Review Article
Volume 3 Issue 1 - 2018
An Overview of some Major Mycotoxins in Food and their Detection Methods
1Centre for Food and Nutrition Research, IMPM, PO Box 6163 Yaoundé, Cameroon
2Department of Biochemistry, University of Yaoundé I, PO Box 812, Yaoundé, Cameroon
3Department of Microbiology, University of Yaoundé I, PO Box 812, Yaoundé, Cameroon
2Department of Biochemistry, University of Yaoundé I, PO Box 812, Yaoundé, Cameroon
3Department of Microbiology, University of Yaoundé I, PO Box 812, Yaoundé, Cameroon
*Corresponding Author: Evelyne Nguegwouo, Centre for Food and Nutrition Research, IMPM, PO Box 6163 Yaoundé, Cameroon.
Received: May 28, 2018; Published: June 09, 2018
Abstract
Molds are one of the main groups of microorganisms associated with food spoilage and postharvest food losses. During their growth on food and feed commodities, some of them produce secondary metabolites with a potentially toxic effect on humans and animals known as “mycotoxins”. These compounds which are known for their carcinogenic, teratogenic, mutagenic and immune-toxic effects have become a global concern. Many associated outbreaks have been reported. International food trade nowadays obeys strict legislations on their presence in food in order to protect consumers’ health. From an economic and sanitary point of view, the most important or reported agriculture-oriented mycotoxins are Aflatoxins, Fumonisins, Ochratoxin A, Zearalenone and Trichothecenes. This review describes these major toxins that are associated to food commodities and the analytical methods available nowadays for their detection in the interest of researchers, food manufacturers, laboratory managers or anyone else concerned.
Keywords: Foods; Major mycotoxins; Analysis; Quantification
Introduction
Fungal contamination constitutes a major problem for food preservation all over the world, this especially as it usually leads to their spoilage. The ability of molds to grow on crops is one of greatest causes of post-harvest losses. Factors influencing this fungal growth and sporulation include the nature of the substrate, humidity and climate [1]. Substrates differ in their ability to support this growth due to the variability of their physicochemical characteristics (water activity aw, nutrient content like carbohydrates, fat, proteins, trace elements and amino acid composition). Moisture content is another key factor determining this growth [2]. It enables molds to break down complex macromolecular compounds for their growth and metabolism. Excess of humidity in the field of storage, high temperature, drought, oxygen availability and insect infestations are some of the major environmental factors that determine the severity of fungal contamination [3].
During their growth, some of these fungal contaminants may produce and secrete toxic secondary metabolites called “mycotoxins”. Indeed, from the Greek, “Mycos” which means mushroom and Latin, “toxicum” which means poison, the term mycotoxin refers to some chemical substances produced by fungi developing on foodstuffs, mainly of plant origin [4,5]. These substances can be harmful both for humans and animals. Indeed, when absorbed even in small amounts, these substances can lead to an acute or chronic disease termed mycotoxicoses [1]. They have been reported as carcinogens, mutagens (genotoxic), teratogens, or immuno-toxins based on some effects observed on the liver, kidney, lungs, and the nervous, endocrine and immune systems [6,7]. The International Agency for Research on Cancer (IARC) has classified them into 5 groups: Group 1 (human carcinogen); Group 2A (probable human carcinogen; Group 2B (possible human carcinogen), Group 3 (inadequate information); Group 4 (No evidence). Such products are also toxic for some plants (phytotoxins) or for other microorganisms (antibiotics for bacteria) [6,8]. In contrast to bacterial toxins, which are mainly proteins with antigenic properties, mycotoxins are a variety of low-molecular-weight compounds with diverse chemical structures and biological activities.
The presence of molds in food or feed products does not necessarily suggest the presence of mycotoxins. In fact, not all molds are toxigenic and not all secondary metabolites from molds are toxic. Furthermore, these toxins are only produced under certain conditions. Generally, stressful conditions for fungal growth (acidic conditions, low temperatures, stressful aw, nitrogen-starving conditions and oxidative stress) enhance production of mycotoxins [9,10]. Examples of mycotoxins of greatest public health and agro-economic significance include aflatoxins, ochratoxins, trichothecenes, zearalenone, fumonisins, deoxynivalenol, nivalenol and T-2 Toxin; tetramic acids and the ergot alkaloids. In monetary terms, these toxins account for millions of US dollars lost annually worldwide due to their negative impact on human health, animal productivity and agricultural products trade [11]. Cases of food destruction due to high mycotoxin levels have been reported [11]. The Food and Agricultural Organization has estimated that at least 25% of the world food crops are significantly contaminated with mycotoxins per year [12]. In order to limit the health risks that can be associated to their presence in food, strict standards on the maximum tolerable concentration of different mycotoxins in foods have been fixed at both national and international levels. This review describes the major toxins that are associated to food commodities and the analytical methods available for their detection.
The major mycotoxins in foods
Out of the 400 mycotoxins produced by more than 100 fungal species [1,13], the five most agriculturally-important fungal toxins are Aflatoxins (AFs) Fumonisins (FUMs), Ochratoxins, Zearalenone (ZEA) and Trichothecenes especially Deoxynivalenol (DON) [13].Aflatoxins are the most present in African countries, followed by Fumonisins, Ochratoxins, Zearalenone and Deoxynivalenol [14].
Out of the 400 mycotoxins produced by more than 100 fungal species [1,13], the five most agriculturally-important fungal toxins are Aflatoxins (AFs) Fumonisins (FUMs), Ochratoxins, Zearalenone (ZEA) and Trichothecenes especially Deoxynivalenol (DON) [13].Aflatoxins are the most present in African countries, followed by Fumonisins, Ochratoxins, Zearalenone and Deoxynivalenol [14].
Aflatoxins
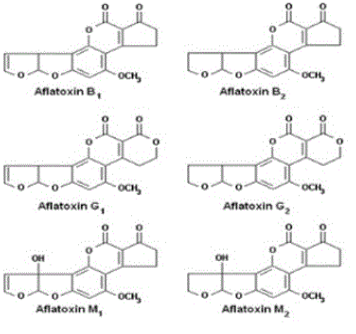
AFs were first discovered in the 1960s when moulded peanuts were identified as the cause of a disease called aflatoxicosis, which killed turkeys, ducks and pheasants [15]. They are the most studied group of mycotoxins. AFs are mainly produced by different species of the genus Aspergillus such as Aspergillus flavus, A. parasiticus, A. nomius, A. arachidicola and in some cases by those of the genus Emericella like Emericella astellata, E. venezuelensis, E. olivicola [16,17]. Their production is influenced by environmental factors such as moisture content and temperature. The optimal temperature and water activity for their production were reported to be 33°C and 0.99, respectively [2]. There are about 20 types of AFs with the most important being Aflatoxin B1 (AFB1), Aflatoxin B2 (AFB2), Aflatoxin G1 (AFG1), Aflatoxin G2 (AFG2), Aflatoxin M1 (AFM1) and Aflatoxin M2 (AFM2). Their chemical structures are presented in (Figure 1). Concerning their physicochemical properties, AFs are chemical compounds which appear as colourless to pale-yellow crystals. They can be classified according to the fluorescence they emit in presence of ultraviolet (UV) light. AFs of group B (B1, B2) appear blue, those of group G (G1, G2) appear green and those of group M (M1, M2) appear blue-violet. They are heat tolerant, insoluble in non-polar organic solvents, slightly soluble in water and moderately in polar organic solvents [18]. These toxins generally occur in foodstuffs like corn, peanuts and derived products, cotton seeds, peppers, rice, pistachios, tree nuts (Brazilian nuts, almonds, pecans), sunflower seeds and other oil seeds, copra, spices, dried fruits (figs, raisins) and yams [7]. Their reported adverse effects on human and animal health are their hepatocarcinogenic, genotoxic, carcinogenic, immunosuppressive and oncogenic abilities [16,17,19]. The IARC has classified AFB and AFM1 in Group 1 and Group 2B, respectively. In 1969, FDA had fixed 20 µg/kg as maximum level of AFs for all foodstuffs [7]. Nowadays, more than 50 countries have formulated their own legislation on the maximum permitted levels of AFs in food and feeds. They range from 0 to 50 μg/kg of food [20,21]. For instance, the level of AFM1 which is mainly found in milk intended for human consumption has been fixed at 0.05-0.5 μg/kg [12].
AFs were first discovered in the 1960s when moulded peanuts were identified as the cause of a disease called aflatoxicosis, which killed turkeys, ducks and pheasants [15]. They are the most studied group of mycotoxins. AFs are mainly produced by different species of the genus Aspergillus such as Aspergillus flavus, A. parasiticus, A. nomius, A. arachidicola and in some cases by those of the genus Emericella like Emericella astellata, E. venezuelensis, E. olivicola [16,17]. Their production is influenced by environmental factors such as moisture content and temperature. The optimal temperature and water activity for their production were reported to be 33°C and 0.99, respectively [2]. There are about 20 types of AFs with the most important being Aflatoxin B1 (AFB1), Aflatoxin B2 (AFB2), Aflatoxin G1 (AFG1), Aflatoxin G2 (AFG2), Aflatoxin M1 (AFM1) and Aflatoxin M2 (AFM2). Their chemical structures are presented in (Figure 1). Concerning their physicochemical properties, AFs are chemical compounds which appear as colourless to pale-yellow crystals. They can be classified according to the fluorescence they emit in presence of ultraviolet (UV) light. AFs of group B (B1, B2) appear blue, those of group G (G1, G2) appear green and those of group M (M1, M2) appear blue-violet. They are heat tolerant, insoluble in non-polar organic solvents, slightly soluble in water and moderately in polar organic solvents [18]. These toxins generally occur in foodstuffs like corn, peanuts and derived products, cotton seeds, peppers, rice, pistachios, tree nuts (Brazilian nuts, almonds, pecans), sunflower seeds and other oil seeds, copra, spices, dried fruits (figs, raisins) and yams [7]. Their reported adverse effects on human and animal health are their hepatocarcinogenic, genotoxic, carcinogenic, immunosuppressive and oncogenic abilities [16,17,19]. The IARC has classified AFB and AFM1 in Group 1 and Group 2B, respectively. In 1969, FDA had fixed 20 µg/kg as maximum level of AFs for all foodstuffs [7]. Nowadays, more than 50 countries have formulated their own legislation on the maximum permitted levels of AFs in food and feeds. They range from 0 to 50 μg/kg of food [20,21]. For instance, the level of AFM1 which is mainly found in milk intended for human consumption has been fixed at 0.05-0.5 μg/kg [12].
Fumonisins
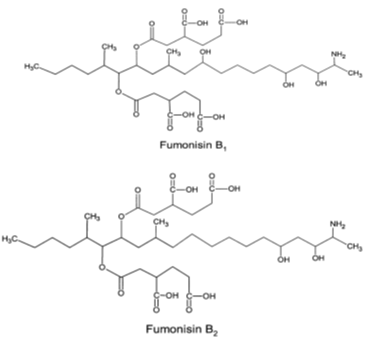
FUMs are mycotoxins produced by some fungal species of the genus Fusarium, including the maize pathogens Fusarium verticillioides and Fusarium proliferatum. Their optimal production was described at aw 0.9–0.995 and temperature15-30°C [2-22]. They were discovered in Southern Africa as the cause of oesophageal cancer [23]. These mycotoxins are a structurally related group of diesters of propane-1,2,3-tricarboxylic acid and various 2-amino-12,16-dimethylpolyhydroxyeicosanes in which the C14 and C15 hydroxyl groups are esterified with the terminal carboxyl group of tricarboxylic acid. More than nine structurally related fumonisins are found in nature, including FB1, FB2, FB3, FB4, FA1 and FA2. Among these diverse FUMs, those of the B series (FB1 and FB2) are the most abundant and most toxic naturally occurring analogues [24]. The (Figure 2) presents their chemical structures. Fumonisins appear as a white hygroscopic powder [25]. They are soluble in water, acetonitrile–water or methanol, and insoluble in chloroform and hexane [18]. Being heat stable compounds [26], FUMs were also notified as stable in buffer solutions over the pH range 4.8–9 at 78°C [2]. They are most frequently found in maize, maize-based foods and other grains (such as sorghum and rice) but peanuts and soybeans are poor substrates. The level of contamination varies considerably from one region to another, with values ranging from negligible to more than 100 ppm. Fumonisin B1 (FB1) is the most common fumonisin in naturally contaminated samples; FB2 generally accounts for 1/3 or less of the total [27]. Several harmful effects of FUMs on human and animal health including esophageal and liver carcinogens, neurotoxicity and genotoxicity have been reported in literature [28,29]. FB1 and FB2 are classified by the IARC in the Group 2B. To avoid their negative impact, fumonisins maximum acceptable level in food and feed has been set at 0.2-4 µg/kg by European Commission (EC) [30] and 2-4 µg/kg by Food and Drug Administration (FDA) [31].
FUMs are mycotoxins produced by some fungal species of the genus Fusarium, including the maize pathogens Fusarium verticillioides and Fusarium proliferatum. Their optimal production was described at aw 0.9–0.995 and temperature15-30°C [2-22]. They were discovered in Southern Africa as the cause of oesophageal cancer [23]. These mycotoxins are a structurally related group of diesters of propane-1,2,3-tricarboxylic acid and various 2-amino-12,16-dimethylpolyhydroxyeicosanes in which the C14 and C15 hydroxyl groups are esterified with the terminal carboxyl group of tricarboxylic acid. More than nine structurally related fumonisins are found in nature, including FB1, FB2, FB3, FB4, FA1 and FA2. Among these diverse FUMs, those of the B series (FB1 and FB2) are the most abundant and most toxic naturally occurring analogues [24]. The (Figure 2) presents their chemical structures. Fumonisins appear as a white hygroscopic powder [25]. They are soluble in water, acetonitrile–water or methanol, and insoluble in chloroform and hexane [18]. Being heat stable compounds [26], FUMs were also notified as stable in buffer solutions over the pH range 4.8–9 at 78°C [2]. They are most frequently found in maize, maize-based foods and other grains (such as sorghum and rice) but peanuts and soybeans are poor substrates. The level of contamination varies considerably from one region to another, with values ranging from negligible to more than 100 ppm. Fumonisin B1 (FB1) is the most common fumonisin in naturally contaminated samples; FB2 generally accounts for 1/3 or less of the total [27]. Several harmful effects of FUMs on human and animal health including esophageal and liver carcinogens, neurotoxicity and genotoxicity have been reported in literature [28,29]. FB1 and FB2 are classified by the IARC in the Group 2B. To avoid their negative impact, fumonisins maximum acceptable level in food and feed has been set at 0.2-4 µg/kg by European Commission (EC) [30] and 2-4 µg/kg by Food and Drug Administration (FDA) [31].
Ochratoxins
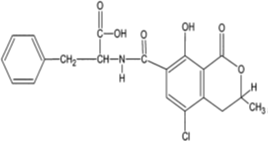
Ochratoxins are chemically described as 3,4-dihydromethylisocoumarin derivatives linked with an amide bond to the amino group of L-β-phenylalanine [32]. They are mainly produced by Aspergillus melleus, A. alutaceus, Penicillium verrucosum, A. ochraceus, A. carbonarius, A. alliaceus, A. albertensis, P. carbonarius [17]. The optimum ecological conditions for Ochratoxin A (OTA) production have been described at temperature 15–30°C and aw 0.98 – 0.99 [2]. Although a wide range of ochratoxin derivatives have been isolated from grains or laboratory cultures of the above-mentioned molds, only OTA (Figure 3) and in extremely rare cases, Ochratoxin B (OTB) and Ochratoxin C (OTC) have been found to occur naturally [33]. Ochratoxin A was first isolated in the mid 1960s in South Africa during laboratory studies in search of new toxic metabolites from A. ochraceus [34] and was later shown as a secondary metabolite of Penicillium spp. in temperate climates [32]. Being a white odourless crystalline solid, OTA in acid or alkaline solutions emits green and blue fluorescence under UV-light. This compound is slightly soluble in water and moderately soluble in polar organic solvents like chloroform, ethanol and methanol [32]. Its stability at temperatures up to 180°C has also been demonstrated [35]. Ochratoxins are often detected in cereals including maize, beans, rice, wheat, rye, oats, barley, coffee, cocoa, pulses, grapes, wine, spices and all kinds of commodities of animal origin [32-37]. OTA is the most prevalent mycotoxin of all ochratoxins [29]. It is a hepatotoxic, mutagenic, teratogenic, neurotoxic and immunotoxic compound [17-38]. The IARC has classified it in Group 2B [8]. In the European Union, OTA maximum acceptable level in cereals, dry fruits, wine, spices, oat, raisins, coffee, cocoa, soybeans and meat has been fixed at 0.5–10 µg/kg [39]. In USA, FDA defined the maximum acceptable level in food intended for human and animal consumption at 4–20 µg/kg [31].
Ochratoxins are chemically described as 3,4-dihydromethylisocoumarin derivatives linked with an amide bond to the amino group of L-β-phenylalanine [32]. They are mainly produced by Aspergillus melleus, A. alutaceus, Penicillium verrucosum, A. ochraceus, A. carbonarius, A. alliaceus, A. albertensis, P. carbonarius [17]. The optimum ecological conditions for Ochratoxin A (OTA) production have been described at temperature 15–30°C and aw 0.98 – 0.99 [2]. Although a wide range of ochratoxin derivatives have been isolated from grains or laboratory cultures of the above-mentioned molds, only OTA (Figure 3) and in extremely rare cases, Ochratoxin B (OTB) and Ochratoxin C (OTC) have been found to occur naturally [33]. Ochratoxin A was first isolated in the mid 1960s in South Africa during laboratory studies in search of new toxic metabolites from A. ochraceus [34] and was later shown as a secondary metabolite of Penicillium spp. in temperate climates [32]. Being a white odourless crystalline solid, OTA in acid or alkaline solutions emits green and blue fluorescence under UV-light. This compound is slightly soluble in water and moderately soluble in polar organic solvents like chloroform, ethanol and methanol [32]. Its stability at temperatures up to 180°C has also been demonstrated [35]. Ochratoxins are often detected in cereals including maize, beans, rice, wheat, rye, oats, barley, coffee, cocoa, pulses, grapes, wine, spices and all kinds of commodities of animal origin [32-37]. OTA is the most prevalent mycotoxin of all ochratoxins [29]. It is a hepatotoxic, mutagenic, teratogenic, neurotoxic and immunotoxic compound [17-38]. The IARC has classified it in Group 2B [8]. In the European Union, OTA maximum acceptable level in cereals, dry fruits, wine, spices, oat, raisins, coffee, cocoa, soybeans and meat has been fixed at 0.5–10 µg/kg [39]. In USA, FDA defined the maximum acceptable level in food intended for human and animal consumption at 4–20 µg/kg [31].
Zearalenone
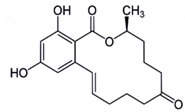
Previously known as F2 toxin [40], the toxin zearalenone (ZEA) is produced by F. graminearum, F. culmorum, F. equiseti, F. poae and some other Fusariumspecies, which frequently colonized wheat, barley maize and maize-based products worldwide [33,40]. Its production mainly occurs when temperature is between 15-25°C and aw between 0.95-0.96 [2-41]. ZEA [6-(10-hydroxy-6-oxo-trans-1-undecenyl) β-resorcylic-acid-lactone] (Figure 4) is a white crystal compound soluble in alkaline solutions, ether, benzene, acetonitrile, ethyl alcohol and insoluble in water. It was also reported to be heat stable [42]. The natural occurrence of ZEA in a variety of agricultural commodities has been extensively reviewed. The most suitable substrates for zearalenone production were reported by Bennett and Klich [43] as wheat and rice. This compound decreases fertility and provokes precocious puberty, breast cancer, endometrial carcinoma and hyperplasia of uterus [44]. ZEA also causes serious oestrogenic disorders, such as cervical cancer due to its mimic effect with 17-beta-oestradiol. This mycotoxin was classified by the IARC in the Group 3 [8]. Its maximum acceptable level has been established at 200 µg/kg for corn and 100 µg/kg for unprocessed cereals by EC [45]. In cereal snacks, breakfast cereals and processed cereal-based foods, and baby foods, this value was fixed at 50 µg/kg, 50 µg/kg, and 20 µg/kg, respectively [39].
Previously known as F2 toxin [40], the toxin zearalenone (ZEA) is produced by F. graminearum, F. culmorum, F. equiseti, F. poae and some other Fusariumspecies, which frequently colonized wheat, barley maize and maize-based products worldwide [33,40]. Its production mainly occurs when temperature is between 15-25°C and aw between 0.95-0.96 [2-41]. ZEA [6-(10-hydroxy-6-oxo-trans-1-undecenyl) β-resorcylic-acid-lactone] (Figure 4) is a white crystal compound soluble in alkaline solutions, ether, benzene, acetonitrile, ethyl alcohol and insoluble in water. It was also reported to be heat stable [42]. The natural occurrence of ZEA in a variety of agricultural commodities has been extensively reviewed. The most suitable substrates for zearalenone production were reported by Bennett and Klich [43] as wheat and rice. This compound decreases fertility and provokes precocious puberty, breast cancer, endometrial carcinoma and hyperplasia of uterus [44]. ZEA also causes serious oestrogenic disorders, such as cervical cancer due to its mimic effect with 17-beta-oestradiol. This mycotoxin was classified by the IARC in the Group 3 [8]. Its maximum acceptable level has been established at 200 µg/kg for corn and 100 µg/kg for unprocessed cereals by EC [45]. In cereal snacks, breakfast cereals and processed cereal-based foods, and baby foods, this value was fixed at 50 µg/kg, 50 µg/kg, and 20 µg/kg, respectively [39].
Trichothecenes
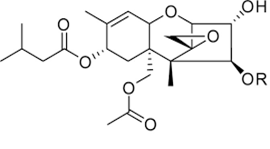
Trichothecenes are a group of mycotoxins mainly produced by molds belonging to the Fusarium genus like Fusarium graminearum, F. culmorum, F. crookwellense, F. sporotrichioides, F. poae, F. tricinctum and F. acuminatum[17]. Other trichothecenes producing species are Cephalosporium sp., Myrothecium sp., Trichoderma sp., Trichothecium sp., and Phomopsis sp. [17]. Trichothecenes consist of a group of closely related compounds designated as sesquiterpenoids. They have a common 12,13-epoxytrichothene skeleton and an olefinic bond with various side chain substitutions [43]. Examples of trichothecenes include T-2 and HT-2 toxins, Diacetoxyscirpenol, Deoxynivalenol (also known as Vomitoxin) and Nivalenol. T-2 and HT-2 toxins (Figure 5) are classified in group A of Trichothecenes. The T-2 toxin was discovered in 1968 from F. tricinctum [46]. The production of these toxins has also been reported with Fusarium sporotrichioides, F. poae, F. equiseti, and F. acuminatum [47]. The optimal aw and temperature conditions for T-2 and HT-2 were reported by Medina and Magan [48] to be between 0.98-0.995 and 20-30ºC, respectively. They are highly soluble in ethyl acetate, acetone, chloroform, dichloromethane and diethyl ether [18]. Toxin T-2 and HT-2 are mostly found in grains like wheat, maize, oats, barley, rice, beans, and soya beans as well as in some cereal-based products [49]. Consumption of food or feed contaminated with T-2 or HT-2 leads to genotoxic effects, cell apoptosis as well as immunodepression [50]. Richard [45] observed that in response to T-2 toxin exposure, experimental animals and livestock developed vomiting, diarrhea, loss of appetite, weight loss, and hemorrhages. Bennett & Klich [43] reported necrosis in the oral cavity, bleeding from the nose, mouth and vagina, and central nervous system disorders in animal exposed to T-2 toxin. Toxins T-2 and HT-2 were classified by IARC in group 3 [8]. The regulation limit of toxin T-2 in food was defined at 100 µg/kg in Russia for food grains, including wheat, rye, triticale, oats, barley, millet, buckwheat, rice, corn, sorghum, oatmeal, flakes, and wheat flour including pasta. In China, this limit is fixed at 80 µg/kg [29].
Trichothecenes are a group of mycotoxins mainly produced by molds belonging to the Fusarium genus like Fusarium graminearum, F. culmorum, F. crookwellense, F. sporotrichioides, F. poae, F. tricinctum and F. acuminatum[17]. Other trichothecenes producing species are Cephalosporium sp., Myrothecium sp., Trichoderma sp., Trichothecium sp., and Phomopsis sp. [17]. Trichothecenes consist of a group of closely related compounds designated as sesquiterpenoids. They have a common 12,13-epoxytrichothene skeleton and an olefinic bond with various side chain substitutions [43]. Examples of trichothecenes include T-2 and HT-2 toxins, Diacetoxyscirpenol, Deoxynivalenol (also known as Vomitoxin) and Nivalenol. T-2 and HT-2 toxins (Figure 5) are classified in group A of Trichothecenes. The T-2 toxin was discovered in 1968 from F. tricinctum [46]. The production of these toxins has also been reported with Fusarium sporotrichioides, F. poae, F. equiseti, and F. acuminatum [47]. The optimal aw and temperature conditions for T-2 and HT-2 were reported by Medina and Magan [48] to be between 0.98-0.995 and 20-30ºC, respectively. They are highly soluble in ethyl acetate, acetone, chloroform, dichloromethane and diethyl ether [18]. Toxin T-2 and HT-2 are mostly found in grains like wheat, maize, oats, barley, rice, beans, and soya beans as well as in some cereal-based products [49]. Consumption of food or feed contaminated with T-2 or HT-2 leads to genotoxic effects, cell apoptosis as well as immunodepression [50]. Richard [45] observed that in response to T-2 toxin exposure, experimental animals and livestock developed vomiting, diarrhea, loss of appetite, weight loss, and hemorrhages. Bennett & Klich [43] reported necrosis in the oral cavity, bleeding from the nose, mouth and vagina, and central nervous system disorders in animal exposed to T-2 toxin. Toxins T-2 and HT-2 were classified by IARC in group 3 [8]. The regulation limit of toxin T-2 in food was defined at 100 µg/kg in Russia for food grains, including wheat, rye, triticale, oats, barley, millet, buckwheat, rice, corn, sorghum, oatmeal, flakes, and wheat flour including pasta. In China, this limit is fixed at 80 µg/kg [29].
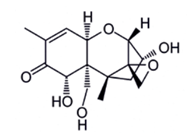
In contrast to T-2 or HT-2 toxin, Deoxynivalenol (DON) belongs to the type B of Trichothecenes [18], which also includes other toxins like 3- and 15-Acetyldeoxynivalenol and Nivalenol [50]. This compound is soluble in chloroform, ethanol, methanol and ethyl acetate. Highly stable to heat treatment, DON (figure 6) was also notified by Lauren and Smith [42] as relatively stable in buffer solutions over the pH range 1–10. This toxin is the most common Trichothecene. It is commonly found world-wide in cereals such as maize, wheat, barley and oat [51]. Temperatures between 15 and 30ºC and aw between 0.95 and 0.99 are the most suitable for its production [2-41]. This toxin has been reported to cause immunodepression, anorexia, leukaemia, rectal bleeding, and diarrhoea [29-52]. Its genotoxic effect and its ability to inhibit the synthesis of nucleic acids and proteins, cell division and mitochondrial function as well as destabilize cell membranes were also reported [50]. As toxins T-2, HT-2 and Nivalenol, DON is classified by the IARC in Group 3 [8]. An amount of 500 µg/kg for bread, other bakery products and breakfast cereals and 1750 µg/kg for wheat, oats and unprocessed cornare the maximum values tolerated [30].
Assessment of the occurrence of mycotoxins in foods
Analysis of mycotoxins in food matrices globally follows four steps: sampling, extraction, purification and analysis. Each of these steps determines the quality of the final results.
Analysis of mycotoxins in food matrices globally follows four steps: sampling, extraction, purification and analysis. Each of these steps determines the quality of the final results.
Sampling
Since mycotoxinogenic filamentous fungi do not grow uniformly on the food matrix they colonized, the mycotoxins they produce during this growth are also not homogenously distributed through the contaminated food [5]. Hence, collecting samples for mycotoxin analysis is a critical step which significantly impacted the final results. Many sampling approaches have been developed to make the final results as representative as possible [53-56]. Norms taking in consideration the weight of the lot and the type of food were established. The most used norms reported in literature are those of the European commission (EC) No 401/2006 of 2006, revised in 2014 [57-58].
Since mycotoxinogenic filamentous fungi do not grow uniformly on the food matrix they colonized, the mycotoxins they produce during this growth are also not homogenously distributed through the contaminated food [5]. Hence, collecting samples for mycotoxin analysis is a critical step which significantly impacted the final results. Many sampling approaches have been developed to make the final results as representative as possible [53-56]. Norms taking in consideration the weight of the lot and the type of food were established. The most used norms reported in literature are those of the European commission (EC) No 401/2006 of 2006, revised in 2014 [57-58].
Extraction
Due to their micro (ppm) or nano (ppb) quantities in foods, mycotoxins are usually firstly extracted from samples before analysis. Two methods are generally used: Liquid-Liquid Extraction (LLE) for liquid samples like milk, wine, juice [1] or Solid-Liquid Extraction (SLE) for solid samples like cereals, leguminous and other solid materials [59-60]. For both LLE and SLE techniques, many factors affect the mycotoxin extraction yield. The most important parameter is the solvent used which should have a great affinity with the specific mycotoxins chosen and little affinity with interfering compounds. During extraction, mycotoxins will move from the matrix to the extraction solvent until an equilibrium is established. Organic solvents like ethyl acetate, methanol, chloroform, acetonitrile, acetone, dichloromethane or a mixture of these solvents are the most commonly used [36-61]. In most cases, extraction is performed two or three times, and the extracted sample pooled before analysis. In addition to the nature of the solvents used, other important parameters are pH [59-62], the ratio of solvent/sample, the presence of water in the extraction solution [63], the particle sizes of samples after grinding, the presence of salts, the speed of agitation and the temperature during extraction [64]. Apart from LLE and SLE traditional methods, other methods like microwave-assisted extraction (MAE), supercritical fluid extraction (SFE) and accelerated solvent extraction (ASE) have been developed recently to enhance extraction yield [59-65]. However, these new methods are still very expensive [66].
Due to their micro (ppm) or nano (ppb) quantities in foods, mycotoxins are usually firstly extracted from samples before analysis. Two methods are generally used: Liquid-Liquid Extraction (LLE) for liquid samples like milk, wine, juice [1] or Solid-Liquid Extraction (SLE) for solid samples like cereals, leguminous and other solid materials [59-60]. For both LLE and SLE techniques, many factors affect the mycotoxin extraction yield. The most important parameter is the solvent used which should have a great affinity with the specific mycotoxins chosen and little affinity with interfering compounds. During extraction, mycotoxins will move from the matrix to the extraction solvent until an equilibrium is established. Organic solvents like ethyl acetate, methanol, chloroform, acetonitrile, acetone, dichloromethane or a mixture of these solvents are the most commonly used [36-61]. In most cases, extraction is performed two or three times, and the extracted sample pooled before analysis. In addition to the nature of the solvents used, other important parameters are pH [59-62], the ratio of solvent/sample, the presence of water in the extraction solution [63], the particle sizes of samples after grinding, the presence of salts, the speed of agitation and the temperature during extraction [64]. Apart from LLE and SLE traditional methods, other methods like microwave-assisted extraction (MAE), supercritical fluid extraction (SFE) and accelerated solvent extraction (ASE) have been developed recently to enhance extraction yield [59-65]. However, these new methods are still very expensive [66].
Purification
During extraction, due to the complexity of some food matrices, undesirable compounds like lipids, sugars, pigments or proteins will be extracted together with the mycotoxins and interfere later in their detection [1-13]. As a consequence, the specificity, sensitivity, accuracy and precision of the analytic method will be modified [4]. Many purification methods known as “clean-up” exist. They include column chromatography, solid phase extraction (SPE), immune-affinity columns (IAC), ion-exchange columns, and multifunctional cleanup columns such as Mycosep cartridges, DON prep columns, columns containing charcoal, celite and alumina [60,67,68]. Among all these methods, SPE and IAC are the most widely used [69]. IAC is more specific and sensitive than SPE, but when samples contain high amounts of mycotoxins, SPE is more appropriate [63].
During extraction, due to the complexity of some food matrices, undesirable compounds like lipids, sugars, pigments or proteins will be extracted together with the mycotoxins and interfere later in their detection [1-13]. As a consequence, the specificity, sensitivity, accuracy and precision of the analytic method will be modified [4]. Many purification methods known as “clean-up” exist. They include column chromatography, solid phase extraction (SPE), immune-affinity columns (IAC), ion-exchange columns, and multifunctional cleanup columns such as Mycosep cartridges, DON prep columns, columns containing charcoal, celite and alumina [60,67,68]. Among all these methods, SPE and IAC are the most widely used [69]. IAC is more specific and sensitive than SPE, but when samples contain high amounts of mycotoxins, SPE is more appropriate [63].
Analytical methods
Several analytical methods exist to assess the presence of mycotoxins in foods. They can be classified as qualitative or quantitative. Qualitative methods are used only for screening purposes (presence or absence of mycotoxins). They allow a rapid discrimination of contaminated samples. Samples are detected as positive above a certain concentration of the toxin which is defined by the manufacturers. Analytical methods for mycotoxins are mainly separated in two groups: chromatographic methods and rapid methods [1,69,70].
Several analytical methods exist to assess the presence of mycotoxins in foods. They can be classified as qualitative or quantitative. Qualitative methods are used only for screening purposes (presence or absence of mycotoxins). They allow a rapid discrimination of contaminated samples. Samples are detected as positive above a certain concentration of the toxin which is defined by the manufacturers. Analytical methods for mycotoxins are mainly separated in two groups: chromatographic methods and rapid methods [1,69,70].
Methods using chromatographic principles
Thin Layer Chromatography (TLC)
TLC is one of the oldest methods used for detection of mycotoxins in a food matrix due to its ability to screen a large number of samples and provide qualitative, semi quantitative and quantitative information on mycotoxins [4]. TLC technics have been improved year after year through the introduction of spraying agents which by reacting with mycotoxins enhance their fluorescence or produce colored compounds leading to an increase of the detection limit. The recent improvement in TLC method for mycotoxins determination is High-Performance Thin Layer Chromatography (HPTLC). This technic allows an enhancement of the resolution and accuracy of TLC [70].
Thin Layer Chromatography (TLC)
TLC is one of the oldest methods used for detection of mycotoxins in a food matrix due to its ability to screen a large number of samples and provide qualitative, semi quantitative and quantitative information on mycotoxins [4]. TLC technics have been improved year after year through the introduction of spraying agents which by reacting with mycotoxins enhance their fluorescence or produce colored compounds leading to an increase of the detection limit. The recent improvement in TLC method for mycotoxins determination is High-Performance Thin Layer Chromatography (HPTLC). This technic allows an enhancement of the resolution and accuracy of TLC [70].
Conventional High-Performance Liquid Chromatography (HPLC)
In order to improve detection and quantification of mycotoxins with high selectivity, sensitivity and accuracy, the HPLC method was developed. Detection of mycotoxins is carried out here with ultraviolet (UV), diode array (DAD), fluorescence (FLD), mass spectrometry (MS) or photodiode array (PDA) detectors [60-69]. Among these detectors, FLD is the most popular and widely used in mycotoxins analysis, because the major mycotoxins generally found in foods like Ochratoxin A, Aflatoxins, and Zearalenone exhibit a natural fluorescence [69,72-76]. Regarding the non-fluorescent mycotoxins like Trichothecenes and Patulin, UV/DAD detectors are often used [69]. In some case, a derivatization step which transforms the mycotoxin into a fluorescent compound and ease its detection is performed [5]. The most significant limit of conventional HPLC methods is its inability to detect trace levels of toxins in foods [71].
In order to improve detection and quantification of mycotoxins with high selectivity, sensitivity and accuracy, the HPLC method was developed. Detection of mycotoxins is carried out here with ultraviolet (UV), diode array (DAD), fluorescence (FLD), mass spectrometry (MS) or photodiode array (PDA) detectors [60-69]. Among these detectors, FLD is the most popular and widely used in mycotoxins analysis, because the major mycotoxins generally found in foods like Ochratoxin A, Aflatoxins, and Zearalenone exhibit a natural fluorescence [69,72-76]. Regarding the non-fluorescent mycotoxins like Trichothecenes and Patulin, UV/DAD detectors are often used [69]. In some case, a derivatization step which transforms the mycotoxin into a fluorescent compound and ease its detection is performed [5]. The most significant limit of conventional HPLC methods is its inability to detect trace levels of toxins in foods [71].
Gas chromatography
Gas Chromatography (GC) is also used for mycotoxins determination in foods. Its accuracy and sensitivity depend on the type of detector used. Generally, it is flame ionization detection (FID), electron capture detection (ECD) or MS detections. GC coupled with MS or ECD detection is the most widely used method to simultaneous quantify a great diversity of mycotoxins in complex food matrices even in lower range [5,67,77]. However, this technique requires a preliminary clean-up step. Furthermore, the targeted mycotoxins need to be both thermally stable and volatile [4,77,78].
Gas Chromatography (GC) is also used for mycotoxins determination in foods. Its accuracy and sensitivity depend on the type of detector used. Generally, it is flame ionization detection (FID), electron capture detection (ECD) or MS detections. GC coupled with MS or ECD detection is the most widely used method to simultaneous quantify a great diversity of mycotoxins in complex food matrices even in lower range [5,67,77]. However, this technique requires a preliminary clean-up step. Furthermore, the targeted mycotoxins need to be both thermally stable and volatile [4,77,78].
Liquid Chromatography (LC) coupled with mass analyzers
In contrast to conventional HPLC, LC coupled with mass analyzers was recently introduced in mycotoxin analysis and allows a simultaneous screening, identification and quantification of a large variety of chemically diverse mycotoxins [77,79-81]. Numerous mass analyzers were reported in literature including triple and quadruple time-of-flight, ion-trap, magnetic sector mass spectrometers (MS/MS), Fourier transformation ion cyclotron resonance and Fourier transformation Orbitrap [82,83]. According to Rahmani., et al. [59], ion-trap, triple and quadruple time-of-flight are the most important mass analyzers used for determination of mycotoxins in food matrices. The widespread use of these high-resolution mass spectrometry methods is hampered by the prior sample purification step with MycoSep® or immunoaffinity columns [77].
In contrast to conventional HPLC, LC coupled with mass analyzers was recently introduced in mycotoxin analysis and allows a simultaneous screening, identification and quantification of a large variety of chemically diverse mycotoxins [77,79-81]. Numerous mass analyzers were reported in literature including triple and quadruple time-of-flight, ion-trap, magnetic sector mass spectrometers (MS/MS), Fourier transformation ion cyclotron resonance and Fourier transformation Orbitrap [82,83]. According to Rahmani., et al. [59], ion-trap, triple and quadruple time-of-flight are the most important mass analyzers used for determination of mycotoxins in food matrices. The widespread use of these high-resolution mass spectrometry methods is hampered by the prior sample purification step with MycoSep® or immunoaffinity columns [77].
Capillary Electrophoresis (CE)
The electrical potential of mycotoxins is used here as the basis of their separation, and the laser induced fluorescence or UV as detection systems [84]. When CE is coupled with a purification step using an IAC column, its accuracy, sensitivity and precision are higher compared to HPLC methods [85]. Moreover, the short time and the very low quantity of solvents it requires to be performed make this method a good and low-cost alternative to HPLC [77].
The electrical potential of mycotoxins is used here as the basis of their separation, and the laser induced fluorescence or UV as detection systems [84]. When CE is coupled with a purification step using an IAC column, its accuracy, sensitivity and precision are higher compared to HPLC methods [85]. Moreover, the short time and the very low quantity of solvents it requires to be performed make this method a good and low-cost alternative to HPLC [77].
Rapid tests for mycotoxins screening and quantification
Rapid methods are less expensive, easier to use and can be moved to an on-site environment. They are useful to determine the effectiveness of food safety measures, to determine legal compliance, to achieve logistical and operational goals, to keep commodities and products moving rapidly through marketing channels, to save time and thus costs, to save investments in complex instruments and to employ staff with less technical training [86]. Most rapid methods provide qualitative or semi-quantitative results and are recommended for use in screening samples.
Rapid methods are less expensive, easier to use and can be moved to an on-site environment. They are useful to determine the effectiveness of food safety measures, to determine legal compliance, to achieve logistical and operational goals, to keep commodities and products moving rapidly through marketing channels, to save time and thus costs, to save investments in complex instruments and to employ staff with less technical training [86]. Most rapid methods provide qualitative or semi-quantitative results and are recommended for use in screening samples.
Immunochemical methods
Immunochemical methods are among the most used techniques for rapid screening and quantification of mycotoxins on foods. Radioimmunoassay (RIA), Enzyme Linked Immouno-Sorbent Assay (ELISA), and immuno-affinity column assay (ICA) belong to this group [59]. ELISA has gained an increase interest all over the world because of its speed and sensitivity [87]. Moreover, it is easy to use. Typically, no clean-up or analyte enrichment steps are required [69]. Nowadays, ELISA kits for most of mycotoxins commonly found in foods like Aflatoxins, Fumonisins, Trichothecenes, Zearalenone and Ochratoxin A are already available in markets [88].Direct ELISA is hampered by the fact that the immunoreactivity of the primary antibody may be reduced as a result of labeling, and signal amplification becomes difficult. Moreover, the cross-reactivity which may occur with the secondary antibody, during indirect ELISA could lead to a non-specific signal [4].
Immunochemical methods are among the most used techniques for rapid screening and quantification of mycotoxins on foods. Radioimmunoassay (RIA), Enzyme Linked Immouno-Sorbent Assay (ELISA), and immuno-affinity column assay (ICA) belong to this group [59]. ELISA has gained an increase interest all over the world because of its speed and sensitivity [87]. Moreover, it is easy to use. Typically, no clean-up or analyte enrichment steps are required [69]. Nowadays, ELISA kits for most of mycotoxins commonly found in foods like Aflatoxins, Fumonisins, Trichothecenes, Zearalenone and Ochratoxin A are already available in markets [88].Direct ELISA is hampered by the fact that the immunoreactivity of the primary antibody may be reduced as a result of labeling, and signal amplification becomes difficult. Moreover, the cross-reactivity which may occur with the secondary antibody, during indirect ELISA could lead to a non-specific signal [4].
Biosensor techniques
The biosensors techniques are based on the reaction between the mycotoxins and a biologically sensitive element such as enzyme, nucleic acids or antibodies, leading to the formation of a signal which can easily be detected by a transducer. The transducer will then transform the signal into a measurable variable [13]. The biosensor technique with DNA as aptamer (molecules that can bind a specific analyte) was successfully used by Dinckaya., et al. [89] to assess the level of Aflatoxin M1 in milk samples.
The biosensors techniques are based on the reaction between the mycotoxins and a biologically sensitive element such as enzyme, nucleic acids or antibodies, leading to the formation of a signal which can easily be detected by a transducer. The transducer will then transform the signal into a measurable variable [13]. The biosensor technique with DNA as aptamer (molecules that can bind a specific analyte) was successfully used by Dinckaya., et al. [89] to assess the level of Aflatoxin M1 in milk samples.
Bead-based assays
With this technique, a bead marked with an antibody against a specific mycotoxin and which possesses a magnetic code or a specific color code is added to sample solution. The complex antibody-antigen on the bead is then measured using a laser. This emerging technique is not widely used in the analysis of mycotoxins in food because of its high cost [13].
With this technique, a bead marked with an antibody against a specific mycotoxin and which possesses a magnetic code or a specific color code is added to sample solution. The complex antibody-antigen on the bead is then measured using a laser. This emerging technique is not widely used in the analysis of mycotoxins in food because of its high cost [13].
Electronic nose (EN)
EN is a variant of GC which is non-destructive, rapid and cheap for the analysis of mycotoxins in foods [90]. The method assimilated as a mimic of human olfactory sensory system, is based on the interaction of a volatile mycotoxin with an array of chemical sensors with different specificities leading to generation of a signal. The obtained signal is then used as a fingerprint of the volatile molecules and will serve to identify the mycotoxins [91]. The limit of this method which is still embryonic is the fact that many mycotoxins are not volatile and thus they cannot be detected [1].
EN is a variant of GC which is non-destructive, rapid and cheap for the analysis of mycotoxins in foods [90]. The method assimilated as a mimic of human olfactory sensory system, is based on the interaction of a volatile mycotoxin with an array of chemical sensors with different specificities leading to generation of a signal. The obtained signal is then used as a fingerprint of the volatile molecules and will serve to identify the mycotoxins [91]. The limit of this method which is still embryonic is the fact that many mycotoxins are not volatile and thus they cannot be detected [1].
Conclusion
Mycotoxins contamination of foods has become a global concern that calls for urgent actions. The challenge is complex and deserves coordinated efforts. Proper detection is the basis to deal with their potential negative impact on human and animal health. Rapid test methods easy to use, reliable and accessible exist nowadays as alternative to conventional methods which are more expensive. Considering the limited laboratory infrastructures and capacities in most of the developing countries, these rapid methods will therefore be very useful to control dietary exposure of populations to these toxins. Furthermore, they will also be helpful for these countries to reduce the rejection risk of the agricultural products they export.
References
- Alshannaq A., et al. “Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food”. International Journal of Environmental Research and Public Health 14 (2017): 632.
- Milani JM. “Ecological conditions affecting mycotoxin production in cereals: a review”. Veterinary Medicina 58.8 (2013): 405-411.
- Wu F., et al. “Climate change impacts on mycotoxin risks in US maize”. World Mycotoxin Journal 4.1 (2011): 79-93.
- Turner NW., et al. “Analytical methods for determination of mycotoxins: A review”. Analitica Chimica Acta 632 (2009): 168-180.
- Zhang L., et al.“A Review of Current Methods for Analysis of Mycotoxins in Herbal Medicines”. Toxins 10 (2018): 65.
- Bhatnagar D., et al. “Toxins of filamentous fungi”. Chemistry and Immunology 81 (2002): 167–206.
- CAST Council for Agricultural Science and Technology. “Mycotoxins: Risks in Plant, Animal, and Human Systems”. Task force report 139 (2003): 217.
- IARC International Agency for Research on Cancer Evaluation of carcinogenic risks of chemical to humans. In ‘‘Some naturally-occurring substances: Food Items and Constituents. Heterocyclic Aromatic Amines and Mycotoxins”. IARC monographs, Lyon, France (1993): 359-362.
- Jurado M., et al. “Relationship between solute, matric potential stress, temperature, growth, and FUM1 gene expression in two Fusariumverticillioidesstrains from Spain”. Applied and Environmental Microbiology 74 (2008): 2032-36.
- Kohut G., et al. “N-starvation stress induced FUM gene expression and fumonisin production is mediated via the HOG-type MAPK pathway in Fusariumproliferatum”. International Journal of Food Microbiology 130 (2009): 65-69.
- Marin S., et al. “Mycotoxins: Occurrence, toxicology, and exposure assessment”. Food Chemistry and Toxicology 60 (2013): 218-237.
- FAO Food and Agricultural Organization. Mycotoxins. “Food Safety and Quality, United Nations Environmental Program (UNEP) GRID Adrenal, Food Demand and Need”. (2013).
- Wolf K., et al. “Mycotoxin Analysis: A Focus on Rapid Methods”. Partnership for Aflatoxin Control in Africa, African Union Commission, Addis Ababa, Ethiopia (2018).
- DarwishWS., et al. “An Overview on Mycotoxin Contamination of Foods in Africa”. Journal of Veterinary and Medical Science 76.6 (2014): 789-797.
- Betina V. “Mycotoxins: chemical, biological and environmental aspects, in “Bioactive molecules”. Elsevier, Amsterdam, The Netherlands, 9 (1989).
- Williams JH., et al. “Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions”. American Journal Clinical Nutrition 80 (2004): 1106-1122.
- Abrunhosa L., et al. “Review of Mycotoxins in Food and Feed Products in Portugal and Estimation of Probable Daily Intakes”. Critical Reviews in Food Science and Nutrition 56:2 (2016): 249-265.
- Brera C., et al. “Mycotoxins. In Comprehensive Analytical Chemistry; Picó, Y., Ed.” Elsevier: Amsterdam, The Netherlands 51 (2008): 363-427.
- IARC International Agency for Research on Cancer. “Monograph on the Evaluation of Carcinogenic Risk to Humans, World Health Organization. Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene”. Summary of Data Reported and Evaluation, Lyon 82 (2002): 171-175.
- IARC International Agency for Research on Cancer. “Chemical agents and related occupations: A review of human carcinogens, International Agency for Research on Cancer (IARC) 100F”. Lyon, France (2012).
- Kensler TW., et al. “Aflatoxin: a 50-year odyssey of mechanistic and translational toxicology”. Toxicology Science 120.1 (2011): 28-48.
- Sinai W., et al. “Investigate the optimal production conditions of Fumonisin B1 from local isolation of Fusariumverticillioides”. International Journal of Advanced biological Research 5.3 (2015): 209-215.
- Marasas WF., et al. “Leukoencephalomalacia in a horse induced by fumonisin B1 isolated from Fusariummoniliforme”. Onderstepoort Journal Official Veterinary Research 55 (1988): 197-203.
- Sydenham EW., et al. “Natural occurrence of some Fusariummycotoxins in corn from low and high oesophageal cancer prevalence areas of the Transkei, Southern Africa”. Journal of Agricultural Food Chemistry 38 (1990): 1900-1903.
- WHO World Health Organization. “Evaluation of certain food additives and contaminants. Zearalenone”. Fifty-third report of the Joint FAO/WHO Expert Committee on Food Additives, WHO Geneva, WHO Technical Report Series 896 (2000): 93-96.
- Jackson LS., et al. “Effects of baking and frying on the fumonisin B1 content of corn-based foods”. Journal of Agricultural and Food Chemistry 45 (1997): 4800-4805.
- FAO Food and Agriculture Organization. “Worldwide regulations for mycotoxins in food and feed in 2003”. FAO Food and Nutrition Paper 81 (2004) Rome, Italy.
- Voss KA., et al. “Fumonisins: Toxicokinetics, mechanism of action and toxicity”. Animal and Feed Science Technology 137 (2007): 299-325.
- Anukul N., et al. “Significance of regulation limits in mycotoxin contamination in Asia and risk management programs at the national level”. Journal of Food and Drug Analysis 21(2013): 227-241.
- EU European Union. “Commission Regulation (EU) No 178/2010 of 2 March 2010. amending Regulation (EC) No 401/2006 as regards groundnuts (peanuts), other oilseeds, tree nuts, apricot kernels, liquor ice and vegetable oil”. Official Journal of European Union 52(2010): 32-43.
- FDA Food and Drug Administration. “U.S. Food and Drug Administration (FDA) mycotoxin regulatory guidance”. In: U.S. Food and Drug Administration (FDA), Editor. Washington, D.C.: National Grain and Feed Association (2011): 1-9.
- El Khoury A., et al. “Ochratoxin A: General Overview and Actual Molecular Status”. Toxins 2 (2010): 461-493.
- Pittet A. Revue de medécine veterinaire, 149 (1998): 479.
- Van der Merwe KJ., et al. “Ochratoxin A, a toxic metabolite produced by Aspergillus ochraceus Wilh”. Nature 205 (1965): 1112-1113.
- Raters M., et al. “Thermal stability of Aflatoxin B1 and Ochratoxin A”. Mycotoxin Research 24.3 (2008):130-4.
- Nganou DN., et al. “Fungal flora and ochratoxina associated with coffee in Cameroon”. British Microbiology Research Journal 4 (1): 2014.17 p.
- Nguegwouo E., et al. “Ochratoxin A in black pepper, white pepper and clove sold in Yaoundé (Cameroon) markets: contamination levels and consumers’ practices increasing health risk”. International Journal of Food Contamination 5 (2018): 1-7.
- Pfohl-Leszkowicz A., et al. “Ochratoxin A: An overview on toxicity and carcinogenicity in animals and humans”. Molecular Nutrition Food Research 51 (2007): 61-99.
- EC European Commission. “Commission recommendation No 2006/576/EC on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding”. Official Journal of the European Union (2006): 229/7-229/9.
- Srianujata S. “Regulatory update and control measures for prevention and reduction of mycotoxins contamination in foods and feeds”. Proceedings of FFTCeKU conference, International seminar on risk assessment and risk management of mycotoxins for food safety in Asia. Thailand: Kasetsart University (2011).
- Garcia-Cela E., et al. “Interacting Environmental Stress Factors Affects Targeted Metabolomic Profiles in Stored Natural Wheat and That Inoculated with F. graminearum”. Toxins 10 (2018):56.
- Lauren DR., et al. “Stability of the fusariummycotoxinsnivalenol, deoxynivalenol and zearalenone in ground maize under typical cooking environments”. Food Additives Contamination 18.11 (2001): 1011-6.
- Bennett JW., et al. “Mycotoxins”. Clinical Microbiology Reviews 16 (2003): 497-516.
- Zinedine A., et al. “Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenicmycotoxin”. Food Chemistry and Toxicology 45 (2007): 1-18.
- Richard JL. “Some major mycotoxins and their mycotoxicosis: an overview”. International Journal of Food Microbiology 119 (2007): 3-10.
- Bamburg JR., et al.“The structure of toxins from two strains of Fusariumtricinctum”. Tetrahedron 24 (1968): 3329-36.
- Creppy EE. “Update of survey, regulation and toxic effects of mycotoxins in Europe”. Toxicology Letters 127 (2002): 19-28.
- Medina A. “Temperature and water activity effects on production of T-2 and HT-2 by Fusarium langsethiae strains from north European countries”. Food Microbiology (2010).
- Zinedine., et al. “Occurrence and legislation of mycotoxins in food and feed from Morocco”. Food Control 20 (2008): 334-344
- Foroud., et al. “Trichothecenes in cereal grains”. Journal of Molecular Science 10(2009): 147-173.
- JECFA-Joint FAO/WHO Expert Committee on Food Additives. “Safety evaluation of certain mycotoxins in food”. Prepared by the 56th Meeting of the Food Additives Series No. 47. Geneva (2001).
- Pestka JJ., et al. “Deoxynivalenol: toxicology and potential effects on humans”. Journal of Toxicology and Environmental Health B Critical Review 8 (2005): 36-9.
- Whitaker TB., et al. “Variability and distribution among sample test results when sampling unprocessed oat lots for ochratoxin A”. World Mycotoxin Journal 8 (2015): 511-524.
- Armorini S., et al. “Occurrence of aflatoxin B 1 in conventional and organic flour in Italy and the role of sampling”. Food Control 50 (2015): 858-863.
- Wesolek N., et al. “Assessing aflatoxin B1 distribution and variability in pistachios: validation of a Monte Carlo modeling method and comparison to the Codex method”. Food Control 59 (2016): 553-560.
- McElhinney C., et al. “Variation associated with sampling bale or pit silage for mycotoxins and conventional chemical characteristics”. World Mycotoxin Journal 9 (2016): 331-342.
- EC European Commission. “Guidance document for the sampling of cereals for mycotoxins final version”. http://ec.europa.eu/food/food/chemicalsafety/contaminants/guidance-sampling-final.pdf (2010). Access date: 05.11.2010
- EU European Union. “Commission Regulation (EU) No 519/2014 of 16 May 2014 amending Regulation (EC) No 401/2006 as regards methods of sampling of large lots, spices and food supplements, performance criteria for T-2, HT-2 toxin and citrinin and screening methods of analysis”. Official Journal of European Union 147 (2014): 29-43.
- Rahmani A., et al. “Qualitative and quantitative analysis of mycotoxins”. Comprehensive Review Food Science and Food Safety 8 (2009): 202-251.
- Pereira VL., et al. “Mycotoxins in cereals and related foodstuffs: A review on occurrence and recent methods of analysis”. Trends in Food Science and Technology 36 (2014): 96-136.
- Ridgway K and Scientific R. Sample preparation for food contaminant analysis. LC-GC Europe 25 (2012): 1–8.
- Monbaliu S., et al. “Development of a multi-mycotoxin liquid chromatography/tandem mass spectrometry method for sweet pepper analysis”. Rapid Communications in Mass Spectrometry 23.1 (2009): 3-11.
- Hinojo MJ., et al. “Fumonisin production in rice cultures of Fusariumverticillioides under different incubation conditions using an optimized analytical method”. Food Microbiology 23.2 (2006): 119-27.
- Andersen B., et al. “Automated and unbiased image analyses as tools in phenotypic classification of small sporedAlternariaspp”. Phytopathology 95 (2005): 1021-1029.
- Maragos CM and Busman M. “Rapid and advanced tools for mycotoxin analysis: A review”. Food Additives Contamination Part A 27 (2010): 688–700.
- Kralj CI. et al. “An overview of conventional and emerging analytical methods for the determination of mycotoxins”. International Journal Molecular Science 10 (2009): 62-115.
- Krska R., et al. “The state-of-the-art in the analysis of type-A and -B trichothecene mycotoxins in cereals”. Fresenius Journal of Analytical Chemistry 371 (2001): 285-299.
- Kostelanska M., et al. “The study of deoxynivalenol and its masked metabolites fate during the brewing process realised by UPLC–TOFMS method”. Food Chemistry 126 (2011): 1870-1876.
- Hajslova J., et al. “Analysis of Multiple Mycotoxins in Food”. In Jerry Zweigenbaum (ed.), Mass Spectrometry in Food Safety: Methods and Protocols, Methods in Molecular Biology 747 (2011): 233-258.
- Aiko V., et al. “Occurrence, detection and detoxification of mycotoxins”. Journal of Biosciences 40 (2015): 943-954.
- Pittet A. “Modern methods and trends in mycotoxin analysis”. Mitteilungen aus Lebensmitteluntersuchung und Hygiene 96 (2005): 424-444.
- EPC European Pharmacopoeia Commission. “Determination of Aflatoxin B1 in herbal drugs”. In European Pharmacopoeia 9th Edition 2.8.18; Council of Europe: Strasbourg, France, 1 (2016): 289p.
- CPC Chinese Pharmacopoeia Commission. “Chinese Pharmacopoeia 2015 Edition volume IV” Chinese Medicine Science and Technology Press: Beijing, China, (2015).
- USPC United States Pharmacopeial Convention. “USP 38-NF 33 Chapter 561: Articles of Botanical Origin”. United States Pharmacopeial Convention: Rockville, MD, USA, (2014).
- KFD Korean Food and Drug. “General Tests, Processes and Apparatus” Korean Food & Drug: Chungcheongbuk-do, Korea. Korean Pharmacopoeia 10th Edition (2012): 1673-1675.
- JPCEC Japanese Pharmacopoeia Commentary Editorial Committee. “Analytical Methods for Aflatoxins in Crude Drug and Crude Drug Preparations”. Japanese Pharmacopoeia Commentary Editorial Committee: Tokyo, Japan 17th Edition (2016) 2513-2515.
- Bueno D., et al. “Determination of mycotoxins in food: a review of bioanalytical to analytical methods”. Applied Spectroscopy Reviews 50 (2015): 728-774.
- Li P., et al. “Advanced hyphenated chromatographic-mass spectrometry in mycotoxin determination: Current status and prospects”. Mass Spectrometry Review 32 (2013): 420-452.
- Zhang K., et al. “Perspective on advancing FDA regulatory monitoring for mycotoxins in foods using liquid chromatography and mass spectrometry”. Journal of AOAC International 99 (2016): 890-894.
- Berthiller F., et al. “Developments in mycotoxin analysis: an update for 2014-2015”. World Mycotoxin Journal 9 (2016): 5-30.
- Tolosa J., et al. “Multimycotoxin analysis in water and fish plasma by liquid chromatography-tandem mass spectrometry”. Chemosphere 145 (2016): 402-408.
- Berthiller F., et al. “Masked mycotoxins: determination of a deoxynivalenolglucoside in artificially and naturally contaminated wheat by liquid chromatography-tandem mass spectrometry”. Journal of Agricultural and Food Chemistry 53 (2005): 3421-3425.
- Biancardi A., et al. “A rapid multi-residual determination of type A and type B trichothecenes in wheat flour by HPLC-ESI-MS”. Food Additives & Contaminants 22 (2005): 251-258.
- Maragos CM. “Analysis of mycotoxins with capillary electrophoresis”. Seminars in Food Analysis 3 (1998): 353–373.
- Maragos CM., et al. “Detection of zearalenone and related metabolites by fluorescence polarization immunoassay”. Journal of Food Protection 67 (2004): 1039-1043.
- Scanlan FP. “Why rapid testing?” In: van AmerongenA, Barug D, Lauwaars M, eds. Rapid Methods for Biological, Chemical Contaminants in Food and Feed, Wageningen Academic Publishers, The Netherlands (2005): 19-29.
- Papadopoulou-Bouraoui A., et al. “Screening survey of deoxynivalenol in beer from the European market by an enzyme-linked immunosorbent assay”. Food Additives Contamination 21 (2004): 607-17.
- Sangare-Tigori B., et al. “Cooccurrence of Aflatoxin B1, fumonisin B1, ochratoxin A and zearalenone in cereals and peanuts from Cote d’Ivoire”. Food Additives Contamination 23.10 (2006): 1000-7.
- Dinckaya E., et al. “Development of an impedimetricaflatoxin M1 biosensor based on a DNA probe and gold nanoparticles”. Biosens Bioelectron (2011).
- Keshri G. et al. “Detection and differentiation between mycotoxigenic and non-mycotoxigenic strains of two Fusarium spp. using volatile production profiles and hydrolytic enzymes”. Journal Applied Microbiology 89 (2000): 825-833.
- Feast S. “Potential application of electronic noses in cereals”. Cereal Food World 46 (2001): 159-161.
Citation:
Evelyne Nguegwouo., et al. “An Overview of some Major Mycotoxins in Food and their Detection Methods”. Nutrition and
Food Toxicology 3.1 (2018): 564-576.
Copyright: © 2018 Evelyne Nguegwouo., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.