Research Article
Volume 2 Issue 1 - 2018
Current Epidemiological Status of Bovine Theileriosis in North-Western States of India
Entomology Laboratory, Division of Parasitology, ICAR-Indian Veterinary Research Institute (IVRI)
Izatnagar-243122, Bareilly, UP, INDIA
*Corresponding Author: Sachin Kumar, Entomology Laboratory, Division of Parasitology, ICAR-Indian Veterinary Research Institute (IVRI), Izatnagar-243122, Bareilly, UP, INDIA.
Received: February 26, 2018; Published: March 14, 2018
Abstract
Tick-borne diseases of livestock are responsible for heavy economic losses globally. The protozoan parasite Theileria annulata is the causative agent of the tick-borne disease tropical theileriosis (also known as Mediterranean fever) which causes morbidity in indigenous cattle and severe lethal disease in imported high-grade cattle and crossbreds in a wide geographical area ranging from the Mediterranean littoral regions of Europe and Africa to the near and Middle East to India. Mediterranean fever is an important bovine haemoprotozoan disease in an economic point of view and spreads over North Africa, Southern Europe, The Middle East and Asia. Tropical theileriosis has long been recognized as a hindrance to the development of sound dairy industry in the India and is a cause of major economic losses. Serological surveys indicated that Theileria annulata infection is widespread in the country but the disease mostly affects exotic dairy breeds and their crosses with indigenous breeds. Knowledge of the of these diseases is important for the design and implementation of control strategies Long term carrier animals which were all recovered from previous exposure to the organisms pose thread as source of infection in Hyalomma ticks. The prevalence rate of theileriosis is based on the geographical region and several other associated factors like tick density, climatic conditions, age, gender, management practices and immunity of the host. Theileria caused by Theileria annulata is economically important vector borne haemoprotozoan disease of livestock. Theileria is responsible for causing theileriosis resulting in death of affected animals. The disease is endemic in warmer regions, it is seasonal and the incidence is higher during summer and rainy season when the ticks have higher activity although sporadic outbreaks have been recorded year round. It is a potential killer of livestock and causes economic losses in terms of mortality, morbidity, abortion, infertility, reduced milk yield etc. The disease is underestimated in cattle due to sub clinical nature. The conventional parasitological techniques are less sensitive. More than 80 percent of infections are cryptic and undetectable by direct. This paper briefly discuss about the occurrence, control and the economic importance of Theileria spp. In WesternHimalayan region of Uttar Pradesh.
Keywords: Epidemiology; livestock; Tropical Theileriosis; PCR; Microscopy
Introduction
Ticks and tick-borne diseases (TBDs) cause major economic losses, and affect many domestic animals, mainly cattle and sheep, in tropical and subtropical regions. Tropical theileriosis is a TBD caused by a protozoon called Theileria annulata transmitted by several tick species of the genus Hyalomma (Robinson., et al. 1982). Theileriosis and babesiosisare the most importantanddangerousblood protozoan diseases of the cattle, these are transmitted by ticks especially in countries which have intensive animal industries (Balha, 1989). Tropical theileriosis is a frequent fatal disease of cattle caused by the protozoan parasite Theileria annulata (Preston., et al. 1999).
The temperature of Uttar Pradesh region is favorable for ticks and responsible for the transmission of theileriosis in cattle. Uttar Pradesh is northern Indian state and shares international Himalayan border with Nepal, but the state’s most parts are covered by plains and are different from the Himalayan region. Cattle are the important species in this areas because of dual purpose. They are reared for the supply of draught power for agriculture and for milk production.
This is because of the introduction of new cattle from the surrounding states where this disease is prevalent. The climate of Uttar Pradesh is humid and subtropical and comprises of four seasons. The mean annual rainfall varies from region to region in the state. The winter starts in January and ends in February, summer between March and May, monsoon season between June and September and retreating monsoon season from September to November. The temperature during summer season is extreme and may touch up to 48°C, and during the summer months dry hot winds called loo remains in the summer months. The present paper attempts to present a scenario of theileriosis, their occurrence in Uttar Pradesh, reason of occurrence, effects and their preventive measures. So that dairy venture become more profitable and mortality rate in crossbred cattle due to theileriosis should be reduced.
Theileriosis
The Theileria parasite was first reported by Arnald Theiler and Dschunkowsky first described the disease theileriosis in 1904. Theileriosis caused by Theileria annulata and transmitted through the bites of Hyalomma and Rhipicephalus with higher incidence in the crossbred cow of all age groups with the general epidemiology of the disease in tropical areas (Jithendran., et al. 1997). This disease is seasonal, starts in the second part of April, and adds to its abundance increase in June and July (Assadpour., et al. 1990). Cases of theileriosis are generally observed during summer or rainy season when the ticks have higher activity although sporadic outbreaks have been recorded year round (Meenakshisundaram., et al. 2014). Tropical theileriosis caused by T. annulata may result in 80% mortality in susceptible animals (Gill., et al. 1977).
The Theileria parasite was first reported by Arnald Theiler and Dschunkowsky first described the disease theileriosis in 1904. Theileriosis caused by Theileria annulata and transmitted through the bites of Hyalomma and Rhipicephalus with higher incidence in the crossbred cow of all age groups with the general epidemiology of the disease in tropical areas (Jithendran., et al. 1997). This disease is seasonal, starts in the second part of April, and adds to its abundance increase in June and July (Assadpour., et al. 1990). Cases of theileriosis are generally observed during summer or rainy season when the ticks have higher activity although sporadic outbreaks have been recorded year round (Meenakshisundaram., et al. 2014). Tropical theileriosis caused by T. annulata may result in 80% mortality in susceptible animals (Gill., et al. 1977).
Acute clinical cases of theileriosis were first recorded on 12 June, 1922 in hill bulls. In 1930 outbreak of clinical theileriosis were recorded in imported herds maintained at Lahore, Bangalore, Allahabad and Kirkee. Since then occasional outbreaks of theileriosis have been recorded mainly in cross bred and exotic cattle.
Treated cattle turn out to be long standing carriers, with only a few number of infested erythrocytes, thus posing difficulty in the demonstration of parasites in blood smear (Nayel., et al. 2012). In long standing carrier animals blood smears are negative on microscopy (Aktas., et al. 2006). Carrier animals have an important role in the transmission of infection by the Hylomma ticks (Dóliveira., et al. 1995). Antibodies tend to disappear in long term carrier cattle despite the presence of piroplasms (Burridge., et al. 1974; Papadopoulous., et al. 1996). Transport of carrier cattle to non-endemic areas can lead to disease outbreak (Bilgic., et al. 2013). It is possible for cattle infected with these parasites to maintain carrier state for several years (Young., et al. 1981).
Piroplasms are very small < 2.5m they are ovoid, annular, ring or rod shaped. Radostitset., et al. 1994; Roy., et al. 2004 found highest prevalence in monsoon months. Minjauw and Mcleod (2000) have estimated the cost of T. Annulata in India to be US$ 384.3 million. A recent estimate of US$ 498.7 million per annum has been calculated as the cost of TTBD’s in India (Minjauw and Mcleod 2003)
Subheadingsshould be classified
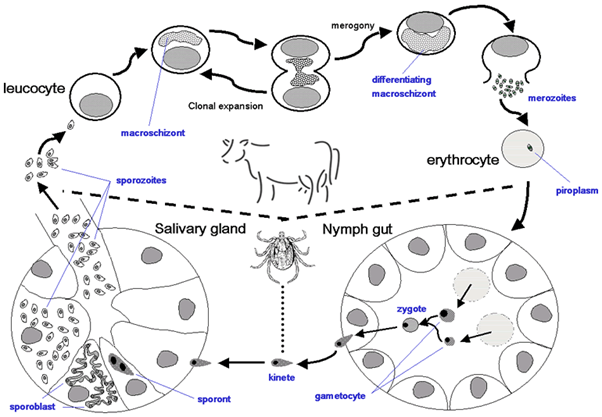
Pathogenesis:The life cycle of T. annulata includes the following stages:
Pathogenesis:The life cycle of T. annulata includes the following stages:
Sporozoite stage: When infected adult ticks attach to cattle, the sporozoites develop in the tick salivary gland and are injected with the tick saliva. The sporozoites invade the lymphoid cells and schizonts are detected in 10-13 days. This is the prepatent period of the disease.
Schizont stage: The schizonts parasitize lymphocytes, proliferate and invade and damage the lymphoid system and produce lesions in the skin, liver and spleen.
Piroplasm stage: The piroplasm parasitizes the erythrocytes and causes destruction of these cells with a decrease in the erythrocyte count and haemoglobin level.
Symptoms: Clinically a rise of body temperature up to 107°F and enlarged superficial lymph nodes accompanied by dullness, anorexia, salivation, lacrimation, and discharge from nostrils, tachycardia, and decreased milk production are the symptoms for theileria (El-DeebYounis, 2009).
Diagnosis of Theileriosis
1. Microscopic Examination: Theileriaannulata infection in cattle is usually based on the detection of macroschizonts in Giemsa’s-stained lymph node biopsy smears in live animals and impression smears of lymph node and spleen in dead animals (Aktas., et al. 2001).
1. Microscopic Examination: Theileriaannulata infection in cattle is usually based on the detection of macroschizonts in Giemsa’s-stained lymph node biopsy smears in live animals and impression smears of lymph node and spleen in dead animals (Aktas., et al. 2001).
2. Serological Examination: Serological tests such as the indirect immunoflourescent antibody test (IFAT) can be used to detect circulating antibodies (Pipano and Shkap, 2000; Musoke., et al. 2001). However, cross-reactivity with antibodies directed against other Theileria species limits the specificity of the IFAT (Burridge., et al. 1974; Kiltz., et al. 1986).
3. DNA Based Examination (PCR & LAMP):
a) PCR: Molecular diagnosis of haemoprotozoan diseases involves several PCR- based diagnosis procedures, which help in the identification of the parasites up to the species or even strain level (Figueora., et al. 1993; Birkenheuer., et al. 2003; Rampersad., et al. 2003 and Criado-Fornelio., et al. 2003). With the availability of sequenced parasite genes and PCR, it is possible to detect parasites within samples of blood (Bishop., et al. 1992; Tanaka., et al. 1993). PCR based technique uses small material which is very relevant because large amount of material is not possible from different stages of parasitic life cycle. (Gasser RB., et al. 2006). This technique reveals a high sensitivity compared to immunological examinations and serological testing (Shah D., et al. 1990). Furthermore, the advent of the polymerase chain reaction (PCR) technique has made it possible to increase the sensitivity of nuclear hybridization techniques, through amplification of target DNA sequences of the parasites in test material, by in situ synthesis of these sequences prior to hybridization with the diagnostic probe. Despite the benefits of PCR based technologies, such as high specificity and sensitivity to detect some parasites the main disadvantage of these methods is that they are very time consuming and do not provide quantitative data (Linn MH., et al. 2003).
a) PCR: Molecular diagnosis of haemoprotozoan diseases involves several PCR- based diagnosis procedures, which help in the identification of the parasites up to the species or even strain level (Figueora., et al. 1993; Birkenheuer., et al. 2003; Rampersad., et al. 2003 and Criado-Fornelio., et al. 2003). With the availability of sequenced parasite genes and PCR, it is possible to detect parasites within samples of blood (Bishop., et al. 1992; Tanaka., et al. 1993). PCR based technique uses small material which is very relevant because large amount of material is not possible from different stages of parasitic life cycle. (Gasser RB., et al. 2006). This technique reveals a high sensitivity compared to immunological examinations and serological testing (Shah D., et al. 1990). Furthermore, the advent of the polymerase chain reaction (PCR) technique has made it possible to increase the sensitivity of nuclear hybridization techniques, through amplification of target DNA sequences of the parasites in test material, by in situ synthesis of these sequences prior to hybridization with the diagnostic probe. Despite the benefits of PCR based technologies, such as high specificity and sensitivity to detect some parasites the main disadvantage of these methods is that they are very time consuming and do not provide quantitative data (Linn MH., et al. 2003).
b) Loop mediated isothermal amplification (LAMP): It is sensitive and specific (Parida M., et al. 2008) and less time consuming method. It is characterized by use of DNA polymerase that has low sensitivity to inhibitors and the set of four primers to recognize six different sequences on target gene (Paris DH., et al. 2007). It can amplify 109 copies in an hour (Notomi T., et al. 2000). It is isothermal technique which uses water bath. It has been used for Babesia and Theileria (Paris D.H., et al. 2007, Nkouawa A., et al. 2009, Bakheit M.A., et al. 2008, and Iseki H, 2007). It can be used without DNA extraction (Njiru Z.K., et al. 2008).
Treatment: There are three effective drugs available for the treatment of Theileriosis namely, parvaquone, buparvaquone, and halofuginone lactate are used worldwide (Ngumi., et al. 1992). Research work regarding the efficacy of these drugs has shown that buparvaquone, second-generation hydroxynaphthoquinone, is more effective so far. Early treatment with buparvaquone was 100% effective in eliminating the protozoan parasites from the blood and lymph nodes and led to an improvement in the clinical state whereas treatment in the later stages of the disease whilst eliminating the parasites failed to improve the clinical condition of the animal (Salama., et al. 2007).
Status of theileriosis in India:-
India being one of the 12 mega biodiversity country contribute significantly to world flora and fauna. As a result India with its tropical climate is hub of several vector borne diseases like bovine tropical theileriosis. T. annulata, the causative agent of tropical thcileriosis has a much wider distribution; it is found in Southern Europe, Northern Africa, and Egypt to the Sudan, the Middle East, India, parts of the former Soviet Union and southern China. T. annulata, originating from Asian water buffalo (Bulbulusbubulis), and transmitted by several Hyalomma tick species, is responsible for tropical theileriosis from Southern Europe to China, a vast region in which an estimated 250 million cattle are at risk. Livestock plays a critical role in the welfare of India. Indigenous cattle are resistant to this disease but cross bred cattle are highly sensitive to theileria (Nair., et al. 2011). The theileriaparasites have detrimental effect on the cows as it causes high mortality in the animals and there is an irreversible loss of production and reproduction. Theileria and Babesia both have same symptoms like high fever and both are fatal diseases, but in Babesia blood comes out with the urine and hence it is also known as Red water disease. Medicine for Babesia is easily available but for theileria it is not easily available as it is very costly. So small holder dairy farmers would prefer to run the risk of tropical theileriosis rather then they pay for the vaccines (Rushton., et al. 1990).
India being one of the 12 mega biodiversity country contribute significantly to world flora and fauna. As a result India with its tropical climate is hub of several vector borne diseases like bovine tropical theileriosis. T. annulata, the causative agent of tropical thcileriosis has a much wider distribution; it is found in Southern Europe, Northern Africa, and Egypt to the Sudan, the Middle East, India, parts of the former Soviet Union and southern China. T. annulata, originating from Asian water buffalo (Bulbulusbubulis), and transmitted by several Hyalomma tick species, is responsible for tropical theileriosis from Southern Europe to China, a vast region in which an estimated 250 million cattle are at risk. Livestock plays a critical role in the welfare of India. Indigenous cattle are resistant to this disease but cross bred cattle are highly sensitive to theileria (Nair., et al. 2011). The theileriaparasites have detrimental effect on the cows as it causes high mortality in the animals and there is an irreversible loss of production and reproduction. Theileria and Babesia both have same symptoms like high fever and both are fatal diseases, but in Babesia blood comes out with the urine and hence it is also known as Red water disease. Medicine for Babesia is easily available but for theileria it is not easily available as it is very costly. So small holder dairy farmers would prefer to run the risk of tropical theileriosis rather then they pay for the vaccines (Rushton., et al. 1990).
Serological surveys conducted indicated that 30-60% of cross bred cattle were positive for antibodies to T. annulata piroplasms, all over India, except in Himalayan regions, where climate is not favorable for tick activity. In India theileriosis has been reported from Punjab, Haryana, Gujarat etc. geographical regions. Anand., et al. (2009) reported the occurrence of T. Annulata among crossbred cattle in Bangalore north. Nair., et al. (2011) reported 16% positive cases of theileriosis in crossbred cattle of Northern Kerala. Vahora., et al. (2012) reported 37% cattle found positive for the haemoprotozoan infection in Kaira and Anand District of Gujrat. Samanta and Dutta (2012) also reported a case of tropical theileriosis from West Bengal. Mahajan., et al. (2013) has reported the outbreaks of theileriosis in cattle of Punjab with 4.86% mortality rate.
If animals suffering from tropical theileriosis are treated with anti-parasitic drugs, T. annulata is removed from lymph nodes and remains in blood at very low numbers (Ahmed and Mehlhorn, 1999; Glass, 2001; Salama and Magdy, 2007) and these animals becomes the carrier of parasites. In carrier animals blood smears are negative on microscopy (Aktas., et al. 2006). Carrier animals have an important role in the transmission of infection by the Hyalomma ticks (Dóliveira., et al. 1995). Negative microscopic examination does not exclude the possibility of infection (Weiland and Reiter, 1988).
Status of theileriosis in Uttar Pradesh:-
Livestock are the important part of the rural population. Uttar Pradesh is specialized in smallholder dairy production system. Cattle have been the important species in the herd. Due to moderate climate of the region, ticks responsible for the blood-borne diseases are not in the active form or found less. No earlier case of theileriosis is reported from the region. But to increase the milk production rate, cross bred cattle have been introduced in the Uttar Pradesh state from the neighboring states like Haryana, Punjab, and Rajasthanetc where these diseases are prominent and many of these animals may be the carriers. These animals are the source of infection. Infection is usually caused by tick that migrates from carrier animals to non-infected animals. Now some cases found positive for theileriosis in a preliminary survey. The reason for the occurrence of theileriosis is the introduction of carrier cattle to the herd of healthy animals. The stress due to extreme of climate may be the contributory factor.
Livestock are the important part of the rural population. Uttar Pradesh is specialized in smallholder dairy production system. Cattle have been the important species in the herd. Due to moderate climate of the region, ticks responsible for the blood-borne diseases are not in the active form or found less. No earlier case of theileriosis is reported from the region. But to increase the milk production rate, cross bred cattle have been introduced in the Uttar Pradesh state from the neighboring states like Haryana, Punjab, and Rajasthanetc where these diseases are prominent and many of these animals may be the carriers. These animals are the source of infection. Infection is usually caused by tick that migrates from carrier animals to non-infected animals. Now some cases found positive for theileriosis in a preliminary survey. The reason for the occurrence of theileriosis is the introduction of carrier cattle to the herd of healthy animals. The stress due to extreme of climate may be the contributory factor.
Infection by theileria limits the movement of cattle between the countries and can result in the production losses and high mortality in susceptible animals. Due to lack of sensitive diagnostic methods and lack of cost effective treatment for the detection of clinical cases and carrier animal majority of cattle positive for theileria are left untreated.
Present status of the animal diseases needs a serious attention in terms of research. Minjauw and Mcleod (2000) have estimated the cost of T. annulata in India to be $384.3 million. Vaccination against this disease is not practiced due to higher cost and non-availability. To reduce the chance of introducing the parasite first is essential screening should be done before introduction of the cross bred cows to the areas where the chance of occurrence of such disease is very low or introduced from the districts where infection is uncommon. The second is treat the cattle for ticks on arrival and don’t mix them with home cattle. So the aim is monitoring cattle for the blood protozoan through microscopic examination and by using Polymerase Chain Reaction (PCR). PCR monitors the presence of parasites which are not visible by microscopic examination and in the preclinical cases.
Effect on milk production
Theileria annulata infection was diagnosed as the cause of severely depressed milk yields in Friesian cows (Michael S A., et al. 1989). It was also found that cows of higher producing breeds were generally susceptive to the tick and the effect on milk production appeared to be greater (R.A.I. Norval, 1991).
Theileria annulata infection was diagnosed as the cause of severely depressed milk yields in Friesian cows (Michael S A., et al. 1989). It was also found that cows of higher producing breeds were generally susceptive to the tick and the effect on milk production appeared to be greater (R.A.I. Norval, 1991).
Effect on reproduction
Previous studies confirmed that theileriosis has an adverse effect on reproduction. Pregnant animals introduced to endemic bush tick areas are especially at risk and should be monitored carefully after introduction for signs of theileriosis. (Rumberia R M., et al. 1993).
Previous studies confirmed that theileriosis has an adverse effect on reproduction. Pregnant animals introduced to endemic bush tick areas are especially at risk and should be monitored carefully after introduction for signs of theileriosis. (Rumberia R M., et al. 1993).
Effect on thyroid hormone
A number of experimental conditions have been used to evaluate hormonal secretion during heat stress including short-term temperature modification using environmental chamber, seasonal comparisons of hormonal-profiles and the use of micro climatic modification during period of heat stress. Johnson and Vanjonack (1976) stated that the thyroid function in the lactating animals showed a general depression in the summer months and was normal or elevated during winter months.
A number of experimental conditions have been used to evaluate hormonal secretion during heat stress including short-term temperature modification using environmental chamber, seasonal comparisons of hormonal-profiles and the use of micro climatic modification during period of heat stress. Johnson and Vanjonack (1976) stated that the thyroid function in the lactating animals showed a general depression in the summer months and was normal or elevated during winter months.
It was also reported that thyroid hormones are affected in cases of tropical theileriosis caused by Theileriaannulata (Badiei and Jaber, 2002; Garg., et al. 2001; Sangwan., et al. 2002). It is stated that thyroid hormones, which affect growth, development, energy and efficiency metabolisms necessary for the development and normal functioning of many cells (Guyton, 1986; Sanli, 1999; Turgut, 2000), are closely associated with the regulation of oxygen consumption (Guyton, 1986; Sawhney and Malhotra, 1990; Sanli, 1999). Sangwan., et al. (2000) and Garg., et al. (2001) reported that thyroid hormones decrease in tropical theileriosis.
Conclusion
A future strategy of dual vaccination with tick antigen and associated recombinant T. annulata antigen can aid to achieve integrated protection in host.
References
- Aktas M., et al. “Field evaluation of PCR in detecting Theileria annulata infections in cattle in the east of Turkey”. Veterinary Record 94.4 (2001): 413-423.
- Badiei K and M Jaber. Changes of hormones (T3, T4 and Cortisol) in Theileriaannulata infected cattle. Proceedings of the 22th World Buiatrics Congress, Nov. 18-23, Germany. (2002): 346-359.
- Bakheit MA., et al. “Sensitive and specific detection of Cryptosporidium species in PCR-negative samples by loop-mediated isothermal DNA amplification and confirmation of generated LAMP products by sequencing”. Veterinary Parasitology 158.1 (2008): 11-22.
- Balha T. “Applied Veterinary Epidemiology 1st Edition.” Elsevier Science Publishers (1989).
- Birkenheurer A., et al. “Development and evalution of a semi nested PCR for detection and differentiation of Babesiagibsoni (Asian genotype) and B. Canis DNA in canine blood samples”. Journal of Clinical Microbiology 41.9 (2004): 4172-4177.
- Burridges MJ., et al. “Theileriaannulata: cross reaction between a cell culture schizont antigen and antigen of east African Theileria species in the indirect fluorescent antibody test”. Experimental Parasitol 35.3 (1994): 374-380.
- Criado-Fornelio A., et al. “Molecular studies on Babesia, Theileria a Hepatozoon in Southern Europe. Part I.Epizootiological aspects”. Veterinary Parasitology 113.3 (2003): 189-01.
- Cytauxzoonoses: a review. Onderstepoort Journal of Veterinary Research.
- Figueroa JV., et al. “Multiplex polymerase chain reaction based assay for the detection of Babesiabigemina, Babesia bovis, and Anaplasma marginale DNA in bovine blood”. Veterinary Parasitology 50.1 (1993): 69–81.
- Garg S L., et al. “Plasma cortisol and thyroid hormone concentration in cross bred cow calves affected with theleriosis”. Indian Veterinary Journal 78.7 (2001): 583-585.
- Gasser R B and Gasser RB. “Molecular tools – advances, opportunities and prospects”. Veterinary Parasitology 136. 2 (2006): 69-89.
- Government of Uttar Pradesh, Luck now, Irrigation Department Uttar Pradesh. "Average rainfall pattern of Uttar Pradesh". 2012.
- Guyton AC. 1986. Textbook of medical physiology 7thEdn. W.B. Saunders Company, Philadelphia, USA.
- Iseki H., et al. “Development of a multiplex loop-mediated isothermal amplification (mLAMP) method for the simultaneous detection of bovine Babesiaparasites”. Journal of Microbiological Methods 71.3 (2007): 281-287.
- Jithendran KP. “Blood protista of cattle and buffaloes in Kangra valley, Himachal Pradesh”. The Indian Journal of Animal Sciences 67 (1997): 207-208.
- Johnson HD and Vanjonack WJ. “Effects of environmental and other stressors on blood hormone patterns in lactating animals “. Journal of Dairy Science 59.9 (1976): 1603- 1617.
- Lin MH., et al. “Real-time PCR for quantitative detection of Toxoplasma gondii”. Journal of Clinical Microbiology 38.11 (2000): 4121-4125.
- Meenakshisundaram A., et al. “Concomitant theileriaannulata and anaplasmamarginale infections in a cross bred dairy herd”. Indian Journal of Animal Health 43.6 (2014): 422 - 425.
- Michael SA., et al. “Effect of treatment of chronic theileriosis with buparvaquone on milk yields”. Tropical Animal Health and Production 21.4 (1989): 218-222.
- Moorhous., et al. “The epidemiology of bovine theileriosis in Zambia: results of a longitudinal study in Southern Province. In: Proceedings of the 4thInternational Symposium on Veterinary Epidemiology and Economics. Singapore”. Singapore Veterinary Association (2001): 389-391.
- Musoke AJ., et al. “A recombinant sporozoit surface antigen of Theileria parvainduced protection in cattle”. Proceedings of the National Academy of Science 89.2 (2001): 514-519.
- Nair AS. “Haemoprotozoan of cattle in Northern Kerala, India”. Tropical Biomedicine 28.1 (2011): 68-75.
- Neitz WO. “Theileriosis, gonderioses and cytauxzoonoses.” Journal of Veterinary Research 27.3 (1957): 275-430.
- Ngumi., et al. “Isolation and preliminary characterization of a previously unidentified Theileria parasite of cattle in Kenya”. Research in Veterinary Science 57.1 (1994): 1–9.
- Njiru ZK., et al. “African trypanosomiasis: sensitive and rapid detection of the sub-genus Trypanozoon by loop-mediated isothermal amplification (LAMP) of parasite DNA”. International Journal for Parasitology 38.5 (2008): 589-599.
- Nkouawa A., et al. “Loop-mediated isothermal amplification method for differentiation and rapid detection of Taenia species”. Journal of Clinical Microbiology 47.1 (2009): 168-174.
- Norval RAI., et al. “Theileria parva: influence of vector, parasite and host relationships on the epidemiology of theileriosis in southern Africa”. Parasitology 102 (1991): 347-356.
- Notomi T., et al. “Loop-mediated isothermal amplification of DNA”. Nucleic Acids Research 28.12 (2000): E63.
- Parida M., et al. “Loop mediated isothermal amplification (LAMP): a new generation of innovative gene amplification technique; perspectives in clinical diagnosis of infectious diseases”. Reviews in Medical Virology 18.6 (2008): 407-421.
- Paris DH., et al. “Loop-mediated isothermal PCR (LAMP) for the diagnosis of falciparum malaria”. The American Journal of Tropical Medicine and Hygiene 77.5 (2007): 972-976.
- Pipano E and Shkap V. “Vaccination against tropical theileriosis”. Annals of the New York Academy of Sciences 916 (2000): 484–500.
- Rampersad J., et al.” field evalution of PCR for the routine detection of Babesiaequi in horses”. Veterinary Parasitology 114.2 (2003): 81-87.
- Robinson PM. “Theileriosis annulata and its transmission-a review”. Tropical Animal Health and Production 14.1 (1982): 3-12.
- Rumberia RM., et al. “The effect of high and low dose Theileria parva infection on the reproductive function of Boran/Friesian heifers”. Theriogenology 40.5 (1993): 977-986.
- Salama AO and Gaabarya MHA. “Clinical, haematological and therapeutic studies on tropical theileriosis in water buffaloes (Bubalusbubalis) in Egypt.” Veterinary Parasitology146.3 (2007): 337-340.
- Sangwan N., et al. “Cortisol and thyroid hormones in relation to bovine tropical theileriosis”. Indian Journal of Animal Science 72.12 (2002): 1098-1099.
- Sanli Y. “Veterinary Clinic Pharmacology and Medicine Therapy Principles. 3rdEdn.Ozkan Matbaacilik Ltd., Ankara (1999).
- Sawhney RC and AS. “Thyroid function during intermittent exposure to hypobaric hypoxia”. International Journal of Biometeorology 34.3 (1990): 161-163.
- Tait A and Hall FR. Theileriaannulata: control measures, diagnosis and the potential use of subunit vaccines. Revue Scientifique ET Technique 9.2 (1990): 387- 403.
- Turgut K. “Veterinary Clinic Laboratory Diagnosis. 2ndEdn”. Printer Gardeners, Konya. (2000).
- UpkarPrakashan - Editorial Board (2008). Uttar Pradesh General Knowledge. UpkarPrakashan. pp. 26–. ISBN 978-81-7482-408-0. 2011.
Citation:
Sachin Kumar., et al. “Current Epidemiological Status of Bovine Theileriosis in North-Western States of India”. Multidisciplinary
Advances in Veterinary Science 2.1 (2018): 293-300.
Copyright: © 2018 Sachin Kumar., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.





























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.