Research Article
Volume 1 Issue 6 - 2017
Antipyretic Potential of Root of Aralia racemosa L.
1Research Scholar, Department of Pharmacy, JNTUK, Kakinada -533 003, Andhra Pradesh, INDIA
2Professor, Department of Pharmaceutical Analysis and Quality Control, Shri Vishnu College of Pharmacy, Bhimavaram, Andhra Pradesh, INDIA
3Professor, Department of Pharmaceutical Chemistry, University College of Pharmaceutical Sciences, Visakhapatnam, INDIA
2Professor, Department of Pharmaceutical Analysis and Quality Control, Shri Vishnu College of Pharmacy, Bhimavaram, Andhra Pradesh, INDIA
3Professor, Department of Pharmaceutical Chemistry, University College of Pharmaceutical Sciences, Visakhapatnam, INDIA
*Corresponding Author: D S N B K Prasanth, Research Scholar, Department of Pharmacy, JNTUK,
Kakinada – 533 003, Andhra Pradesh, INDIA.
Received: December 17, 2017; Published: December 28, 2017
Abstract
The antipyrexia action of the methanol extract of A. racemosa roots had been explored utilizing the yeast evoked pyrexia procedure in rabbits. Paracetamol utilized as a positive control as well as negative control group acquired distilled water. Rectal temperatures of all rabbits had been documented instantly prior to the administration of the extract or vehicle or paracetamol as well as again at 30 min period for 3h utilizing digital thermometer. The extract had been additionally phytochemically tested with regard to alkaloids, tannins, saponins, flavonoids, cardiac glycosides and phenols. At 400 mg/kg dosage the extract revealed considerable decrease in yeast evoked raised temperature when compared with that of standard drug paracetamol where by the extract dose 200 mg/kg had been less effective as compared to higher dose (p < 0.05). Phytochemical testing demonstrated the existence of saponins, alkaloids, sterols, carbohydrates, proteins, flavonoids, tannins, phenols, glycosides and volatile oil. This research confirmed that this methanol extract of A. racemosa roots at a dose of 400 mg/kg owns considerable antipyretic outcome against the yeast-induced raised temperature. The antipyretic activity of A. racemosa extract could be due to its secondary metabolites, which probably consist of phytosterols such as β-Sitosterol. However additional phytochemical, along with biological assessments, are suggested to look for the other active chemical constituents responsible for the antipyrexia action.
Introduction
The bondage among human health and plants can be found from fossils history regarding 60,000 years back [1]. About 215,000 to 500,000 species of higher plants exist on our planet. However, merely 6% of plants are utilized for the pharmacological activity. Nearly 122 compounds have been isolated from 94 species of plants as well as 80% of these compounds have been employed with the same intention or relevant purpose [2].
Aralia racemosa L. (family: Araliaceae) is a plant which is native to the equatorial and fructiferous region of the world. The genus Aralia consists of 71 species of plants distributed all over Asia, Mexico, North America, and South America. In 1994 Smith identified the North American species of Araliaceae and recognized the following eight species of Aralia i.e., A. racemosa, A. californica, A. nudicaulis, A. spinosa, A. hispida, A. humilis, A. regeliana and A. scopulorum. Standley recognized five species of Aralia from Mexico: A. scopulorum, A. regeliana, A. humilis, A. pubescens, and A. racemosa [3]. Traditionally, A. racemosa roots has a wide range of reputed medicinal applications as carminative, antiseptic, in cough preparations, pain in the breast, mortifications, rheumatism, Whooping cough, skin diseases, pleurisy, diaphoretic, diuretic, pulmonary diseases, asthma, diarrhea, stimulant, expectorant, syphilis, inflammation and hay fever [4,5]. Only a few pharmacological properties have been reported from this plant such as antioxidant, antidiabetic [6] and antitubercular [7]. Few phytoconstituents are documented with this plant including triterpenoid saponins i.e., oleanolic acid, sterols i.e., β-sitosterol and Diterpenoids i.e., ent-Kaurenoic acid, continentalic acid [8,9].
Therefore, existing had been carried out to assess the antipyretic potential of methanolic extract of A. racemosa against baker's yeast-induced fever in rabbits as the drugs having antiinflammatory activity can show antipyretic activity too.
Materials and Methods
Animal used
Local strain, healthy male and female adult rabbits (1-1.5 kg) were used for the study and kept in the departmental animal house, V. V. Institute of Pharmaceutical Sciences, Gudlavalleru. Before the study, rabbits had been acclimatized in a controlled temperature of 22-25°C and light/dark cycles for 12/12h for one week. Animals were maintained on a standard diet and water ad libitum. These fasted overnight prior to study nevertheless water was handed in free accessibility. The experiment was carried out according to guidelines “Committee for the Purpose of Control and Supervision” (CPCSEA). (Approval No.: 1847/PO/Re/S/16/CPCSEA).
Local strain, healthy male and female adult rabbits (1-1.5 kg) were used for the study and kept in the departmental animal house, V. V. Institute of Pharmaceutical Sciences, Gudlavalleru. Before the study, rabbits had been acclimatized in a controlled temperature of 22-25°C and light/dark cycles for 12/12h for one week. Animals were maintained on a standard diet and water ad libitum. These fasted overnight prior to study nevertheless water was handed in free accessibility. The experiment was carried out according to guidelines “Committee for the Purpose of Control and Supervision” (CPCSEA). (Approval No.: 1847/PO/Re/S/16/CPCSEA).
Drugs, reagents, and apparatus used
Paracetamol (GlaxoSmithKline), Baker yeast (Loba Chem, Mumbai), Distilled water (Faculty of Pharmacy and alternative medicine, IUB, Pakistan), Digital Thermometer (Medisign MANA).
Paracetamol (GlaxoSmithKline), Baker yeast (Loba Chem, Mumbai), Distilled water (Faculty of Pharmacy and alternative medicine, IUB, Pakistan), Digital Thermometer (Medisign MANA).
Plant Material
The plant material was purchased from Tirupati, Chittoor region of Andhra Pradesh, India throughout the month of March 2016 and identified by Dr. K. Madhava chetty, Taxonomist, Sri Venkateswara University Tirupati, India. Voucher specimen No. 1489 was placed at the herbarium of V. V. Institute of Pharmaceutical Sciences, Gudlavalleru for future reference.
The plant material was purchased from Tirupati, Chittoor region of Andhra Pradesh, India throughout the month of March 2016 and identified by Dr. K. Madhava chetty, Taxonomist, Sri Venkateswara University Tirupati, India. Voucher specimen No. 1489 was placed at the herbarium of V. V. Institute of Pharmaceutical Sciences, Gudlavalleru for future reference.
Preparation of Extract
The freshly gathered plant material was shade dried and pulverized. The powder (1 Kg) was treated with petroleum ether for the removal of fatty and waxy material. After that, it had been air dried and macerated with methanol, strained and concentrated at 45°C in Buchi rotavapor. The weight of methanolic extract obtained was 73g (7.3% w/w yield). The methanolic extract had been revoked in distilled water in a separating funnel and partitioned sequentially with petroleum ether, chloroform, ethyl acetate and n-butanol to acquire fractions in these solvents. Eventually, left residual aqueous fraction at the end was collected. The solvents were removed on a rotary evaporator at low pressure to obtain dried fractions. These extracts were subjected to preliminary phytochemical screening and these extracts were stored in the refrigerator at 4°C for further use [10].
The freshly gathered plant material was shade dried and pulverized. The powder (1 Kg) was treated with petroleum ether for the removal of fatty and waxy material. After that, it had been air dried and macerated with methanol, strained and concentrated at 45°C in Buchi rotavapor. The weight of methanolic extract obtained was 73g (7.3% w/w yield). The methanolic extract had been revoked in distilled water in a separating funnel and partitioned sequentially with petroleum ether, chloroform, ethyl acetate and n-butanol to acquire fractions in these solvents. Eventually, left residual aqueous fraction at the end was collected. The solvents were removed on a rotary evaporator at low pressure to obtain dried fractions. These extracts were subjected to preliminary phytochemical screening and these extracts were stored in the refrigerator at 4°C for further use [10].
Phytochemical Screening
The various extract of A. racemosa was subjected to qualitative chemical analysis by using standard procedures as follows.
The various extract of A. racemosa was subjected to qualitative chemical analysis by using standard procedures as follows.
The phytochemical screening of carbohydrates was detected by molisch's test; Proteins were detected by using two tests namely Biuret test and millon's test and amino acids by Ninhyrdin's test; Steroids was detected by salkowski, Libermann- Burchards and Libermann's test; Alkaloids was identified with freshly prepared Dragendroff's Mayer's, Hager's and Wagner's reagents and observed for the presence of turbidity or precipitation. The flavonoids were detected using four tests namely Shinoda, sulfuric acid, aluminum chloride, lead acetate, and sodium hydroxides. Tannins were detected with four tests namely gelatin, lead acetate, potassium dichromate and ferric chloride. The froth, emulsion, and lead acetate tests were applied for the detection of saponins. The steroids were detected by (acetic anhydride with sulfuric acid) and (acetic chloride with sulfuric acid) tests. Sample extracted with chloroform was treated with sulfuric acid to test for the presence of terpenoids. Ammonia solution and ferric chloride solutions were used to the presence of anthraquinone [11-14].
Isolation of Constituents
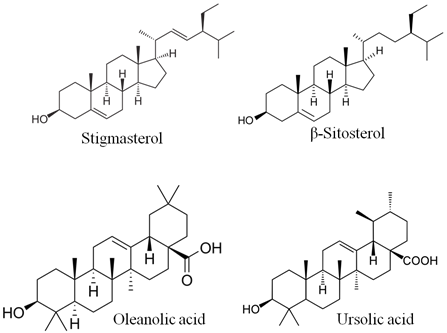
Petroleum ether extract (PEE) was subjected to silica-gel (100-200 mesh) column (length 100 cm and diameter 3 cm) chromatography (elution rate of 2 ml min−1 flow with a total elution of 200 ml) and eluted with Petroleum ether and ethyl acetate in different proportions. The consequent fractions (Fr) were collected and spotted over pre-coated silica gel F254 plates (20 × 20 cm, Merck, Germany). The optimum resolution was achieved in the hexane, ethyl acetate and formic acid (7.5: 2: 0.5 v/v) solvent system and the plates were sprayed with anisaldehyde–sulphuric acid reagent to visualize the spots. The fractions showing similar spots were pooled together and concentrated. The fractions which showed prominent spots were taken up for spectral studies which result in the identification of 4 compounds. The compounds PC-1 (Stigmasterol) and PC-2 (β-Sitosterol) were identified as phytosterols by Libermann-Burchard’s test (Figure 1a). The chloroform fraction was subjected to chromatography on silica gel (60-120 mesh, Merck) eluted with ethyl acetate-hexane (7:3) solvent system. Repeated chromatography to give major two pentacyclic triterpenoids i.e., PC-3 (Oleanolic acid) and PC-4 (Ursolic acid) [15,16].
Petroleum ether extract (PEE) was subjected to silica-gel (100-200 mesh) column (length 100 cm and diameter 3 cm) chromatography (elution rate of 2 ml min−1 flow with a total elution of 200 ml) and eluted with Petroleum ether and ethyl acetate in different proportions. The consequent fractions (Fr) were collected and spotted over pre-coated silica gel F254 plates (20 × 20 cm, Merck, Germany). The optimum resolution was achieved in the hexane, ethyl acetate and formic acid (7.5: 2: 0.5 v/v) solvent system and the plates were sprayed with anisaldehyde–sulphuric acid reagent to visualize the spots. The fractions showing similar spots were pooled together and concentrated. The fractions which showed prominent spots were taken up for spectral studies which result in the identification of 4 compounds. The compounds PC-1 (Stigmasterol) and PC-2 (β-Sitosterol) were identified as phytosterols by Libermann-Burchard’s test (Figure 1a). The chloroform fraction was subjected to chromatography on silica gel (60-120 mesh, Merck) eluted with ethyl acetate-hexane (7:3) solvent system. Repeated chromatography to give major two pentacyclic triterpenoids i.e., PC-3 (Oleanolic acid) and PC-4 (Ursolic acid) [15,16].
Antipyretic activity
The rabbits had been arbitrarily divided into four groups each comprising of six animals (n = 6). Each of the groups was initially administered with baker yeast (Saccharomyces cerevisiae) (3 mL/kg of 10% suspension subcutaneous) for induction of fever [17,18]. Immediately after 4h of yeast administration, Group I animals were given with distilled water and served as negative control. Group II animals were treated with paracetamol by oral administration and considered as positive control. Group III and IV animals were treated with MAR 200 and 400 mg/kg body weight respectively. The dose of A. racemosa was selected by an effective dose fixation study method with slight modification [19]. The rectal temperature had been assessed using the digital thermometer (Medisign) layered with glycerin (as a lubricant). After baker yeast injection, the rectal temperature had been documented half and hourly. The animals exhibited rises in temperature of 0.5-1°C during 4th hr were included in the study. After 4h of yeast injection, each of the screened samples had been given orally by using the syringe. After drug administration, rectal temperature was recorded half an hourly for 3h.
The rabbits had been arbitrarily divided into four groups each comprising of six animals (n = 6). Each of the groups was initially administered with baker yeast (Saccharomyces cerevisiae) (3 mL/kg of 10% suspension subcutaneous) for induction of fever [17,18]. Immediately after 4h of yeast administration, Group I animals were given with distilled water and served as negative control. Group II animals were treated with paracetamol by oral administration and considered as positive control. Group III and IV animals were treated with MAR 200 and 400 mg/kg body weight respectively. The dose of A. racemosa was selected by an effective dose fixation study method with slight modification [19]. The rectal temperature had been assessed using the digital thermometer (Medisign) layered with glycerin (as a lubricant). After baker yeast injection, the rectal temperature had been documented half and hourly. The animals exhibited rises in temperature of 0.5-1°C during 4th hr were included in the study. After 4h of yeast injection, each of the screened samples had been given orally by using the syringe. After drug administration, rectal temperature was recorded half an hourly for 3h.
Statistical Analysis
Statistical analysis was carried out using Graph Pad Prism 5.0 (Graph Pad Software, San Diego, CA). All results were expressed as mean ± SD. The data were analyzed by one-way ANOVA followed by Tukey multiple comparison tests.
Statistical analysis was carried out using Graph Pad Prism 5.0 (Graph Pad Software, San Diego, CA). All results were expressed as mean ± SD. The data were analyzed by one-way ANOVA followed by Tukey multiple comparison tests.
Results
Acute Toxicity Studies
The methanolic extract of A. racemosa roots, when orally administered in the dose of 2000 mg/kg body wt. did not produce any significant changes in the autonomic or behavioral responses, including death during the observation period.
The methanolic extract of A. racemosa roots, when orally administered in the dose of 2000 mg/kg body wt. did not produce any significant changes in the autonomic or behavioral responses, including death during the observation period.
Phytochemical Screening
The phytochemical screening for various extracts viz., petroleum ether, chloroform, ethyl acetate, methanol, n-butanol, and water was carried out and results were displayed in Table 1.
The phytochemical screening for various extracts viz., petroleum ether, chloroform, ethyl acetate, methanol, n-butanol, and water was carried out and results were displayed in Table 1.
| Phytoconstituents | Method | Pet. ether Extract |
Chloroform Extract | Ethyl acetate Extract | Methanolic Extract | n-butanol Extract | Aqueous Extract |
| Flavonoids | Shinoda Test | - | - | + | + | - | + |
| Zn+HCl test | - | - | + | + | - | + | |
| Lead acetate Test | - | - | + | + | - | + | |
| Volatile oil | Stain test | + | - | - | + | - | + |
| Alkaloids | Wagner Test | - | + | - | + | - | + |
| Hager’s Test | - | + | - | + | - | + | |
| Tannins & Phenols | Fecl3 Test | - | - | - | + | + | + |
| Potassium dichromate test | - | + | - | + | + | + | |
| Saponins | Foam Test | - | - | - | + | + | + |
| Phytosterols | Libermann’s test | + | + | - | + | - | - |
| Carbohydrates | Molish test | - | - | - | + | - | - |
| Acid compounds | Litmus test | - | - | - | - | - | - |
| Glycoside | Borntragers test | - | - | - | + | - | + |
| Amino acids | Ninhydrin test | - | - | - | + | - | + |
| Proteins | Biuret test | - | - | - | + | - | + |
| Fixed oils & fats | Spot test | + | - | - | - | - | - |
Table 1: Preliminary phytochemical screening of various extracts of Aralia racemosa.
“+” Present; “-” – Absent
“+” Present; “-” – Absent
Characterization of isolated Phytoconstituents
Stigmasterol
White powder, C29H48O, MW 412.69. UV λmax (CHCl3) nm: 257; IR (KBr) νmax 3418 (-OH), 2934, 2866, 2339, 1602, 1566, 1461, 1409, 1383, 1251, 1191, 1154, 1109, 1089, 1053, 1020, 791 cm-1; ESMS m/z (%): 409.2, 395.3, 335, 161, 144, 121.1, 105.1, 97.1, 85.1, 69, 67.2, 65, 50.2; 1H NMR (400 MHz, CDCl3) δ ppm: 7.25 (1H, s, OH-2), 5.34-5.35 (1H, d), 5.12-5.18 (1H, m), 4.99-5.05 (1H, m), 3.48-3.56 (1H, m), 2.18-2.31 (2H, m), 1.93-2.09 (3H, m), 1.82-1.87 (2H, m), 1.66-1.75 (1H, m), 1.37-1.54 (13H, m), 1.05-1.31 (m, 7H), 0.99-1.01 (m, 8H), 0.90-0.98 (m, 2H), 0.78-0.85 (m, 9H), 0.66-0.70 (3H, t); 13C NMR (400 MHz, CDCl3) δ ppm: 140.85 (C-4), 138.31 (C-19), 129.40 (C-20), 121.72 (C-7), 77.34 (C-2), 71.86 (C-11), 56.95 (C-17), 56.09 (C-21), 51.29 (C-10), 50.29 (C-12), 42.41 (C-3), 42.30 (C-18), 40.46 (C-13), 39.77 (C-5), 37.35 (C-6), 36.59 (C-8), 32 (C-9), 31.96 (C-1), 31.91 (C-22), 31.77 (C-16), 28.91 (C-15), 25.41 (C-24), 24.41 (C-23), 21.24 (C-26), 21.14 (C-14), 21.06 (C-29), 19.42 (C-27), 19.03 (C-25), 12.23 (C-28). PC-01 was identified as Stigmasterol.
Stigmasterol
White powder, C29H48O, MW 412.69. UV λmax (CHCl3) nm: 257; IR (KBr) νmax 3418 (-OH), 2934, 2866, 2339, 1602, 1566, 1461, 1409, 1383, 1251, 1191, 1154, 1109, 1089, 1053, 1020, 791 cm-1; ESMS m/z (%): 409.2, 395.3, 335, 161, 144, 121.1, 105.1, 97.1, 85.1, 69, 67.2, 65, 50.2; 1H NMR (400 MHz, CDCl3) δ ppm: 7.25 (1H, s, OH-2), 5.34-5.35 (1H, d), 5.12-5.18 (1H, m), 4.99-5.05 (1H, m), 3.48-3.56 (1H, m), 2.18-2.31 (2H, m), 1.93-2.09 (3H, m), 1.82-1.87 (2H, m), 1.66-1.75 (1H, m), 1.37-1.54 (13H, m), 1.05-1.31 (m, 7H), 0.99-1.01 (m, 8H), 0.90-0.98 (m, 2H), 0.78-0.85 (m, 9H), 0.66-0.70 (3H, t); 13C NMR (400 MHz, CDCl3) δ ppm: 140.85 (C-4), 138.31 (C-19), 129.40 (C-20), 121.72 (C-7), 77.34 (C-2), 71.86 (C-11), 56.95 (C-17), 56.09 (C-21), 51.29 (C-10), 50.29 (C-12), 42.41 (C-3), 42.30 (C-18), 40.46 (C-13), 39.77 (C-5), 37.35 (C-6), 36.59 (C-8), 32 (C-9), 31.96 (C-1), 31.91 (C-22), 31.77 (C-16), 28.91 (C-15), 25.41 (C-24), 24.41 (C-23), 21.24 (C-26), 21.14 (C-14), 21.06 (C-29), 19.42 (C-27), 19.03 (C-25), 12.23 (C-28). PC-01 was identified as Stigmasterol.
β-Sitosterol
White powder, C29H50O, MW 414.70; UV λmax (CHCl3) nm: 251; IR (KBr) νmax 3424, 2959, 2936, 2867, 1602, 1565, 1465, 1382, 1332, 1242, 1191, 1154, 1051, 779, 450, 432, 416cm-1; ESMS m/z (%): 411.2, 397.3, 383.3, 311.2, 161.1, 81.2; 1H NMR (400 MHz, CDCl3) δ ppm: 7.30 (1H, s), 5.34-5.35 (1H, d), 4.98-5.19 (1H, m), 3.47-3.55 (1H, m), 2.19-2.31 (2H, m), 1.03-1.30 (9H, m), 1.00 (4H, s), 0.90-0.98 (4H, m), 0.76-0.86 (9H, m), 0.68-0.69 (3H, d), 1.94-2.07 (2H, m), 1.79-1.88 (4H, m); 13C NMR (400 MHz, CDCl3) δ ppm: 140.84 (C-4), 121.70 (C-7), 71.82 (C-2), 56.94 (C-11), 56.85 (C-17), 50.25 (C-10), 45.95 (C-21), 42.39 (C-7), 42.36 (C-3), 39.87 (C-13), 37.34 (C-5), 36.57 (C-6), 36.19 (C-18), 33.78 (C-19), 32.15 (C-8), 31.99 (C-9), 31.97 (C-7), 30.39 (C-22), 26.28 (C-20), 25.90 (C-15), 25.40 (C-16), 24.40 (C-24), 23.2 (C-23), 21.17 (C-26), 21.06 (C-14) 21.06 (C-29), 19.32 (C-27), 19.34 (C-25), 12.11 (C-28). PC-02 was identified as β-Sitosterol.
White powder, C29H50O, MW 414.70; UV λmax (CHCl3) nm: 251; IR (KBr) νmax 3424, 2959, 2936, 2867, 1602, 1565, 1465, 1382, 1332, 1242, 1191, 1154, 1051, 779, 450, 432, 416cm-1; ESMS m/z (%): 411.2, 397.3, 383.3, 311.2, 161.1, 81.2; 1H NMR (400 MHz, CDCl3) δ ppm: 7.30 (1H, s), 5.34-5.35 (1H, d), 4.98-5.19 (1H, m), 3.47-3.55 (1H, m), 2.19-2.31 (2H, m), 1.03-1.30 (9H, m), 1.00 (4H, s), 0.90-0.98 (4H, m), 0.76-0.86 (9H, m), 0.68-0.69 (3H, d), 1.94-2.07 (2H, m), 1.79-1.88 (4H, m); 13C NMR (400 MHz, CDCl3) δ ppm: 140.84 (C-4), 121.70 (C-7), 71.82 (C-2), 56.94 (C-11), 56.85 (C-17), 50.25 (C-10), 45.95 (C-21), 42.39 (C-7), 42.36 (C-3), 39.87 (C-13), 37.34 (C-5), 36.57 (C-6), 36.19 (C-18), 33.78 (C-19), 32.15 (C-8), 31.99 (C-9), 31.97 (C-7), 30.39 (C-22), 26.28 (C-20), 25.90 (C-15), 25.40 (C-16), 24.40 (C-24), 23.2 (C-23), 21.17 (C-26), 21.06 (C-14) 21.06 (C-29), 19.32 (C-27), 19.34 (C-25), 12.11 (C-28). PC-02 was identified as β-Sitosterol.
Ursolic acid
White powder, C30H48O3, MW 456.7 ; UV λmax (EtOH) nm: 203; IR (KBr) νmax 3450, 2925, 2869, 2339, 1556, 1456, 1387, 1247, 1157, 822, 444, 433, 422, 415cm-1; ESMS m/z (%): 455.2 (M-1)+, 456.2, 457.3; 1H NMR (400 MHz, DMSO) δ ppm: 11.91 (1H, s), 5.14 (1H, s), 4.27 (1H, s), 3.01 (1H, s), 2.51 (1H, s), 2.10-2.13 (1H, d) 1.85-1.93 (4H, t), 1.26-1.32 (4H, t), 1.05 (1H, s), 0.91-0.92 (8H, d), 0.88 (1H, s) 0.82-0.83 (4H, d), 0.76 (3H, s), 0.69 (4H,s); 13C NMR (400 MHz, DMSO) δ ppm: 178.16 (C-29), 138.17 (C-12), 124.58 (C-13), 76.86 (C-2), 56.01 (C-4), 54.82 (C-18), 52.40 (C-11), 47.05 (C-10), 46.82 (C-17), 41.64 (C-9), 40.41 (C-3), 40.21 (C-22), 40 (C-6), 39.79 (C-5), 39.58 (C-19), 39.37 (C-8), 39.16 (C-20), 38.96 (C-1), 38.49 (C-15), 38.46 (C-16), 38.36 (C-23), 38.28 (C-24), 36.53 (C-14), 36.31 (C-30), 32.73 (C-7), 30.2 (C-28), 28.24 (C-26), 27.55 (C-27), 26.99 (C-15). PC-03 was identified as Ursolic acid.
White powder, C30H48O3, MW 456.7 ; UV λmax (EtOH) nm: 203; IR (KBr) νmax 3450, 2925, 2869, 2339, 1556, 1456, 1387, 1247, 1157, 822, 444, 433, 422, 415cm-1; ESMS m/z (%): 455.2 (M-1)+, 456.2, 457.3; 1H NMR (400 MHz, DMSO) δ ppm: 11.91 (1H, s), 5.14 (1H, s), 4.27 (1H, s), 3.01 (1H, s), 2.51 (1H, s), 2.10-2.13 (1H, d) 1.85-1.93 (4H, t), 1.26-1.32 (4H, t), 1.05 (1H, s), 0.91-0.92 (8H, d), 0.88 (1H, s) 0.82-0.83 (4H, d), 0.76 (3H, s), 0.69 (4H,s); 13C NMR (400 MHz, DMSO) δ ppm: 178.16 (C-29), 138.17 (C-12), 124.58 (C-13), 76.86 (C-2), 56.01 (C-4), 54.82 (C-18), 52.40 (C-11), 47.05 (C-10), 46.82 (C-17), 41.64 (C-9), 40.41 (C-3), 40.21 (C-22), 40 (C-6), 39.79 (C-5), 39.58 (C-19), 39.37 (C-8), 39.16 (C-20), 38.96 (C-1), 38.49 (C-15), 38.46 (C-16), 38.36 (C-23), 38.28 (C-24), 36.53 (C-14), 36.31 (C-30), 32.73 (C-7), 30.2 (C-28), 28.24 (C-26), 27.55 (C-27), 26.99 (C-15). PC-03 was identified as Ursolic acid.
Oleanolic acid
White powder, C30H48O3, MW 456.71; UV λmax (EtOH) nm: 210; IR (KBr) νmax 3443, 2941, 2862, 1694, 1602, 1566, 1462, 1388, 1364, 1304, 1273, 1208, 1185, 1161, 1093, 1028, 960, 791 cm-1; ESMS m/z (%): 455.3, 456.2; 1H NMR (400 MHz, DMSO) δ ppm: 12 (1H, s), 5.16 (1H, s), 4.27 (1H, s), 3 (1H, s), 2.73-2.77 (1H, m), 1.88-1.95 (1H, s), 1.80-1.83 (2H, m), 1.58-1.70 (3H, m), 1.42-1.50 (8H, m), 1.23-1.38 (5H, m), 1.07-1.10 (4H, t), 0.98-1.01 (1H, m), 0.86-0.93 (14H, m), 0.72 (3H, s), 0.68 (5H, s); 13C NMR (400 MHz) 178.52 (C-28), 143.83 (C-12), 121.49 (C-13), 76.83 (C-2), 54.81 (C-4), 47.09 (C-11), 45.70 (C-10), 45.44 (C-22), 41.32 (C-17), 40.82 (C-22), 40.20 (C-18), 39.99 (C-12), 39.58 (C-9), 39.37 (C-6), 39.16 (C-3), 38.95 (C-5), 38.89 (C-8), 38.36 (C-19), 38.07 (C-21), 36.60 (C-1), 33.34 (C-29), 32.80 (C-30), 32.43 (C-16), 32.09 (C-14), 30.35 (C-23), 28.21 (C-24), 27.20 (C-7), 26.94 (C-26), 14.82 (C-27). PC-04 was identified as Oleanolic acid.
White powder, C30H48O3, MW 456.71; UV λmax (EtOH) nm: 210; IR (KBr) νmax 3443, 2941, 2862, 1694, 1602, 1566, 1462, 1388, 1364, 1304, 1273, 1208, 1185, 1161, 1093, 1028, 960, 791 cm-1; ESMS m/z (%): 455.3, 456.2; 1H NMR (400 MHz, DMSO) δ ppm: 12 (1H, s), 5.16 (1H, s), 4.27 (1H, s), 3 (1H, s), 2.73-2.77 (1H, m), 1.88-1.95 (1H, s), 1.80-1.83 (2H, m), 1.58-1.70 (3H, m), 1.42-1.50 (8H, m), 1.23-1.38 (5H, m), 1.07-1.10 (4H, t), 0.98-1.01 (1H, m), 0.86-0.93 (14H, m), 0.72 (3H, s), 0.68 (5H, s); 13C NMR (400 MHz) 178.52 (C-28), 143.83 (C-12), 121.49 (C-13), 76.83 (C-2), 54.81 (C-4), 47.09 (C-11), 45.70 (C-10), 45.44 (C-22), 41.32 (C-17), 40.82 (C-22), 40.20 (C-18), 39.99 (C-12), 39.58 (C-9), 39.37 (C-6), 39.16 (C-3), 38.95 (C-5), 38.89 (C-8), 38.36 (C-19), 38.07 (C-21), 36.60 (C-1), 33.34 (C-29), 32.80 (C-30), 32.43 (C-16), 32.09 (C-14), 30.35 (C-23), 28.21 (C-24), 27.20 (C-7), 26.94 (C-26), 14.82 (C-27). PC-04 was identified as Oleanolic acid.
Antipyretic Activity
Subcutaneous injection of the pyrogenic dose of yeast produced elevated changes in rectal temperature, which is shown in Table 2. The MAR showed a significant (P < 0.05) decrease in rectal temperature at doses 200, and 400 mg/kg when compared with the standard. However, no significant decrease in mean temperature was noted by MAR at 200 and 400 mg/kg when compared with control throughout the study period (Table 1 and Figure 1). Extract at doses of 200, and 400 mg/kg showed a progressive decline in mean temperature pattern with the increase in the dose. Paracetamol showed significant (P < 0.05) decrease in rectal temperature. The onset of action of paracetamol was 30 min. MAR at doses 200 and 400 mg/kg showed its onset of action from 180 min (Figure 2). The line graph (Figure 2) shows that paracetamol registered a phenomenal decrease in mean temperature from 38.66 ± 0.21 to 38.22 ± 0.4. Figure 2 illustrates that the most significant (P < 0.05) decrease in round-the-clock mean temperature in this study was shown by paracetamol followed by MAR at 400 mg/kg. Statistical analysis was performed using one-way analysis of variance followed by Tukey's test. The consequences of extract at a dose of 400 mg/kg were nearly much like that of standard drug paracetamol. The results were recorded as mean ± SD. Out of all the groups apart from negative control, the temperature became normal through 3h of study (Figure 2, Table 2).
Subcutaneous injection of the pyrogenic dose of yeast produced elevated changes in rectal temperature, which is shown in Table 2. The MAR showed a significant (P < 0.05) decrease in rectal temperature at doses 200, and 400 mg/kg when compared with the standard. However, no significant decrease in mean temperature was noted by MAR at 200 and 400 mg/kg when compared with control throughout the study period (Table 1 and Figure 1). Extract at doses of 200, and 400 mg/kg showed a progressive decline in mean temperature pattern with the increase in the dose. Paracetamol showed significant (P < 0.05) decrease in rectal temperature. The onset of action of paracetamol was 30 min. MAR at doses 200 and 400 mg/kg showed its onset of action from 180 min (Figure 2). The line graph (Figure 2) shows that paracetamol registered a phenomenal decrease in mean temperature from 38.66 ± 0.21 to 38.22 ± 0.4. Figure 2 illustrates that the most significant (P < 0.05) decrease in round-the-clock mean temperature in this study was shown by paracetamol followed by MAR at 400 mg/kg. Statistical analysis was performed using one-way analysis of variance followed by Tukey's test. The consequences of extract at a dose of 400 mg/kg were nearly much like that of standard drug paracetamol. The results were recorded as mean ± SD. Out of all the groups apart from negative control, the temperature became normal through 3h of study (Figure 2, Table 2).
| Groups | Rectal Temperature (°C) after yeast injection | ||||||
| 0 min | 30 min | 60 min | 90 min | 120 min | 150 min | 180 min | |
| Paracetamol (150 mg/Kg) | 38.66 ± 0.21 | 38.53 ± 0.15 | 38.48 ± 0.16 | 38.45 ± 0.15 | 38.33 ± 0.3 | 38.23 ± 0.39 | 38.22 ± 0.4 |
| Negative control | 38.63 ± 0.04* | 38.66 ± 0.04* | 38.68 ± 0.03* | 38.72 ± 0.04* | 38.78 ± 0.055* | 38.75 ± 0.06* | 38.72 ± 0.06* |
| A.racemosa Methanolic Extract (400 mg/Kg) | 38.57 ± 0.04a | 38.7 ± 0.02a | 38.75 ± 0.09a | 38.81 ± 0.08a | 38.89 ± 0.11a | 38.98 ± 0.11a | 38.62 ± 0.34a |
| A.racemosa Methanolic Extract (200 mg/Kg) | 38.58 ± 0.05b | 38.66 ± 0.05b | 38.75 ± 0.08b | 38.81 ± 0.07b | 39.01 ± 0.13b | 39.21 ± 0.08b | 38.97 ± 0.13b |
Table 2: Effect of Methanolic extract of Aralia racemosa on yeast extract induced pyrexia in Rabbits.
All values expressed as mean ± SEM; n = 6 rabbits in each group, by one-way ANOVA followed by Tukey’s Multiple Comparison Test. *, p < 0.05 Vs Paracetamol (150 mg/Kg); a, p < 0.01 Vs Paracetamol (150 mg/Kg) and b p < 0.05 Vs Control
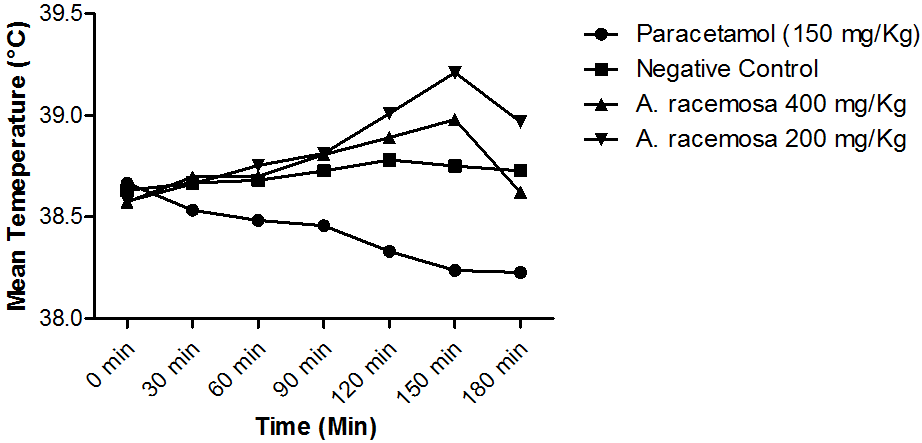
Figure 2: Line graph showing the effect of paracetamol and methanolic
extract of Aralia racemosa on yeast-induced pyrexia.
Discussion
Fever is the main defensive reaction referred to as the “acute phase reaction”, which happen throughout inflamed processes of various sources. Brewer’s yeast (lipopolysaccharide, that is the cell wall element of Gram negative bacteria) is an exogenous pyrogen which binds to the immunological protein referred to as lipopolysaccharide binding protein. This kind of binding leads to the synthesis as well as the release of numerous endogenous cytokine factors, for example, interleukin (IL)-1, IL-6, and TNFa, that trigger the arachidonic acid pathway, and also eventually result in the synthesis and release of prostaglandin E2 (PGE2). Yeast-induced pyrexia is termed as pathogenic fever [20].
Based on the traditional perspective, fever is evoked through inflamed mediators (I L-1, IL-2, TNFα, others) unveiled through the peripheral mononuclear macrophages and other immune cells [21, 22]. These fever-promoting cytokines tend to be moved through the bloodstream to the brain by particular carriers [23]. Cytokines are carried through the blood stream and also enter the brain through the circumventricular organs [24]. On the other hand, the cytokines might interact with their receptors on brain endothelial cells or perivascular tissue [25]. This presumed mechanism of fever induction is called the humoral hypothesis of fever induction. These pro-inflammatory mediators address the preoptic/anterior hypothalamus activating the release of PGE2 made out of cyclooxygenase (COX-2), and therefore increasing the body temperature [26].
An efficient antipyretic like paracetamol serves through embarrassing the effect of these pyrogens in the temperature-sensitive neurons in the preoptic region of the hypothalamus to COX formation of PGE2 [27]. An efficient antipyretic like paracetamol serves through embarrassing the effect of these pyrogens in the temperature-sensitive neurons in the preoptic region of the hypothalamus to COX formation of PGE2.
In this study, orally administered paracetamol at 150 mg/kg significantly attenuated baker's yeast-induced fever in rabbits. Our study results are matching to other studies that have also shown the reduction of temperature in rabbits by paracetamol at the same dose [28]. Antipyretics and non-steroidal anti-inflammatory drugs (NSAIDS) reduce temperature by inflammation reduction in the peripheral and CNS thermoregulatory sites [29].
In the current study, A. racemosa methanolic extract reduced baker's yeast-induced fever in rabbits significantly. The preliminary phytochemical study indicated that extract contains flavonoids, alkaloids, tannins, phenols, steroids, fixed oils, carbohydrates, glycosides, amino acids, and proteins. The presence of these bioactive compounds may be responsible for the antipyretic activity of this extract as sterols like β-Sitosterol [31] have the antipyretic effect. Alkaloids such as bolidine are able to reduce the raised temperature by suppressing the prostaglandin E2 synthesis [32]. In the same manner, flavonoids such as baicalin have antipyretic outcome by curbing TNF- α [33].
It could be assumed that A. racemosa has antipyretic effect by reducing the concentration of PGE2 in the hypothalamus or by interrupting the steps that connect the peripheral inflammation with the central production of PGE2 or both [34,35].
Conclusion
It is concluded that methanolic extract of A. racemosa has significant antipyretic activity. This property was attributed to the presence of β-Sitosterol in the MAR. So, the traditional use of A. racemosa in fever is supported by this study and it would encourage its use in fever with the greater degree of assurance of its efficacy. It is recommended to determine the other active chemical constituents accountable for the antipyretic activity.
References
- Fabricant DS and Farnsworth NR. “The value of plants used in traditional medicine for drug discovery”. Environmental Health Perspectives 109.Suppl 1 (2001): 69-75.
- Farnsworth J. “Screening plants for new medicines”. Biodiversity.
- Wen J. “Systematics and Biogeography of Aralia L. (Araliaceae): Revision of Aralia Sects. Aralia, Humiles, Nanae, and Sciadodendron”. Contributions from the United States National Herbarium 57 (2011): 1-172.
- Duke JA. “Handbook of medicinal herbs”. Boca Raton, FL: CRC Press; 2006.
- Quattro chi U. “CRC world dictionary of medicinal and poisonous plants: common names, scientific names, eponyms, synonyms, and etymology”. CRC Press (2012):
- McCune LM and Johns T. “Antioxidant activity in medicinal plants associated with the symptoms of diabetes mellitus used by the indigenous peoples of the North American boreal forest”. Journal of Ethnopharmacology 82(2-3) (2002): 197-205.
- Grange JM and Davey RW. “Detection of antituberculous activity in plant extracts”. Journal of Applied Microbiology 68.6 (1990): 587-591.
- Clement JA., et al. “Diterpenoids and acetylenic lipids from Aralia racemosa”. Biochemical Systematics and Ecology 51(Supplement C) (2013): 4-7.
- Ahmed D., et al. “Antimicrobial activities of methanolic extract of Carissa opaca roots and its fractions and compounds isolated from the most active ethyl acetate fraction”. Asian Pacific Journal of Tropical Biomedicine 5.7 (2015): 541-545.
- Alam F and Najum us Saqib Q. “Pharmacognostic standardization and preliminary phytochemical studies of Gaultheria trichophylla”. Pharmaceutical Biology 53.12 (2015): 1711-1718.
- Harborne JB. “Phytochemical methods; a guide to modern techniques of plant analysis [by] J.B. Harborne”. London: Chapman & Hall (1973).
- Khandelwal KR. “Practical pharmacognosy techniques and experiments”. Maharashtra: Niral Prakashan (2008).
- Raaman N. “Phytochemical techniques”. New India Publ. Agency (2006):
- Hossain MA and Ismail Z. “Isolation and characterization of triterpenes from the leaves of Orthosiphon stamineus”. Arabian Journal of Chemistry 6.3 (2013): 295-298.
- Vasconcelos MAL., et al. “In vivo analgesic and anti-inflammatory activities of ursolic acid and oleanoic acid from Miconia albicans (Melastomataceae)”. Zeitschrift für Naturforschung C - De Gruyter 61.(7-8) (2006): 477-482.
- Hossain E., et al. “Phytochemical screening and in-vivo antipyretic activity of the methanol leaf-extract of Bombax malabaricum DC (Bombacaceae)”. Tropical Journal of Pharmaceutical Research 10.1 (2011):
- Sultana S., et al. “Phytochemical screening and antipyretic effects of hydro-methanol extract of Melia azedarach leaves in rabbits”. Bangladesh Journal of Pharmacology 8.2 (2013): 214-217.
- Pandikumar P., et al. “Hypoglycemic and antihyperglycemic effect of Begonia malabarica Lam. in normal and streptozotocin induced diabetic rats”. Journal of Ethnopharmacology 124.1 (2009): 111-115.
- Bhattacharya A., et al. “Antipyretic effect of ethanolic extract of Moringa oleifera leaves on albino rats”.Tanta Medical Journal. 42.2 (2014): 74-78.
- Zeisberger E. “From humoral fever to neuroimmunological control of fever.” Journal of Thermal Biology 24.5 (1999): 287-326.
- Roth J. “Endogenous antipyretics”. Clinical Infectious Diseases. 371.1 (2000): 13-24.
- Banks WA., et al. “Permeability of the blood-brain barrier to soluble cytokine receptors”. Neuroimmunomodulation 2.3 (1995): 161-165.
- Roth J., et al. “signaling the brain in systemic inflammation: role of sensory circumventricular organs”. Frontiers in Bioscience 9.7 (2004): 290-300.
- Schiltz JC and Sawchenko PE. “Signaling the brain in systemic inflammation: the role of perivascular cells”. Frontiers in Bioscience 8 (2013): 1321-1329.
- Saper CB and Breder CD. “The neurologic basis of fever”. New England Journal of Medicine 330.26 (1994):1880-1886.
- Ashok B., et al. “Antipyretic activity of Guduchi Ghrita formulations in albino rats”. AYU (An International Quarterly Journal of Research in Ayurveda) 31.3 (2013): 367-370.
- Ahmad S., et al. “Effects of homoeopathic ultrahigh dilutions of Aconitum napellus on Baker's yeast-induced fever in rabbits”. Journal of Integrative Medicine 15.3 (2017): 209-213.
- Jongchanapong A., et al. “Antipyretic and antinociceptive effects of Ben-cha-Lo-Ka-Wi-Chian remedy”. Journal of Health Research 24.1 (2010): 15-22.
- Eldahshan OA and Abdel-Daim MM. “Phytochemical study, cytotoxic, analgesic, antipyretic and anti-inflammatory activities of Strychnos nux-vomica”. Cytotechnology 67.5 (2015): 831-844.
- Gupta MB., et al. “Anti-inflammatory and antipyretic activities of beta-sitosterol”. Biochemistry | Planta Medica 39.2 (1980): 157-163.
- Backhouse N., et al. “Anti-inflammatory and antipyretic effects of boldine.” Agents and Actions 42.3-4 (1994): 114-117.
- Chang CP., et al. “The flavonoid baicalin protects against cerebrovascular dysfunction and brain inflammation in experimental heatstroke”. Neuropharmacology 52.3 (2007): 1024-1033.
- Li S., et al. “Acetaminophen: antipyretic or hypothermic in mice? In either case, PGHS-1b (COX-3) is irrelevant”. Prostaglandins & Other Lipid Mediators 85.3 (2008): 89-99.
- Park EJ., et al. “Inhibition of lipopolysaccharide-induced cyclooxygenase-2 and inducible nitric oxide synthase expression by 4-[(2'-O-acetyl-alpha-L-rhamnosyloxy)benzyl]isothiocyanate from Moringa oleifera”. Nutrition and Cancer 63.6 (2011): 971-982.
Citation:
D S N B K Prasanth., et al. “Antipyretic Potential of Root of Aralia racemosa L.”. Chronicles of Pharmaceutical Science 1.6
(2017): 360-368.
Copyright: © 2017 D S N B K Prasanth., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.