Research Article
Volume 2 Issue 1 - 2018
The Role of Maternal Asthma in the Pregnancy Outcome and Its Relationship to Cd35 and Cd57 Expression in the Placenta
1Assistant professor of the Department of obstetrics and gynecology North-Western State Medical University named after I.I. Mechnikov, 191015, Saint-Petersburg, Kirochnaya st.41
2Leading researcher of the Laboratory of genetic processes of lung diseases, Research Institute of Pulmonology. St. Petersburg 197022 L. Tolstogo street d. 6/8
3A senior researcher at the Department of Pathology of pregnancy D.O. Ott Research Institute RAMS, St. Petersburg, 194034, Mendeleevskaya linia d. 3
4A senior researcher of the department of pathomorphology of FSBSI “The Research Institute of Obstetrics, Gynecology and Reproductology named after D.J. Ott”, 199034, Saint-Petersburg, Mendeleevskaya l., d. 3
2Leading researcher of the Laboratory of genetic processes of lung diseases, Research Institute of Pulmonology. St. Petersburg 197022 L. Tolstogo street d. 6/8
3A senior researcher at the Department of Pathology of pregnancy D.O. Ott Research Institute RAMS, St. Petersburg, 194034, Mendeleevskaya linia d. 3
4A senior researcher of the department of pathomorphology of FSBSI “The Research Institute of Obstetrics, Gynecology and Reproductology named after D.J. Ott”, 199034, Saint-Petersburg, Mendeleevskaya l., d. 3
*Corresponding Author: Dymarskaya YR, Assistant professor of the Department of obstetrics and gynecology North-Western State Medical University named after I.I. Mechnikov, 191015, Saint-Petersburg, Kirochnaya st. 41.
Received: March 15, 2018; Published: April 07, 2018
Summary
Background: Studies in recent years have shown that the severity of maternal asthma directly affects the course and outcome of pregnancy, and many complications of pregnancy are more common in the absence of control of the mother's disease. Being an allergic disease, asthma is associated with the activation of the immune system, thus involvement of the placenta as the main immunocompetent organ in pregnancy, is of great scientific interest. Role and expression of CD35 (a marker of immature dendritic cells) and CD57 (a marker of natural killers) in the placenta of patients with asthma have not been adequately studied.
Aim of the study: Studying the pregnancy outcomes in women with asthma and features expression of CD 35 and CD57 in the placenta of women with asthma.
Materials and methods: The study involved 1800 pregnant women with asthma. All patients were conducted and surveyed throughout the gestational period simultaneously with pulmonologist and obstetrician, and a complex of therapeutic and preventive measures were formed. All measures were continued during labor and postpartum. We performed immunohistochemical study of placental samples of 134 patients for the presence of expression of CD 35 and CD57 on cryostat sections and immunomorphological study of the same placental samples with determination of the fixation of pathogenic immune complexes (PIC), IL-6, IL- 4, and IL-10 in the placenta.
Results: The obstetric and perinatal complications were higher among patients with a lack of control of asthma during pregnancy. The frequency of the most common obstetric and perinatal complications was the greatest in the presence of exacerbation of the disease in the 1st trimester of pregnancy. CD35 expression in the placenta of women with asthma was significantly higher in comparison with control group. Interestingly, in asthmatics with preeclampsia, CD35 expression was lower, especially in cases of uncontrolled asthma in the 3rd trimester of pregnancy. In all cases with persistent and incompletely controlled asthma, the expression of CD57 in the placenta correlated with the presence of PIC, IL-6, IL-4, IL-10 and inflammatory changes in the placenta, that we confirmed by histological study.
Conclusions: In patients with lack of control of asthma during pregnancy, especially in the 1st trimester, high cytotoxicity of NK-cells (CD57) and suppression of immune response (immature activated dendritic cells – CD35) lead to the development of immune inflammation in the placental unit that is associated with the placental insufficiency and drawbacks on the pregnancy outcome. Timely appointment, preferably, preconception period, could decrease the risk of pregnancy and perinatal complications in all patients with asthma.
Keywords: Asthma; Pregnancy; Preeclampsia; Placenta; Dendritic cells; Natural killers
Introduction
The prevalence of asthma and other allergic diseases, based on immunologically mediated mechanisms of hypersensitivity, increases. According to WHO, more than 300 million people all over the world are suffering from asthma and 250,000 people die of this disease every year [1]. The prevalence of the disease among women of reproductive age is increasing every year. Schatz and Zeiger published the most representative data in 2011 [2]. These authors showed that the diagnosis of asthma has been established in 13.9% of the respondents, and 15.8% of women noted symptoms of the disease.
Studies in recent years have shown that the severity of maternal asthma directly affects the course and outcome of pregnancy for both, mother and child; and many complications of pregnancy are more common in the absence of control of the mother's disease [3-6]. At the same time, it is believed that in cases of mild and moderate asthma the outcome of birth is favorable, if treated according the existing standards [7]. Despite the existence of a large number of publications on this topic, the role of the disease itself in the development of complications of pregnancy, that can significantly influence the course and outcome of gestation for the mother and newborn child, remains unclear. We are searching for connecting threads between the presence of an allergic disease of Broncho pulmonary localization and the quality of the gestational process.
Physiological course of pregnancy and normal fetal development are mainly determined by the correct formation of the vascular network of the placenta, maintaining a certain hormonal balance during pregnancy. It is known that endothelial dysfunction, activation of the platelet-vascular units, which are the basis for the development of preeclampsia, are regulated also by immune homeostasis [8]. Along with this, it is proved that the placental pathway is the main one in the prenatal transmission of cytokines from the mother to the fetus [9].
During the implantation period, populations of dendritic cells that, like macrophages, perform an antigen-presenting function for mature T-lymphocytes, and activate naïve T cells [10] perform a number of essential functions, which are necessary for the prolongation of pregnancy and the development of the fetus. At the same time, it is described that in cases with intrauterine growth restriction of the fetus their concentration in the circulating blood was significantly reduced, indicating that these cells possibly effect on the vasculogenesis of the placenta [11]. It is believed that dendritic cells induce an inflammatory response, but there is evidence of their ability to maintain tolerance, which is pathogenically essential in allergic diseases such as asthma [12-14].
At later stages of pregnancy, a number of functions to protect the fetus from infection is performed by NK cells, which can induce cell lysis without developing immune response through interaction with macrophages and the release of pro-inflammatory cytokines. In experimental studies, it has been shown that NK cells play a regulator role in placentation period. They influence the transformation of spiral arteries by production of proangiogenic factors (such as VEGF) [15]. The involvement of NK cells in the pathogenesis of asthma cannot be considered unambiguous - there are data of their mobilization into pulmonary tissue with exacerbation of infectious-dependent asthma [16]. Awad (2014) showed the multidirectional involvement of NK cells in eosinophilic inflammation: NK cells can either activate eosinophils or induce their apoptosis [17].
Immunohistochemical features of the placenta have not been adequately studied. On the one hand, the placenta is an immunocompetent organ, and on the other hand it is the target organ for immunological changes that are taking place in the mother-placenta-fetus system. That is why it is an interesting object of scientific research from the immunological point of view. The study of placenta features is of particular interest in allergic diseases.
Aim of the study: To examine the pregnancy outcomes in women with asthma and features expression of CD 35 and CD57 in the placenta of women with asthma.
Patients and Methods
We analyzed the course of pregnancy and labor of 1,800 women with asthma. Patients were referred to consult a pulmonologist at the Research Institute of Pulmonology of the Pavlov First Saint Petersburg State Medical University. Since the moment of their first visit, we performed supervision by a pulmonologist and obstetrician throughout the period of pregnancy.
More than 60% of patients were from 20 to 30 years old, about a 1/3 of patients were over 30 years old. The severity of asthma was mild in the majority of women – 31.1% of patients (560 people) with mild intermittent asthma and 42.7% (769 people) with mild persistent asthma. 389 women had asthma of moderate severity (21.6%), and 4.6% of patients suffered from severe asthma. The nature of asthma was allergic in 93.4% of patients.
The control group consisted of 100 pregnant women without any pulmonary and allergic diseases. Considering the fact that preeclampsia was the most common and at the same time serious complication of gestation in women with asthma, we found it necessary to divide all pregnant women with asthma into two groups: the main group without preeclampsia and the comparison group, in which the symptoms of preeclampsia were noted.
The type of therapy has been adjusted in accordance with the stepwise approach to therapy of asthma. To all patients with an exacerbation of mild intermittent asthma, as well as to patients with mild persistent asthma, were prescribed inhaled glucocorticosteroids (IGS) in combination with short- or long-acting β2-agonists. Most of the pregnant women were assigned budesonide (60.5%). In cases when pregnant women inhaled other IGS (beclomethasone, fluticasone) with good effect that were prescribed before the consultation of a pulmonologist, this type of therapy was preserved (10%).
In the main group 92% of patients with mild persistent asthma were prescribed IGS in combination with β2-agonists (short-acting or long-acting) from the 1st trimester, while in the comparison group this kind of therapy received only 60% of patients (χ2 = 10.07, p = 0.018). In cases of moderate and severe asthma of the main group, IGS were used significantly more often from the 1 trimester of pregnancy than in the comparison group (p = 0.005). In both groups with persistent asthma, the number of patients who used IGS significantly increased as pregnancy progressed up to 3 trimester (p <0.001).
Persistent asthma was treated by a combination of IGS (budesonide) and short-acting β2-agonist or IGS with long-acting β2-agonist - budesonide/formoterol or fluticasone/salmeterol. The dose of IGS in these drugs was significantly lower than the dose of budesonide in combination with short-acting β2-agonists.
For further investigation of the placenta we performed randomization of all examined patients. Thus, we studied 134 placental samples of women with asthma: 28 patients with mild intermittent asthma, 37 with mild persistent asthma, 41 with moderate and 13 with severe asthma. For comparison, we selected 15 placental samples of the control group. It should be noted that the clinical criteria described below (complications of pregnancy, childbirth, state of newborns) in the selected group of asthmatics were close to those for the general population of examined patients.
Histological examination of the placenta was performed by semi-thin section method, which were made on a material filled with epoxy resin (Epon-Araldite). The slices were made on an ultratom (LKB, Sweden), stained with toluidine blue, analyzed in a Nikon Eclipse E400 microscope (Japan), and photographed with a Nikon-DXM 1200 camera (Japan).
Immunohistochemical analysis of the placenta was carried out on cryostat sections with thickness 5-8 μm. Primary monoclonal mouse anti-CD35 antibodies (1: 40-1: 80, Novocastra, UK), CD57 (1:50, Novocastra, UK) were used. As secondary antibodies were used biotinylated anti-mouse immunoglobulins – a reagent from the DAKO EnVisionTM + System. Visualization was provided by a complex of avidin with biotinylated peroxidase (ABC-Kit), followed by the horseradish peroxidase with diaminobenzidine (all reagents - Dako Citamation, Denmark). To measure the area of expression (S%) and the optical density (conventional units – С. U.) we used a computer analysis system for microscopic images consisting of an Eclipse E400 microscope (Nikon, Japan), a DXM 1200 digital camera (Nikon, Japan) a personal computer based on Intel Pentium 4, software AC 1, version 2.12 and "Morphology 5.2" (Videotest, Russia).
Immunomorphological examination of the central and peripheral parts of the placenta was carried out on cryostat sections processed according to the A. Cronenberger method (1988) using direct and indirect immunofluorescence method with the use of specific sera for the proinflammatory cytokines IL-4, IL 6, IL-10, Human complement (ICN, USA) labeled with FITC (Protein Contour, Russia). The samples were studied using an Axiostar plus HBO 50/AC fluorescent microscope (Zeiss, Germany) and photographed with a DXM 1200 camera (Nikon, Japan). In the obtained pictures, using the Video-Test-Master computer program (Videotest, RF), the intensity of the luminescence was estimated in conventional units (units), which was considered to be significant at more than 30 C. U. (maximum degree of luminescence of 320 units).
Statistical processing was carried out using Microsoft Excel 2013 application software package (Microsoft Corporation, USA) and Statistica 7.0 (Stat soft, USA).
Results
In our study 73.5% of all patients reported about a worsening of the course of the disease, only 26.5% of women noted that the course of asthma did not change or improved in the onset of pregnancy. As pregnancy progressed, due to the monitoring of women and due to prescribed therapy, the number of patients with well-controlled asthma increased and about 73% of all asthmatics had achieved the control of the disease to the time of delivery.
The hyperemesis gravid arum occurred in 35.9% of patients and its frequency and severity increased in parallel with the severity of asthma (reached 40% in cases of severe asthma). Another complication of pregnancy – threatened miscarriage in the first trimester of pregnancy occurred in 29.5% of all pregnant asthmatic women, and in a half of pregnant women with moderate asthma. Moderate and severe maternal asthma was significantly associated both with threatened miscarriage and lack of control of asthma (p <0.01), in the group with preeclampsia (36.4% - comparison group) than in the absence of this complication (9.1% - the main group). More than 80% of pregnant women receiving IGS did not suffer from threatened miscarriage in the 2nd trimester, and inhaled therapy with combination of fluticasone/salmeterol was associated with 2.5 times less often (10.5%) occurrence of threatened miscarriage than the combination of budesonide/formoterol (23 %).
Chronic placental insufficiency occurred in 16.5% of cases and the complication rate was higher in the group of patients with asthma and preeclampsia (20.5%) than in the main group of asthmatics (12.6%), in both groups its frequency correlated with severity of asthma (r = 0.22, p < 0.05). The risk of chronic placental insufficiency was almost 2.5 times higher (OR 2.45, 95% CI 1.04-5.87) if there was lack of control of asthma in the 1st trimester of pregnancy. Chronic placental insufficiency did not develop in 81.1% of patients who used IGS. When fluticasone/salmeterol was used, chronic placental insufficiency occurred significantly (p <0.05) less often (5.3%) than in budesonide/formoterol (21.5%).
The diagnosis of chronic placental insufficiency was confirmed by a dopplerographic study of uteroplacental and fetoplacental blood flow. We observed the correlation between the severity of asthma and the increase of the systolic-diastolic ratio (SDR) and resistance index (IR) in the uterine arteries (r = 0.41, p = 0.000000004) and umbilical cord artery (r = 0.15 p = 0.04). IR in the uterine arteries correlates with the lack of control of asthma in 2nd trimester (r = 0.21, p < 0.05). When fluticasone/salmeterol were used SDR in umbilical cord was significantly lower than in budesonide/formoterol (F = 5.87 p = 0.017).
The vast majority of patients delivered at term (96.5%), premature births occurred in 3.5% of cases at 34-37 weeks, in all of them there was reported the lack of control of asthma in the 2nd trimester of pregnancy. The rate of caesarean delivery was 37.7%, correlated with the severity of asthma (r = 0.14, p = 0.048). Cesarean section rate in patients with mild intermittent asthma was comparable to the control group. At the same time in cases of moderate and severe asthma with preeclampsia, caesarean delivery rate reached 50% (Table 1). In the control group, the rate of delivery by caesarean section was 27%. Among patients with cesarean delivery, 57.8% had lack of control of asthma in the 1st trimester, 59.4% in the 2nd trimester, 45.3% in the 3rd and 35.9% shortly before the delivery.
The most common indications for cesarean section were fetal hypoxia (22%), scar on the uterus (20%), a combination of several obstetric indications (pelvic presentation of the fetus, large fetus, narrow pelvis, uterine contractile activity abnormalities, etc.). The lack of control of asthma in the 1st trimester was highly associated with fetal hypoxia (OR 7.89, 95% CI 2.6-24.0). More than 60% of patients who used IGS for asthma control during the gestation period did not suffer from these complications.
| Group | Cesarean section (%) |
| Mild intermittent asthma | 21 |
| Mild persistent asthma | 42 |
| Moderate and severe asthma | 34 |
| Mild intermittent asthma + preeclampsia | 28 |
| Mild persistent asthma+ preeclampsia | 46 |
| Moderate and severe asthma+ preeclampsia | 50 |
| Control group | 27 |
Table 1: The frequency of cesarean section depending on the severity of asthma and presence or absence of preeclampsia.
The average weight of newborns of asthmatic women was 3311.2 ± 45.1g, the average length -51.2 ± 0.2 cm, which was similar to the average body weight and body length of newborns in St. Petersburg (3350 g and 50.5 cm).
It should be noted that in Russia in recent years there has been a steady decline in the proportion of births "3500g or more", which 36.3% [18] is currently. Ratio of newborns with a weight of 3500 g or more was 39%, so we can conclude that there is no direct negative effect of asthma on the mass of newborns. Our data are similar to the data of recent studies [2]. We found negative correlation between the severity of asthma and body mass of newborns (Table 2), these data did not depend on the presence or absence of preeclampsia, and this relationship was revealed both in the main group (r = -0.22, p <0.05), and the comparison group (r = -0.24, p <0.05).
| Group | Weight of newborns (g) | |
| Mild intermittent asthma | 1 | 3437.9 |
| Mild persistent asthma | 2 | 3385.1 |
| Moderate asthma | 3 | 3335.8 |
| Severe asthma | 4 | 3087.1 |
| Total | 3311.2 | |
| 1-3 p=0.02 1-4 p=0.0005 2-4 p=0.003 3-4 p=0.01 |
||
Table 2: The average weight of newborns depending on the severity of maternal asthma.
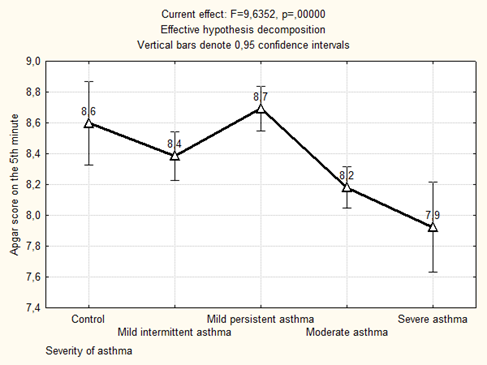
The Apgar score at the 5th minute of life was significantly lower in newborns from mothers who suffered from moderate and severe asthma in group with preeclampsia (p = 0.000034 compared with mild persistent asthma + preeclampsia, p = 0.0013 compared with the control) and without preeclampsia (p = 0.000037 compared with mild persistent asthma, p = 0.008 compared with the control) (Figure 1).
Figure 1: The average Apgar score on the 5th minute of newborns depending on the severity of maternal asthma.
We found significant differences in the weight and Apgar score at the 5th minute of life of the newborns from asthmatic mothers depending on the course of asthma in the 1st trimester of pregnancy (Table 3).
| Parameter | Group | Uncontrolled asthma or less of control in the 1st trimester (M ± m) (I) |
Well-controlled asthma in the 1st trimester (M ± m) (II) |
P | |
| Weight of newborns (g) | Mild intermittent asthma | 1 | 3310.0 ± 266.4 | 3405.6 ± 115.4 | N/S |
| Mild persistent asthma | 2 | 3342.3 ± 128.0 | 3426.3 ± 139.1 | N/S | |
| Moderate and severe asthma | 3 | 3212.3 ± 90.5 | 3240.5 ± 108.7 | N/S | |
| Mild intermittent asthma + preeclampsia | 4 | 3596.8 ± 98.4 | 3933.3 ± 124.4 | p<0.05 | |
| Mild persistent asthma+ preeclampsia | 5 | 3434.6 ± 99.1 | 3732.3 ± 107.0 | p<0.05 | |
| Moderate and severe asthma+ preeclampsia | 6 | 3279,1 ± 94,2 | 3493,3 ± 188,4 | N/S | |
| P | 2-4 p = 0.047 2-5 p = 0.032 3-4 p = 0.01 3-5 p = 0.001 4-6 p = 0.022 5-6 p = 0.004 |
3-4 p = 0.016 | |||
| Apgar score at the 5th minute of life (points) | Mild intermittent asthma | 1 | 8.33 ± 0.21 | 8.56 ± 0.12 | P < 0.05 |
| Mild persistent asthma | 2 | 8.84 ± 0.14 | 8.54 ± 0.16 | N/S | |
| Moderate and severe asthma | 3 | 8.15 ± 0.10 | 8.22 ± 0.12 | N/S | |
| Mild intermittent asthma + preeclampsia | 4 | 8.33 ± 0.31 | 8.27 ± 0.11 | N/S | |
| Mild persistent asthma+ preeclampsia | 5 | 8.53 ± 0.14 | 8.8 ± 0.13 | N/S | |
| Moderate and severe asthma+ preeclampsia | 6 | 8.08 ± 0.11 | 8.0 ± 0.22 | N/S | |
| P | 2-3 p = 0.00022 2-6 p = 0.000065 3-5 p = 0.03 5-6 p = 0.015 |
1-6 p = 0.02 2-6 p = 0.048 3-5 p = 0.0025 4-5 p = 0.004 5-6 p = 0.0025 |
|||
Table 3: Average weight and Apgar score at the 5th minute of life of newborns from mothers with asthma depending on the severity, control of the disease in the 1st trimester of pregnancy and the presence of preeclampsia.
We found statistically significant differences in the average weight of newborns depending on the severity of the disease and different types of therapy. Thus, the smallest average weight of newborns was noted in the group of women suffering from severe asthma who received IGS in combination with short-acting β2-agonists (2895.6g). The average weight of newborns in the group of mothers who received IGS in combination with long-acting β2-agonists was above the average weight for the whole group of asthmatic women (Table 4).
| Group | Weight of newborns (g) | ||
| Asthma in a whole | IGS + short-acting β2-agonists | IGS + long-acting β2-agonists | |
| Mild intermittent asthma | 3437.9 | 3483.5 | 3720.0 |
| Mild persistent asthma | 3385.1 | 3467.1 | 3708.3 |
| Moderate asthma | 3335.8 | 3402.0 | 3446.7 |
| Severe asthma | 3087.1 | 2895.6 | 3216.5 |
| Total | 3311.2 | 3312.1 | 3522.9 |
Table 4: Average weight of newborns from mothers with asthma depending on the severity and different types of therapy of the disease.
Results of the study of the placenta
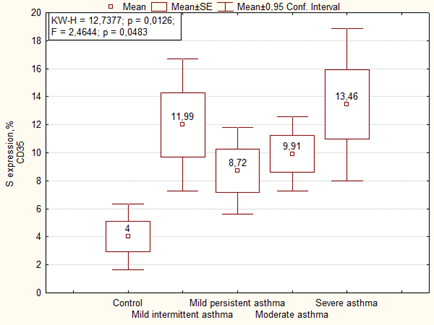
An immunohistochemical study showed that in the placenta of women with asthma there is significantly higher expression of CD35+ immature activated dendritic cells (DC) in comparison with the control (Figure 2). The similar data were observed in the optical density of expression of CD35, where it was higher in cases with moderate and severe asthma (Figure 3).
An immunohistochemical study showed that in the placenta of women with asthma there is significantly higher expression of CD35+ immature activated dendritic cells (DC) in comparison with the control (Figure 2). The similar data were observed in the optical density of expression of CD35, where it was higher in cases with moderate and severe asthma (Figure 3).
Interestingly, in the main group of patients (without preeclampsia), the area and optical density of CD35 expression in the placenta correlated with the severity of asthma, whereas in the presence of preeclampsia, on the contrary, the area of CD35 expression negatively correlated with the severity of the disease, and the optical density was the highest in placenta of women with moderate and severe asthma (Table 5, Figure 4).
| Parameter | Group | Mean (M ± m) | P | |
| Area of expression CD35, % | Mild intermittent asthma | 1 | 8.97 ± 2.66 | F = 2.44, p = 0.02869 2-4 p = 0.030 3-7 p = 0.0034 4-6 p = 0.047 4-7 p = 0.0024 |
| Mild persistent asthma | 2 | 7.24 ± 2.24 | ||
| Moderate and severe asthma | 3 | 12.63 ± 1.66 | ||
| Mild intermittent asthma + preeclampsia | 4 | 14.26 ± 2.30 | ||
| Mild persistent asthma+ preeclampsia | 5 | 9.97 ± 2.06 | ||
| Moderate and severe asthma+ preeclampsia | 6 | 8.24 ± 1.92 | ||
| Control | 7 | 4.00 ± 2.38 | ||
| Optical density of expression CD35, C.U. | Mild intermittent asthma | 1 | 0.16 ± 0.15 | F = 1.25, p = 0.28 4-6 p = 0.04 6-7 p = 0.034 |
| Mild persistent asthma | 2 | 0.19 ± 0.12 | ||
| Moderate and severe asthma | 3 | 0.28 ± 0.09 | ||
| Mild intermittent asthma + preeclampsia | 4 | 0.16 ± 0.13 | ||
| Mild persistent asthma+ preeclampsia | 5 | 0.19 ± 0.11 | ||
| Moderate and severe asthma+ preeclampsia | 6 | 0.49 ± 0.11 | ||
| Control | 7 | 0.13 ± 0.13 | ||
Table 5: Expression of CD35 in the placenta depending on the severity of maternal asthma and the presence or absence of preeclampsia.
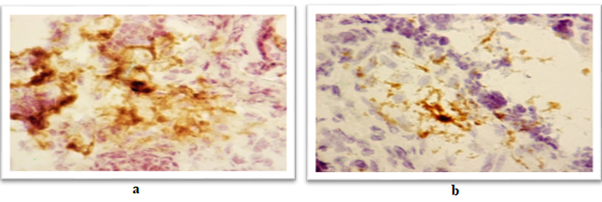
Figure 4: Expression of a marker of immature dendritic cells (CD35) in the placenta:
А) A woman of the main group with severe asthma. The area of expression 26.98%, the optical density 0.30 C.U.
B) A woman of the comparison group with severe asthma and moderate preeclampsia. The area of expression 3.29%, the optical density 0.33 C.U.
А) A woman of the main group with severe asthma. The area of expression 26.98%, the optical density 0.30 C.U.
B) A woman of the comparison group with severe asthma and moderate preeclampsia. The area of expression 3.29%, the optical density 0.33 C.U.
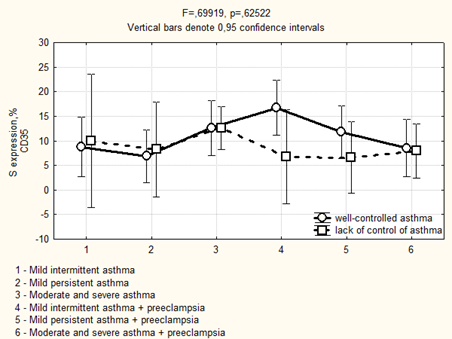
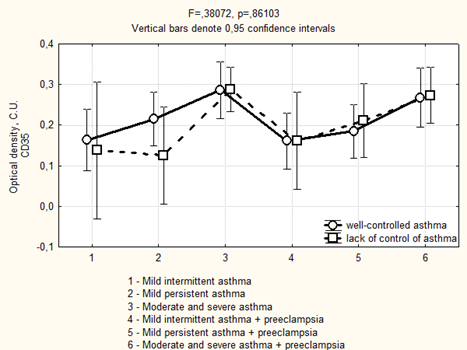
Avidin-biotinic immunoperoxidase method. × 400 we found that if asthma was well-controlled in the 3rd trimester in the group of patients with preeclampsia, the area of CD35 expression had been higher, while the optical density of expression have not significantly differed (Figure 5, 6). We completely agree with the literature data concerning the localization of CD35 + cells in the stroma of stem and intermediate villi, as well as in the stromal canals [19].
Figure 5: Area of CD35 expression in placenta depending on the severity and the control of asthma in the 3rd trimester.
Figure 6: Optical density of CD35 expression in placenta depending on the severity and the control of asthma in the 3rd trimester.
The expression of CD35 in the placenta did not correlate with different types of therapy: in the group of patients who did not use IGS, the area of expression of CD35 was 11.6 ± 1.67%, and the optical density was 0.16 ± 0.09 C.U., in the group of women who received IGS the area of expression was 9.93 ± 1.05%, and the optical density was 0.30 ± 0.05 C.U.
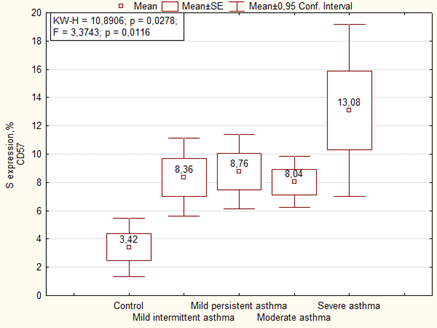
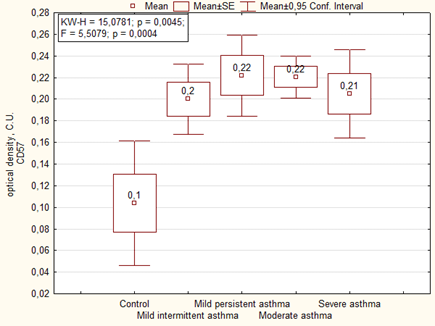
Immunohistochemical analysis of the placenta for the ability to express the marker of NK cells (CD57) showed significantly more high area and optical density of expression than in the control group. We noted the highest level of expression of CD57 in placenta of women with severe asthma (Figures 7, 8).
Expression of CD57 in placenta of women of the main group showed that the highest level of expression of the marker was in cases with mild persistent asthma, as well as in the comparison group the highest level of expression was observed in cases with moderate and severe asthma (Figure 9). The optical density of CD57 was also significantly higher in the placenta of patients with asthma than the control group. There were no significant differences in the optical density of CD57 between the main group and the comparison group, but it is necessary to note that it was higher in placenta of patients with mild persistent asthma than in mild intermittent asthma (Table 6).
Figure 9: Expression of the natural killer marker (CD57) in the placenta of a woman with moderate asthma and mild preeclampsia. The area of expression is 19.05%, the optical density is 0.22 C.U. Avidin-biotinic immunoperoxidase method. × 400.
| Parameter | Group | Mean (M ± m) | P | |
| Area of expression CD57, % | Mild intermittent asthma | 1 | 8.86 ± 2.01 | F = 1.9268. p = 0.08134 1-7 p = 0.049 2-7 p = 0.006 3-7 p = 0.032 6-7 p = 0.003 |
| Mild persistent asthma | 2 | 10.49 ± 1.73 | ||
| Moderate and severe asthma | 3 | 8.26 ± 1.28 | ||
| Mild intermittent asthma + preeclampsia | 4 | 7.99 ± 1.78 | ||
| Mild persistent asthma+ preeclampsia | 5 | 7.28 ± 1.59 | ||
| Moderate and severe asthma+ preeclampsia | 6 | 10.59 ± 1.48 | ||
| Control | 7 | 3.42 ± 1.84 | ||
| Optical density CD57. C.U. | Mild intermittent asthma | 1 | 0.17 ± 0.03 | F = 3.09. p = 0.00727 1-2 p = 0.03 2-7 p = 0.0003 3-7 p = 0.003 4-7 p = 0.003 5-7 p = 0.012 6-7 P = 0.0012 |
| Mild persistent asthma | 2 | 0.25 ± 0.02 | ||
| Moderate and severe asthma | 3 | 0.21 ± 0.02 | ||
| Mild intermittent asthma + preeclampsia | 4 | 0.22 ± 0.02 | ||
| Mild persistent asthma+ preeclampsia | 5 | 0.20 ± 0.02 | ||
| Moderate and severe asthma+ preeclampsia | 6 | 0.22 ± 0.02 | ||
| Control | 7 | 0.12 ± 0.02 | ||
Table 6: CD57 expression in the placenta of women with asthma depending on the severity of asthma and the presence or absence of preeclampsia.
We found a positive correlation between the area of expression of CD57 and the deposition of pathogenic immune complexes (PIC - C3-complement fraction) -r = 0.32 (p <0.05), IL-4 (r = 0.29, p <0.05), IL-6 (r = 0,23, p <0,05), IL-10 (r = 0,27, p <0,05) identified during the immunomorphological study of the placenta, as well as the inflammatory signs (r = 0,23, p <0, 05) identified by the histological study of the placenta of patients with asthma. Importantly, we noted a negative correlation between the CD57 expression in the placenta with the Apgar score at the 5th minute of life of newborns from asthmatic mothers (r = -0.22, p <0.05).
The area of expression of CD57 was slightly associated with the lack of control of asthma in the 3rd trimester (11.81 ± 2.34%) compared to the well-controlled cases (8.62 ± 0.82%, N/S). The optical density of CD57expression was significantly higher in the placenta of women with lack of asthma control in 3rd trimester (0.22 ± 0.009 C.U.) than in placenta of well-controlled asthmatic mothers (0.16 ± 0,026 C.U. p = 0.083). In the placenta of patients of main group with well-controlled mild persistent asthma in 3rd trimester the optical density of CD57 expression was significantly higher (0.24 ± 0.02 C.U.) when compared to mild intermittent asthma without exacerbation (0.17 ± 0.02 C.U., p = 0.03).
The expression of CD57 in the placenta did not significantly differ depending on the type of therapy: area of CD57 expression in placenta of patients who did not use IGS was 9.01 ± 1.27%, and the optical density of expression was 0.20 ± 0.01 C.U., among those who received IGS therapy, the area of expression was 8.83 ± 0.8%, and the optical density was 0.21 ± 0.009 C.U.
Discussion
The lack of control of asthma in the 1st trimester of pregnancy is associated with the 2.5 times risk of chronic placental insufficiency. We observed the correlation between the severity of asthma and the increase of the dopplerographic parameters in the uterine arteries. The smallest weight of newborns was in the subgroup with severe asthma, who received short-acting β2-agonists. The low weight of newborns and low Apgar score at the 5th minute of life were associated with a lack of control of asthma in the 1st trimester of pregnancy.
The last issue interested us most and we tried to find a link between the lack of asthma control and the perinatal outcomes. It is known that many factors influence the state of newborns. However, making sure that maternal asthma is really involved in and associated with the development of many complications of pregnancy, especially if not well-controlled, we decided to go deeper into our study and looked for the morphological and immunohistochemical features of the placenta in patients with asthma.
Dendritic cells (DC) play an important role in maintaining the immune balance between the mother and fetus and are able both to induce an inflammatory response and to show the immunological tolerance that is necessary for the prolongation of pregnancy [12-14]. In the available literature, we were not able to find information about the role of DC in the placenta in patients with asthma, but summarizing the results obtained in our study, it can be assumed that in conditions of allergic ensitization in patients with persistent forms of asthma without preeclampsia, high quantity of CD35-positive cells provide the immune tolerance of the placenta in order to protect the fetus from incoming antigens from mother’s blood flow.
The literature data showed that intrauterine growth retardation of the fetus was associated with the decrease of the activity of the DC of peripheral blood flow. Authors suggest their involvement in angiogenesis of the placenta [11]. In our study, we found low level of CD35 in cases with persistent asthma and preeclampsia that seems to indicate to the depletion of the immune response in conditions of lack of asthma control.
In the 1st trimester of pregnancy NK cells perform a number of functions to protect the fetus from infection by interacting with macrophages and expression of cytokines [12]. In our study we found a correlation between the CD57 expression and the deposition of pathogenic immune complexes, IL-4, IL-6, IL-10, as well as the inflammatory signs in the placenta of asthmatic women. These findings suggest the cytotoxic role of NK cells while an allergic phenotype of the mother. It was recently showed that the number of NK cells increases while obstructive or restrictive changes occur [20], as well as it is proved that exacerbation of infectious-dependent asthma leads to the mobilization NK-cells into pulmonary tissue [16].
Therefore, it can be suggested that NK cells of the placenta play a cytotoxic role in the presence of persistent and incompletely controlled asthma. A high level of their expression results in the above-mentioned morphological and immune changes in the placenta that adversely affect the developing fetus and the state of the newborn. This hypothesis may be confirmed by the negative correlation between the CD57 expression and the Apgar score at the 5th minute of life of newborn.
Conclusion
In patients with lack of control of asthma during pregnancy, especially in the 1st trimester, high cytotoxicity of NK-cells (CD57) and suppression of immune response (immature activated dendritic cells – CD35) lead to the development of immune inflammation in the placental unit that is associated with the placental insufficiency and drawbacks on the pregnancy outcome. Timely appointment, preferably, preconception period, could decrease the risk of pregnancy and perinatal complications in all patients with asthma.
References
- Global Iniative for Asthma Pocket guide for asthma management and prevention (2012).
- Schatz M and Zeiger RS. “Improving asthma outcomes in large populations”. Journal of Allergy and Clinical Immunology 128.2 (2011): 273-277.
- Дымарская Ю.Р., Лаврова О.В. Особенности течения и исходов беременности у пациенток, страдающих бронхиальной астмой // Практическая медицина. – 2015. – Вып. 1, № 86. – С. 50-55. [Dymarskaya Y.R., Lavrova O.V. “Features of pregnancy course and outcomes in patients with bronchial asthma”. Prakticheskaya meditsina 1.86 (2015): 50-55.]
- Лаврова О.В., Дымарская Ю.Р. Бронхиальная астма и беременность // Практическая пульмонология. – 2015. - № 4. – с. 2-9. [Lavrova O.V., Dymarskaya Y.R. Bronkhial'naya astma i beremennost'. Prakticheskaya pul'monologiya 4 (2015): 2-9 (In Russ).]
- Dombrowski MP. “Outcomes of pregnancy in asthmatic women”. Immunology and Allergy Clinics of North America 26.1 (2006): 81-92.
- Wang G., et al. “The risk of maternal and placental complications in pregnant women with asthma: a systematic review and meta-analysis”. The Journal of Maternal-Fetal & Neonatal Medicine 27.9 (2014): 934-942.
- Шугинин И.О. и др. Ведение родов у беременных с бронхиальной астмой // Практическая медицина. – 2003. – № 3. – с. 27-28 [Shuginin I.O. etal. Vedenie rodov u beremennykh s bronkhial'noy astmoy. Prakticheskaya meditsina 3 (2003): 27-28.].
- Young BC., et al. “Pathogenesis of preeclampsia”. Annual Review of Pathology 5 (2014):173-192.
- Murphy VE., et al. “Severe asthma exacerbations during pregnancy”. Obstetrics & Gynecology106.5.1 (2005):1046-1054.
- Wu L and Liu Y-J. “Development of dendritic cell lineages”. Immunity 26 (2007): 741-750.
- Cappelletti M., et al. “Lack of activation of peripheral blood dendritic cells in human pregnancies complicated by intrauterine growth restriction”. Placenta 34.1 (2013): 35-41.
- Айламазян Э.К., Полякова В.О., Кветной И.М. Функциональная морфология плаценты человека в норме и при патологии (нейроиммуноэндокринологические аспекты). СПб.: Изд-во Н-Л, 2012. [Aylamazyan E.K., Polyakova V.O., Kvetnoy I.M. Funktsional'nayamorfologiyaplatsentychelovekavnormeipripatologii (neyroimmunoendokrinologicheskieaspekty). SPb.: Izd-vo N-L; 2012. (In Russ).]
- Hrusch CL., et al. “The role of dendritic cells and monocytes in the maintenance and loss of respiratory tolerance”. Current Allergy and Asthma Reports 15.1 (2015): 494.
- Vroman H., et al. “Mode of dendritic cell activation: The decisive hand in Th2/Th17 cell differentiation. Implications in asthma severity?” Immunobiology 220.2 (2015): 254-261.
- Hanna J and Mandelboim O. “When killers become helpers”. Trends in Immunology 28.5 (2007): 201–206.
- Koh YI., et al. “The role of natural killer t cells in the pathogenesis of acute exacerbation of human asthma”. International Archives of Allergy and Immunology 158.2 (2012):131-141.
- Awad A., et al. “Natural killer cells induce eosinophil activation and apoptosis”. Plos One 9.4 (2014):e94492.
- Schatz M., et al. “The relationship of asthma medication use to perinatal outcomes”. Journal of Allergy andClinical Immunology 113.6 (2004):1040-1045.
- Лапина Е.А. Сигнальные молекулы как маркеры зрелости и старения плаценты у женщин разного возраста: Дис. … канд. биол. наук. – Санкт-Петербург, 2004. [Lapina E.A. Signal'nye molekuly kak markery zrelosti i stareniya platsenty u zhenshchin raznogo vozrasta. [dissertation] Saint-Petersburg; 2004. (In Russ).]
- Shim JU and Koh YI. “Increased Th2-like invariant natural killer t cells in peripheral blood from patients with asthma”. Allergy, Asthma & Immunology Research 6.5 (2014): 444–448.
Citation:
Dymarskaya YR., et al. “The Role of Maternal Asthma in the Pregnancy Outcome and Its Relationship to Cd35 and Cd57
Expression in the Placenta”. Gynaecology and Perinatology 2.1 (2018): 218-232.
Copyright: © 2018 Dymarskaya YR., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.