Research Article
Volume 2 Issue 6 - 2018
Characterization and Determination of Critical Micelle Concentration of Cisplatin Containing Lipid Based Systems
1European University of Lefke, Turkish Republic of Northern Cyprus
2Department of Chemistry, Faculty of Science, Ege University, Turkey
3Department of Pharmaceutical Technology, Faculty of Pharmacy, Ege University, Turkey
2Department of Chemistry, Faculty of Science, Ege University, Turkey
3Department of Pharmaceutical Technology, Faculty of Pharmacy, Ege University, Turkey
*Corresponding Author: Irfan Akartas, European University of Lefke, Turkish Republic of Northern Cyprus Turkey.
Received: June 13, 2018; Published: July 05, 2018
Abstract
The aim of this study is characterization and determination of critical micelle concentration of a novel lipid based system with Cisplatin by surface tension method. Surfactant solutions which are prepared by different concentrations, could show rapid changes of its osmotic pressure, conductivity, turbidity and surface tension at higher concentrations. McBain associated these rapid changes with the formation of micelles or aggregates. Critical micelle concentration (CMC) is defined as the concentration of surfactants above which micelles form and all additional surfactants added to the system go to micelles. There are several methods to measure the surface tension such as Wilhelmy plate, capillary rise method, drop weight method. In this study Traube Stalogmometer is used for drop weight method. Water, HCl buffer with pH 1,2 and phosphate buffer with pH 6,8 were used as solvents. CMC of Cisplatin containing lipid based system was found 0,0012 g/mL for each solvent.
Keywords: Anticancer; Critical micelle concentration; Cisplatin; Lipid based system
Introduction
Cisplatin is a platinum based antineoplastic agent that is widely used to treat various types of cancer such as bladder, head and neck, lung, ovarian and testicular [1,2]. It is used as a primary chemotherapotic agent for non-small cell lung cancer [2]. It is also used to treat carcinoma, lymphoma and sarcoma. Cisplatin shows its effect crosslinking with purine bases on DNA, interfering with DNA repair mechanism, causing DNA damage and inducing apoptosis of cancer cells [3]. Cisplatin has undesired side effects such as kidney problems, allergic reactions, gastrointestinal problems, hemorrhage, ototoxicity, neurotoxicity which limitates its clinical use [1,3]. In order to overcome drug resistance, combination therapies of Cisplatin have been used [3].
Lipid based systems are one of the suitable systems to enhance the solubility and bioavailability of poorly water-soluble drugs by encapsulating and solubilizing. The absorption of drug from lipid based systems depends on many factors like particle size, emulsification degree, dispersion rate and drug precipitation [4]. Lipid based formulations can be classified in five types. Type I systems are oils or oil mixtures without surfactants, Type II systems are oils and water insoluble surfactants, Type IIIA systems are fine emulsions which consist of oils, surfactants and cosolvents, Type IIIB systems are microemulsions which consist of oils, surfactants and cosolvents, Type IV systems are water soluble surfactant and co-solvent mixtures. Lipid based systems could be formulated in different forms such as oily liquids, mixed micelles, self-emulsifying systems, liposomes, solid lipid nanoparticles [4,5]. There are many factors which affect the choice of excipients such as solubility, dispersion, digestion, absorption [5].
Lipid based systems have many advantages like controlled drug release, targeting, high stability, suitability for both lipophilic and hydrophilic drugs, wide ranged excipient options, biodegradability and biocompability. Drug solubility is a critical parameter for these systems. Systems which contain higher ratio of lipid (> 60%) and lower ratio of surfactant (< 30%) and cosurfactant (< 10%) are lead to be more stabilized for the solubilization of drug after dilution [5].
Surfactants contain a hydrophobic long chain and hydrophilic polar group in their structures [6]. Surfactant solutions which are prepared by different concentrations, could show rapid changes of its osmotic pressure, conductivity, turbidity and surface tension at higher concentrations [7]. McBain associated these rapid changes with the formation of micelles or aggregates. Critical micelle concentration (CMC) is defined as the concentration of surfactants above which micelles form and all additional surfactants added to the system go to micelles [8]. Physicochemical properties of solutions such as surface tension, electrical conductivity, turbidity, osmotic pressure, density, viscosity, light scattering shows rapid changes below and above CMC [6]. CMC shows thermodynamic stability of micelles. Lower CMC shows higher thermodynamic stability. In order to determine CMC, interfacial tension, conductivity, viscosity and osmotic pressure could be measured. There are some advanced methods to measure CMC such as fluorescence spectroscopy (pyrene probe method), gel permeation chromatography (GPC) and light scattering. Surface tension, which is typically measured in dynes/cm, is the force in dynes require to break a film of length 1 cm. There are several methods to measure surface tension such as Wilhelmy plate, capillary rise method, drop weight method. In this study, Traube Stalogmometer is used for drop weight method to measure CMC value [9].
The aim of the present study was to develop a novel cisplatin loaded lipid based system for oral delivery. The physico-chemical characterization and critical micelle concentration were evaluated.
Materials and Methods
Materials
Cisplatin (CDDP) and Kolliphor HS 15 were purchased from Sigma Aldrich, Germany. Isoprpyl myristate, Propylene glycol were purchased from Merck, Germany.
Cisplatin (CDDP) and Kolliphor HS 15 were purchased from Sigma Aldrich, Germany. Isoprpyl myristate, Propylene glycol were purchased from Merck, Germany.
Preparation and Characterization of Cisplatin Containing Lipid Based Systems
In order to prepare lipid based system, oil phase, surfactant and co-surfactant were used. Isopropyl myristate was selected as oil phase, Kolliphor HS 15 was selected as surfactant, Propylene glycol was selected as co-surfactant. After identification of the microemulsion region, 0,11 g Isopropyl myristate, 0,55g Kolliphor HS 15 and 0,34g Propylene glycol were weighed and mixed with a magnet under magnetic stirrer. After that, 2 mg Cisplatin was added and dissolved in this mixture under magnetic stirrer to prepare Cisplatin containing lipid based system.
In order to prepare lipid based system, oil phase, surfactant and co-surfactant were used. Isopropyl myristate was selected as oil phase, Kolliphor HS 15 was selected as surfactant, Propylene glycol was selected as co-surfactant. After identification of the microemulsion region, 0,11 g Isopropyl myristate, 0,55g Kolliphor HS 15 and 0,34g Propylene glycol were weighed and mixed with a magnet under magnetic stirrer. After that, 2 mg Cisplatin was added and dissolved in this mixture under magnetic stirrer to prepare Cisplatin containing lipid based system.
Formulation was characterized by physical appearance, pH, dispersion time, droplet size, polydispersity, centrifugation, heating and cooling studies. Physical appearance was detected by visually. pH was measured with Mettler Toledo pH meter. In order to determine dispersion time, formulation was dropped into 1 L of pH 6,8 phosphate buffer mixing under magnetic stirrer at 100 rpm. Droplet size and polydispersity was measured with Malvern NanoZS. During centrifugation studies, 1 mL of formulation was centrifugated at 10000 rpm for 5 minutes and formulation was checked whether there was phase separation. For the heating and cooling studies, formulation was cooled during 24 hours at 4˚C and heated during 24 hours at 40˚C. Then formulation was checked whether there was phase separation or precipitation.
Determination of CMC of Cisplatin Containing Lipid Based Systems
In order to measure critical micelle concentration, Cisplatin containing lipid based system was prepared with the concentrations of 0,24 x 10-3, 1,2 x 10-3, 2 x 10-3, 4 x 10-3 ve 6 x 10-3 g/ml in water, pH 1,2 HCl buffer and pH 6,8 phosphate buffer. Surface tension of each concentration versus water reference at 25˚C was detected. Surface tension versus concentration was measured at different pH values and the critical micelle concentration was determined. Traube stalogmometer was used to detect surface tension with drop weight method.
In order to measure critical micelle concentration, Cisplatin containing lipid based system was prepared with the concentrations of 0,24 x 10-3, 1,2 x 10-3, 2 x 10-3, 4 x 10-3 ve 6 x 10-3 g/ml in water, pH 1,2 HCl buffer and pH 6,8 phosphate buffer. Surface tension of each concentration versus water reference at 25˚C was detected. Surface tension versus concentration was measured at different pH values and the critical micelle concentration was determined. Traube stalogmometer was used to detect surface tension with drop weight method.
Results and Discussion
Preparation and Characterization of Cisplatin Containing Lipid Based Systems
Lipid-based systems have gained much importance in the recent years due to their ability to improve the solubility and bioavailability of poorly water soluble drugs such as cisplatin [5]. A lipid based system is typically composed of lipids and surfactants, and may also contain a hydrophilic co-solvent. According to the present formulation in this study, Isopropyl myristate was selected as oil phase, Kolliphor HS 15 was selected as surfactant, Propylene glycol was selected as co-surfactant. The physical appearance of the prepared lipid based formulation containing cisplatin is clear. Physical characteristics of the developmented system was determined. pH was measured as 5,95 ± 0,07 (diluted by water with the ratio of 1:10). Dispersion time of formulation was found 10 seconds in 1 L of pH 6,8 phosphate buffer. Droplet size was found 25,4 ± 1,9 nm, polydispersity value was found 0,312 ± 0,021. Phase separation wasn’t observed during centrifugation, heating and cooling studies.
Lipid-based systems have gained much importance in the recent years due to their ability to improve the solubility and bioavailability of poorly water soluble drugs such as cisplatin [5]. A lipid based system is typically composed of lipids and surfactants, and may also contain a hydrophilic co-solvent. According to the present formulation in this study, Isopropyl myristate was selected as oil phase, Kolliphor HS 15 was selected as surfactant, Propylene glycol was selected as co-surfactant. The physical appearance of the prepared lipid based formulation containing cisplatin is clear. Physical characteristics of the developmented system was determined. pH was measured as 5,95 ± 0,07 (diluted by water with the ratio of 1:10). Dispersion time of formulation was found 10 seconds in 1 L of pH 6,8 phosphate buffer. Droplet size was found 25,4 ± 1,9 nm, polydispersity value was found 0,312 ± 0,021. Phase separation wasn’t observed during centrifugation, heating and cooling studies.
In a study of F. Martinez-Martinez., et al. ferricyanide ions containing reverse microemulsions were developed and characterized. According to the results, droplet size of the formulations were found between 2,6 ± 0,0 and 6,7 ± 1,6 nm [10].
In a study of Kandasamy., et al. acyclovir and methotrexate containing acetate based ionic liquid in oil microemulsions were developed and characterized. According to the results, mean diameter was found between 9,3 ± 0,4 and 39,7 ± 0,3 nm for different weight ratios. Polydispersity index values was found between 0,115 ± 0,014 and 0,207 ± 0,003 for different weight ratios [11].
Determination of CMC of Cisplatin Containing Lipid Based Systems
Lipid based drug delivery systems should be stable enough to provide drug targeting and controlled release of drug at targeted tissues or cells. In vitro and in vivo stability of lipid based systems such as microemulsions, micelles etc. depends on CMC of these systems [12].
Lipid based drug delivery systems should be stable enough to provide drug targeting and controlled release of drug at targeted tissues or cells. In vitro and in vivo stability of lipid based systems such as microemulsions, micelles etc. depends on CMC of these systems [12].
Surface tension of each concentration in different mediums was measured and graphed in Figure 1. According to the results, CMC value was found 0,0012 g/mL for all pH values and different mediums weren’t affected the CMC values of lipid based systems.
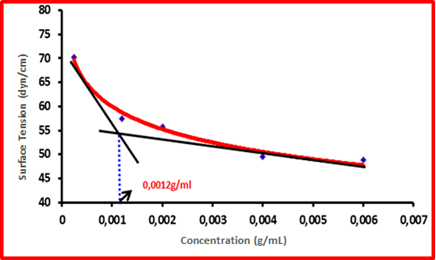
Figure 1: Surface tension-concentration graph for lipid based system
with cisplatin in Water, pH 1, 2 HCl buffer and pH 6, 8 phosphate buffer.
In a study of Ö. Topel., et al. critical micelle concentration of an amphiphilic diblock copolymer was determined by fluorescence spectroscopy and dynamic light scattering (DLS) techniques. CMC of diblock copolymer was calculated as 3 ± 1 × 10-7 and 6 ± 2 × 10-7 mol/L with these two techniques respectively [13]. In a study of Lukyanov., et al. critical micelle concentrations of PEG-PE conjugates with PEG blocks with the range of 750-5000 Da were found less than 10-5 M. PEG-PE conjugates were found stabilized which were demonstrated prolonged circulation in blood [14].
Polarity and molecular weight are the most important characteristic properties in determining CMC. CMC values of copolymers are generally increase with increasing hydrophobicity and molecular weight of copolymere [13].
In our study, Cisplatin containing lipid based system has a very low CMC value that makes this formulation attractive for therapotic use. Drop weight method is a well proven method to determine critical micelle concentration. CMC gives important informations about the in vivo stability of lipid based systems. Orally administered formulations are exposed to high dilution ratios. Low CMC valued formulations (< 10-6 M) keep their in vivo stability in case of dilution. So that, it provides prolonged circulation and prevent drug release before reaching targeted cells and tissues [15].
Conclusion
In this study, Cisplatin containing lipid based systems were prepared and characterized. Critical micelle concentration was determined. According to the characterization and CMC results, it could be stated that formulation was stable. Also this formulation has higher in vivo stability due to its low CMC value. Cisplatin containing lipid based system is an advantageous formulation with its characterization properties and stability. This formulation could be a promising alternative for the treatment.
References
- Cao B-B., et al. “Effect of cisplatin on the clock genes expression in the liver, heart and kidney”. Biochemical and Biophysical Research Communications 501 (2018): 593-597.
- Gotov O., et al. “Hyaluronic acid coated cisplatin conjugated gold nanoparticles for combined cancer treatment”. Journal of Industrial and Engineering Chemistry.
- Dasari S., et al. “Cisplatin in cancer therapy: Molecular mechanisms of action”. European Journal of Pharmacology 740 (2014): 364–378.
- Kalepu S., et al. “Oral lipid-based drug delivery systems – an overview”. Acta Pharmaceutica Sinica B 3(6): (2013): 361–372.
- Shrestha H., et al. “Lipid-Based Drug Delivery Systems” Journal of Pharmaceutics801820 (2014).
- Lavkush Bhaisare M., et al. “Fluorophotometric determination of critical micelle concentration (CMC) of ionic and non-ionic surfactants with carbon dots via Stokes shift”. Talanta 132 (2015): 572–578.
- Deshmukh AS., et al. “Polymeric micelles: Basic research to clinical practice”. International Journal of Pharmaceutics 532 (2017): 249–268.
- Zdziennicka A., et al. “Critical micelle concentration of some surfactants and thermodynamic parameters of their micellization” Fluid Phase Equilibria 322–323 (2012): 126–134.
- Braun AC., et al. “Predicting critical micelle concentration and micelle molecular weight of polysorbate 80 using compendial methods”. European Journal of Pharmaceutics and Biopharmaceutics 94 (2015): 559–568.
- Martinez-Martinez F., et al. “Characterization of reverse microemulsion formed with functionalized surfactants based on ferrycianide ions” Colloids and Surfaces A541 (2018): 10–16.
- Kandasamy S., et al. “Formulation and characterization of acetate based ionic liquid in oil microemulsion as a carrier for acyclovir and methotrexate”. Separation and Purification Technology 196 (2018): 149–156.
- Lukyanov A.N., et al. “Micelles from lipid derivatives of water-soluble polymers as
delivery systems for poorly soluble drugs” Advanced Drug Delivery Reviews 56 (2004): 1273–1289. - Topel Ö et al. “Determination of critical micelle concentration of polybutadiene-block-poly(ethyleneoxide) diblock copolymer by fluorescence spectroscopy and dynamic light scattering”. Journal of Molecular Liquids 177 (2013): 40–43.
- Lukyanov AN., et al. “Polyethylene Glycol-Diacyllipid Micelles Demonstrate Increased Acculumation in Subcutaneous Tumors in Mice”. Pharmaceutical Research 19.10 (2002).
- Torchilin VP. “Micellar Nanocarriers: Pharmaceutical Perspectives”. Pharmaceutical Research 24.1(2006): 1-16.
Citation:
Irfan Akartas., et al. “Characterization and Determination of Critical Micelle Concentration of Cisplatin Containing Lipid
Based Systems”. Chronicles of Pharmaceutical Science 2.6 (2018): 705-709.
Copyright: © 2018 Irfan Akartas., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.