remains to be done (Dietrich-Muszalska & Olas, 2009).
Free radicals induce OS with damage to a variety of molecules which might play a role in SCZ. A result can be membrane deformation caused by lipid peroxidation. The byproducts of enzymatic peroxidation of arachidonic acid were determined to result in substances such as isoprostanes and TBARS (Dietrich-Muszalska & Olas, 2009). Haloperidol caused a marked increase in lipid peroxidation, which significantly reduced AO polyphenols (Dietrich-Muszalska & Olas, 2010). Relevant drug examples of the class are resveratrol and quercetin.
In 2009, a report examined the biomarkers of OS in SCZ by oxidative/nitrative alteration of plasma proteins (Dietrich-Muszalska., et al. 2009). The amount of thiol was also ascertained. The data obtained indicated that the amount of carbonyl groups and 3-nitrotyrosine in proteins may be significant indicators of protein damage.
The effect of the AO vitamin C on SCZ patients was investigated (Dakhale., et al. 2005). Supplementation reverses ascorbic acid levels, reduces OS and improves the brief psychiatric rating scale. Hence, clinical use of the AO is recommended.
Recently, a 2017 article has found a potential application for the treatment of SCZ (Samad & Haleem, 2017). The study assessed the protective properties, such as AO effects, of rice bran oil (RBO). A beneficial role was noted for RBO in attenuation of haloperidol induced tardive dyskinesia which is associated with antipsychotic drugs.
In relation to the mechanistic aspects of SCZ, further evidence indicates that free radicals or OS may be involved. The AO ability of six antipsychotics was determined by the ability to scavenge ROS (Sadowska-Bartosz., et al. 2016). Clozapine and olanzapine displayed AO effects against ROS. Olanzapine demonstrated the greatest activity, followed by clozapine and then aripoprazole. The top two drugs showed limited toxicity and protected against the toxic action of NaOCl. Both drugs reduced protein nitration by peroxynitrite. Since SCZ is linked to oxidative and nitrosative stress, the AO activity of the two drugs may have therapeutic value.
SCZ is a mental illness with involvement of OS or excessive free radical production. Polyphenols, such as curcumin, may alleviate serious side effects associated with neuroleptics in patients with SCZ (Trebaticka & Durackova, 2015). Polyphenols in the diet have the potential to act as drugs in mental health. For example, neuroprotective mechanisms, such as AO action, are described for a natural product, ginkgo biloba, in relation to potential therapeutic effects on psychiatric disorders, including SCZ (Montes., et al. 2015). The potential use is proposed for psychiatric disorders, alone or combined with other medication treatment. The brain is a major organ for the metabolism of oxygen with little protection by AOs. Alteration of the prooxidant - antioxidant balance can be used in therapy for neuroprotection against OS (Popa-Wagner., et al. 2013). AO therapy comprises a novel strategy in treatment.
OS is an important aspect in SCZ. Ketamine induces SCZ-like symptoms. α-Lipoic acid (ALA) is an important natural AO. ALA and clozapine reverses SCZ-like behavioral alterations induced by ketamine (Vasconcelos., et al. 2015). The drugs reverse decrease in GSH and increase in lipid peroxidation. The combination reverses behavioral and some neurochemical aspects. The mechanism partially involves an AO pathway.
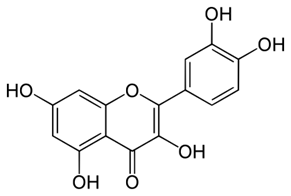
Brain diseases are pathologies with large social and economic impacts. Treatment by drugs only alleviates the conditions. Flavonoids, such as quercetin (Figure 3), are phenolic AOs that counter OS which is involved in the disorders. In a 2013 review, Grosso et al. discuss the benefit of these diet drugs in the therapy of brain diseases, including SCZ. The AO effect of this class is reviewed and the structure-activity relationships are also addressed. AO capacity of a given flavonoid depends upon its chemical structure. This review demonstrates the protective AO effects of flavonoids in countering the OS linked to the neuropathological conditions of SCZ.
Evidence indicates that excess radical generation or OS may be harmful to SCZ making for increase in ROS or decrease in AO protection (Wu, Kosten, & Zhang, 2013). Lipid peroxidation plays an adverse role and abnormalities due to the radicals may result. Novel therapy could be based on OS mechanisms.
A related article proposes that potent AOs may serve as useful drugs in treatment of SCZ (Shirai, Fujita, & Hashimoto, 2012). Evidence indicates that sulforaphane, present in vegetables, may exert a beneficial effect. Redox regulated processes can contribute to our understanding of SCZ (Bokkon & Antal, 2011). Such knowledge, including participation of ROS, is essential for gaining insight into signal pathways in the brain, as well as design of new drugs.
This concept is further explored in another relevant article that deals with nitric oxide (NO) as an important neurotransmitter and neurotoxin, based on involvement of ROS and ET (Kovacic & Jacintho, 2003). Evidence demonstrates that ROS play an important role in SCZ. A study by Akyol., et al. (2002) involves nitric oxide (NO) and other aspects. Their findings indicate the possible role of increased OS and decreased AO enzymes, which may be relevant to the pathophysiology of SCZ. Increased enzymatic production of NO suggests a possible role for NO, as well as new treatment strategies.
SCZ patients exhibit damage to membrane metabolism, excess free radicals, and harm to AO enzymes (Tylec., et al. 2007). The alteration of genes and proteins is related to GSH and OS pathways. The brain is unusually prone to oxidative insult. Antipsychotic treatment affects the oxidative state. Research on AO enzymes could contribute to knowledge in the design of new drugs.
Postmortem of the SCZ brain revealed increases in superoxide generation which would contribute to OS (Marchbanks., et al. 2003). AO glycosides, such as quercetin rutoside, are beneficial in quenching superoxide production without adverse interference in ET activity.
SCZ, whose etiology is unknown, is a major mental illness that affects young people worldwide Marchbanks., et al. 2003. OS can involve neuronal peroxidation. This oxidative neuronal injury can be prevented by dietary AOs. OS is related to altered AO defenses, increased lipid peroxidation, and reduced levels of polyunsaturated fatty acids (PUFAs) (Mahadik, Evans, & Lal, 2001). There is improvement in patients supplemented with PUFAs and AOs. Use of AOs at the onset of psychosis may reduce OS injury and improve outcome.
Acknowledgement
This article is dedicated to the memory of son Eric Kovacic, a brilliant victim of SCZ.
This article is dedicated to the memory of son Eric Kovacic, a brilliant victim of SCZ.
References
- Akyol, O., et al. “The indices of endogenous oxidative and antioxidative processes in plasma from schizophrenic patients. The possible role of oxidant/antioxidant imbalance”. Progress in Neuro-Psychopharmacology & Biological Psychiatry 26.5 (2002): 995-1005.
- Akyol., et al. “Increasedserum G72 protein levels in patients with schizophrenia: a potential candidate biomarker”. Acta Neuropsychiatrica 29.2 (2017): 80-86.
- Bentall, R. Schizophrenia. In G. Davey, Encyclopaedic dictionary of psychology. London, UK: Routledge. Credo Reference Online (2006).
- Bókkon I and Antal I. “Schizophrenia: Redox regulation and volume neurotransmission”. Current Neuropharmacology 9.2 (2011): 289-300.
- Cruz TS., et al. “On the mechanisms of phenothiazine-induced mitochondrial permeability transition: Thiol oxidation, strict Ca2+ dependence, and cyt c release”. Biochemical Pharmacology 80.8 (2010): 1284-1295.
- Dakhale GN., et al. “Supplementation of vitamin C with atypical antipsychotics reduces oxidative stress and improves the outcome of schizophrenia”. Psychopharmacology (Berl) 182.4 (2005): 494-498.
- Da Silva Araujo T., et al. “Reversal of schizophrenia-like symtoms and immune alterations in mice by immunomodulatory drugs”. Journal of Psychiatric Research 84 (2017): 49-58.
- Dietrich-Muszalska A., et al. “Quetiapine, olanzapine, and haloperidol affect human plasa ipid peroxidation in vitro”. Neuropsychobiology 63.4 (2011): 197-201.
- Dietrich-Muszalska A., et al. “Beta-glucan from Saccharomyces cerevisiae reduces plasma induced by haloperidol”. International Journal of Biological Macromolecules 49.1 (2011): 113-116.
- Dietrich-Muszalska A and Olas B. “Isoprostenes as indicators of oxidative stress in schizophrenia”. The World Journal of Biological Psychiatry 10.1 (2009): 27-33.
- Dietrich-Muszalska A and Olas B. (2010). “Inhibitory effects of polyphenol compounds on lipid peroxidation caused by antipsychotics (haloperidol and amisulpride) in human plasma in vitro”. The World Journal of Biological Psychiatr 11.2.2 (2010): 276-281.
- Dietrich-Muszalska A., et al. “Oxdative/nitrative modifications of plasma proteins and thiols from patients with schizophrenia”. Neuropsychobiology 59.1 (2009): 1-7.
- Drews E., et al. “Involvement of the primate specific gene G72 in schizophrenia: From genetic studies to pathomechanisms”. Neuroscience & Biobehavioral Reviews 37.10 (2013): 2410-2417.
- Grosso C., et al. “The use of flavonoids in central nervous system disorders”. Current Medicinal Chemistry 20.37 (2013): 4694-4719.
- Halliwell B and Gutteridge J. Free radicals in biology and medicine (3rd ed., Oxford science publications). Oxford: New York: Clarendon Press; Oxford University Press (1999). 192-194.
- Heiser P., et al. “Effects of antipsychotics and vitamin C on the formation of reactive oxygen species”. Journal of Psychopharmacology 24.10 (2010): 1499-1504.
- Khan A., et al. “Adolecent GBR12909 exposure induces oxidative stress, disrupts parvalbumin-positive interneurons, and leads to hyperactivity in adult mice”. Neuroscience 14 (2017): 166-175.
- Kovacic P., et al. “Unifying electrostatic mechanism for phosphates and sulfates in cell signaling”. Journal of Receptors and Signal Transduction 27.5.6 (2007): 433-443.
- Kovacic P and Jacintho JD. “Reproductive toxins. Pervasive theme of oxidative stress and electron transfer”. Current Medicinal Chemistry 8 (2001): 863-892.
- Kovacic P and Jacintho JD. “Mechanisms of carcinogenesis: focus on oxidative stress and electron transfer”. Current Medicinal Chemistry 8.7 (2003): 773-796.
- Kovacic P Osuna JA. “Mechanisms of anticance agents: Emphasis on oxidative stress and electron transfer”. Current Pharmaceutical Design 6.3 (2000): 277-309.
- Kovacic P., et al. “Mechanism of mitochondrial uncouplers, inhibitors, and toxins: Focus on electron transfer, free radicals, and structure-activity relationships”. Current Medicinal Chemistry 12.22 (2005): 2601-2623.
- KovacicP and Somanathan R. “Ototoxicity and noise trauma: Electron transfer, reactive oxygen species, cell signaling, electrical effects, and protection by antioxidants: Practical medical aspects”. Medical Hypotheses 70.5 (2008): 914-923.
- Kovacic P and Somanathan R. “Cell Signaling and Cancer: Integrated, Fundamental Approach Involving Electron Transfer, Reactive Oxygen Species, and Antioxidants”. Cell Signaling & Molecular Targets in Cancer Springer 12 (2012): 273-297.
- Kovacic P and Somanathan R. “Nitroaromatic compounds: environmental toxicity, carcinogenity, mutagenicity, therapy and mechanism”. Journal of Applied Toxicology 34 (2014): 810-824.
- Kovacic P Somanathan R. “Unifying mechanism for nutrients as anticancer agents: Electron transfer, reactive oxygen species and oxidative stress”. Global Journal of Health Science 9.8 (2017): 66-83.
- Kovacic P and Somanathan R. “Various factors involving aging: Electron transfer, reactive oxygen species and oxidative stress”. International Journal of Current Research 9.7 (2017): 53518-53528.
- Kovacic P and Thurn LA. “Cardiovascular toxicity from the perspective of oxidative stress, electron transfer, and prevention by antioxidants”. Current Vascular Pharmacology 3.2 (2005): 107-117.
- Kovacic P and Weston W. “Treatment of Parkinson’s disease with phenolic antioxidant drugs: Oxidative stress, reactive oxygen species and selectivity”. Chronicles of Pharmaceutical Science 1.4 (2017): 193-198.
- Kovacic P and Weston W. “Phenolic antioxidants as drugs for Alzheimer’s disease: oxidative stress and selectivity”. Novel Approaches in Drug Designing & Development 3.20 (2017): 555-606.
- Lewine HE. Schizophrenia. In Harvard Medical School (Ed.), Health reference series: Harvard Medical School health topics A-Z. Boston, MA: Harvard Health Publications. Credo Reference Online (2017).
- Mahadik S.P., et al. “Oxidative stress and role of antioxidant and omega-3 essential fatty acid supplementation in schizophrenia”. Progress in Neuro-Psychopharmacology & Biological Psychiatry 25.3 (2001): 463-493.
- Marchbanks RM., et al. “A mitochondrial DNA sequence variant associated with schizophrenia and oxidative stress”. Schizophrenia Research 65.1 (2003): 33-38.
- Markovic B., et al. “Long-tern effects of maternal deprivation on redox regulation in rat brain: Involvement of NADPH oxidase”. Oxidative Medicine and Cellular Longevity (2017): 7390516.
- Mhillaj E., et al. “Early life and oxidative stress in psychiatric disorders: What can we learn from animal models”. Current Pharmaceutical Design 21.11 (2015): 1396-1403.
- Montes P., et al. “Ginkgo biloba extract 761: A review of basic studies and potential clinical use in psychiatric disorders”. CNS Neurol. Disord. Drug Targets 14.1 (2015): 132-149.
- Onaolapo AY., et al. “Melatonin attenuates behavioural deficits and reduces brain oxidative stress in a rodent model of schizophrenia”. Biomedicine & Pharmacotherapy 92 (2017): 373-383.
- Popa-Wagner A., et al. “ROS and brain diseases: the good, the bad, and the ugly”. Oxidative Medicine and Cellular Longevity (2013): 963520.
- Reddy R and Reddy R. “Antioxidant therapeutics for schizophrenia”. Antioxidants & Redox Signaling 15.7 (2011): 2047-2055.
- Sadowska-Bartosz I., et al. “Antioxidant properties of atypical antipsychotic drugs used in the treatment of schizophrenia”. Schizophrenia Research 176.2.3: 245-251.
- Samad N and Haleem DJ. “Antioxidant effects of rice bran oil mitigate repeated haloperidol-induced tardive dyskinesia in male rats”. Metabolic Brain Disease 32.4 (2017): 1099-1107.
- Shirai, Y., et al. “Effects of the antioxidant sulforaphane on hyperlocomotion and prepulse inhibition deficits in mice after phencyclidine administration”. Clinical Psychopharmacology and Neuroscience 10.2 (2012): 94-98.
- Shireen E. “Experimental treatment of antipsychotic-induced movement disorders”. Journal of Experimental Pharmacology 8 (2016): 1-10.
- Sorce S., et al. “Targeting NOX enzymes in the central nervous system: therapeutic opportunities”. Cellular and Molecular Life Sciences 69.14 (2012): 2387-2407.
- Trebatická J and Ďuračková Z. “Psychiatric disorders and polyphenols: Can they be helpful in therapy?” Oxidative Medicine and Cellular Longevity (2015): 248529.
- Tsai MC., et al. “Changes in oxidative stress markers in patients with schizophrenia: The effect of antipsychotic drugs”. Psychiatry Research 209.3 (2013): 284-290.
- Tylec A., et al. “Oxidative stress in schizophrenia”. Polski Merkuriusz Lekarski 23.133 (2011): 74-77.
- Vasconcelos GS., et al. “Alpha-lipoic acid alone and combined with clozapine reverses schizophrenia-like symptoms induced by ketamine in mice: Participation of antioxidant, nitrergic and neurotrophic mechanisms”. Schizophrenia Research 165.2.3 (2015): 163-170.
- Walton JC., et al. “Neuronal nitric oxide synthase and NADPH oxidase interact to affect cognitive, affective, and social behaviors in mice”. Behavioural Brain Research (2013): 320-326.
- Wang M., et al. “Idetification of pLG72-induced oxidative stress using systemic approaches”. BioMed Research International (2015): 429253.
- Wu JQ., et al. “Free radicals, antioxidant defense systems, and schizophrenia. Prog. Neuropsychopharmacol”. Biological Psychiatry 46 (2013):200-206.
Citation:
Peter Kovacic and Wil Weston. “Cause and Treatment of Schizophrenia: Electron Transfer, Reactive Oxygen Species, Oxidative
Stress, Antioxidants, and Unifying Mechanism.” Chronicles of Pharmaceutical Science 1.6 (2017): 332-340.
Copyright: © 2017 Peter Kovacic and Wil Weston. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.