Editorial
Volume 2 Issue 3 - 2018
The Medicinal Value of Pyrimidines Structural Similarity, But Different Mechanisms and Clinical Applications
Department of Pharmaceutical Organic Chemistry, Faculty of Pharmacy, Helwan University, Cairo, Egypt
*Corresponding Author: Mosaad S Mohamed, Department of Pharmaceutical Organic Chemistry, Faculty of Pharmacy, Helwan University,
Cairo, Egypt.
Received: April 24, 2018; Published: May 08, 2018
An old-recent question;” Does structural similarity exactly reflect the same mechanism of action and clinical application?” Brief discussion
of the medicinal value of some pyrimidine analogues, generally classified as antimetabolites may provide an answer.
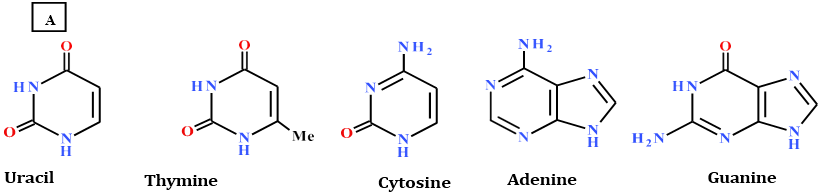
Pyrimidines and purines have long beem discovered. [1,2] such molecules make living creatures’ life possible being the basic constituents
of DNA and RNA. Uracil, its 6-methyl analogue (thymine), its 4-amino analogue (cytosine) are pyrimidine derivatives, while adenine
and guanine are imidazolopyrimidine derivatives (Chart 1).
That is, simply pyrimidine ring is the highest common factor, constituting more than 70% of the living creatures’ genetic materials.
Perhaps, this is one of the factors that explain the tremendous clinical applications of Pyrimidines as therapeutic agents. Literature [3]
indicates diverse medicinal use of pyrimidine derivatives including the anticancer, antibacterial, antiviral, antifungal, anti- inflammatory,
anti-allergic, and anti-diabetic, antihypertensive, and GABARs, GlyRs modulators.
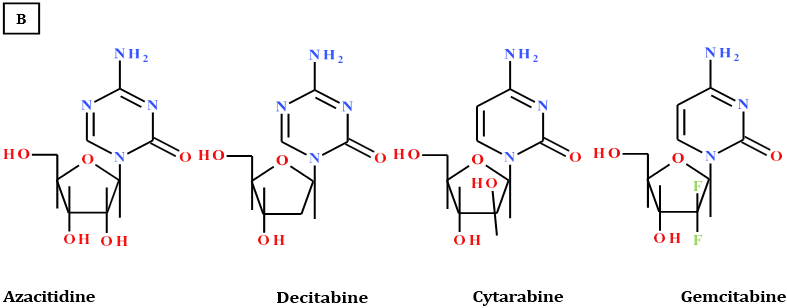
Azacitidine®, Decitabine®, Cytarabine® and Gemcitabine® (chart 1) are cytosine analogues, but have different mechanisms [4,5]
and clinical applications as antineoplastic antimetabolites, interfering or competing with nucleoside triphosphates required for the synthesis
of DNA or RNA or both. However, the first two drugs behave uniquely by blocking or inhibiting the DNA methyl transferase and
mostly used for the treatment of Myelodysplastic syndrome. Cytarabine [6] is one of the cytosine group, but the sugar moiety is unique
being arabinose in which the 2’-hydroxy is in β-configuration. Its mode of action is due to fast conversion into cytosine arabinoside triphosphate,
which damages DNA when the cell cycle holds in the S phase (synthesis of DNA). It is used to treat different types of Leukemia
and non-Hodgkin’s lymphoma.
The fourth analogue of cytosine group, Gemcitabine® [7] acts by incorporating into cell’s DNA creating an irreparable error that leads
to inhibition of further DNA synthesis and consequently cell death. Clinically, Gemcitabine® is used to treat breast, ovarian, non-small
cell lung, pancreatic and bladder cancer.
In contrast, Uracil analogues, 5-fluorouracil® (5-FU) [8] and Floxuridine® [9] have more typically antineoplastic activity and are
important agents in regimens for several solid tumors. 5-FU inhibits thymidylate synthetase, thus blocking the synthesis of thymidine,
which is needed for DNA replication. Floxuridine® interferes with DNA synthesis and to lesser extent inhibits RNA formation through its incorporation into RNA, forming “fraudulent” RNA. Finally, Capecetabine® [10] is used to treat breast, gastric and colorectal cancer. It
is metabolized to 5-FU which is thymidylate synthetase inhibitor, hence inhibiting the synthesis of thymine monophosphate which is
needed for the de novo synthesis of DNA.
In conclusion, chemical structural similarity is not, by necessity, leads to identical mechanisms of action or the same clinical use.
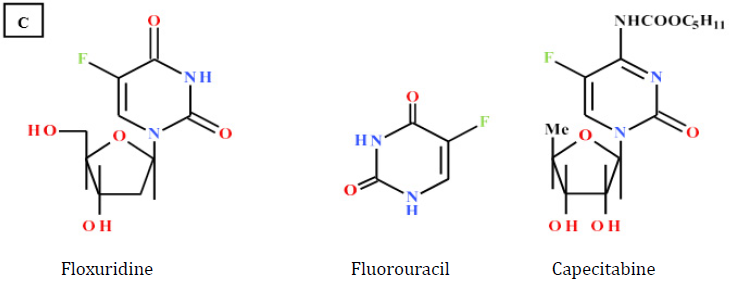
Chart 1: A: Natural bases B: Cytosine analogues anticancer drugs
C: Uracil analogues anticancer drugs
Acknowledgement
The author would like to thank Dr. Rania Helmy for her assistance to produce this text.
The author would like to thank Dr. Rania Helmy for her assistance to produce this text.
References
- Pinner., et al. A18, 759, 1885.
- Fischer., et al. 17, 328, 1880.
- Fischer., et al. 31, 2550, 1898.
- Sharma V., et al. International Journal of Medicinal Chemistry (2014).
- Martens UM. "Small molecules oncology”. Recent results in cancer research 184 (2010). 159-170.
- Orange Book. FDA gov. United states food and drug Administration (2016).
- Peny MJ. “The chemotherapy source book”. Philadelphia Wolter Klower Health/Lippincott Williams and Wilkins P 80 (2008).
- Alvaretlos, ML., et al. Phamaco-genetics and genomic 24 (2014): 564.
- Mimi, E. et al. Annals of oncology 17 (2006): 7.
- Alvarez P., et al. Expert Gpinion on therapeutic patents 22 (2012): 107.
- Canadian Institute of Health Research Drug Bank (2017).
- Med scope References WebMD (2014).
Citation:
Mosaad S Mohamed. “The Medicinal Value of Pyrimidines Structural Similarity, But Different Mechanisms and Clinical
Applications”. Chronicles of Pharmaceutical Science 2.3 (2018): 585-587.
Copyright: © 2018 Mosaad S Mohamed. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.