Research Article
Volume 1 Issue 1 - 2017
Digital Image Analysis of the Foveal Avascular Zone in Albinism
1Departments of Ophthalmology and Visual Neurosciences
2Pediatrics, University of Minnesota, Minneapolis, MN
3Department of Ophthalmology, Hennepin County Medical Center, Minneapolis, MN
2Pediatrics, University of Minnesota, Minneapolis, MN
3Department of Ophthalmology, Hennepin County Medical Center, Minneapolis, MN
*Corresponding Author: C Gail Summers MD, Departments of Ophthalmology and Visual Neurosciences and Pediatrics, University of Minnesota, Minneapolis, United States.
Received: January 30, 2017; Published: April 17, 2017
Abstract
Purpose: Albinism is an inherited disorder of melanin biosynthesis. Patients with albinism have foveal maldevelopment and decreased visual function. The purpose of this study was to use color retinal images to describe the foveal avascular zone (FAZ) in albinism compared to controls, and to correlate the results with best-corrected visual acuity (BCVA) and refractive error.
Methods: A retrospective chart review of 46 individuals with albinism was performed after IRB approval. Data from the ophthalmic examination were gathered systematically. Retinal images of the macula obtained in the group with albinism and in 46 controls with no retinal disease were optimized for vessel visualization and evaluated for six parameters: height, width, and area of the FAZ; distance from the temporal optic nerve border to the center of the FAZ and to the first vessel to traverse the horizontal meridian; and the distance between the major macular vascular arcades thru the FAZ center.
Results: Our data showed that the height, width and area of the FAZ is significantly smaller in individuals with albinism (p < 0.001 for all). The distance from the temporal optic nerve border to the FAZ center was significantly increased (p < 0.001). The distance from the temporal optic nerve to the first vessel traversing the horizontal meridian, and the distance between the macular arcades was similar in both the group with albinism and the control group. Correlation between BCVA and the FAZ parameters was poor as was the spherical equivalent and the FAZ parameters.
Conclusions: The abnormalities of the FAZ identified in this study could not explain the subnormal BCVA in individuals with albinism. The more temporal location of the center of the FAZ may explain the positive angle kappa seen in albinism.
Supported, in part, by an unrestricted grant from Research to Prevent Blindness, Inc., New York, NY, to the Department of Ophthalmology and Visual Neurosciences at the University of Minnesota.
Introduction
Albinism is an inherited disorder of abnormal melanin production with phenotypic heterogeneity. Those who produce no melanin have oculocutaneous albinism (OCA) 1A and those who are born with white hair but develop melanin pigment due to residual tyrosinase enzyme activity have OCA1B. OCA2 occurs in individuals with mutation of the P gene (OCA2 gene) on chromosome 15q. The more unusual gene abnormalities in individuals with OCA3 and OCA4 affect the tyrosinase-related protein 1 and membrane-associated transporter protein, respectively [1]. Types OCA5, OCA6, and OCA7 lack good phenotypic and/or genotypic description. Patients with X-linked ocular albinism (OA1) share the ocular structure and function of OCA but the hair and skin coloration are not affected [2].
The ocular phenotype for the most common types of albinism is well described and an invariable feature is an abnormal fovea, first described by Nettleship in 1876 [3]. The normal human fovea underlies the majority of our visual function and is characterized by an avascular zone, an increase in cone photoreceptor density, and an excavation of inner retinal neurons [4,5]. It is normally surrounded by perifoveal vascular wreathing that arises equally from the superior and inferior macular branches. Despite the known ocular manifestations of albinism, including iris transillumination, high refractive errors, nystagmus, impaired stereopsis, altered retinostriate decussation, and macular abnormalities [4], the cause(s) of subnormal vision is incompletely understood [6].
To our knowledge, there are no prior studies correlating the foveal avascular zone (FAZ) with visual acuity in patients with albinism. The aim of this study is to compare the macular vascular abnormalities in albinism to controls with digital fundus photography, and to determine if the extent of the foveal vascular abnormalities correlates with best-corrected visual acuity (BCVA) or refractive error in individuals with albinism.
Methods
A retrospective chart review of 46 individuals with albinism was performed after IRB approval. This study was compliant with the United States Health Information Portability and Accountability Act. The diagnosis of albinism was made based on clinical phenotype determined by a pediatric ophthalmologist and geneticist. When the clinical examination was not definitive, visual evoked potentials (VEP) to show excessive retino-striate decussation and/or gene testing to look for mutations in one of the genes known to cause albinism were performed.
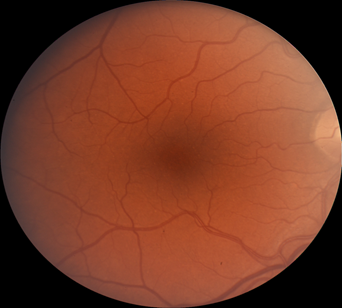
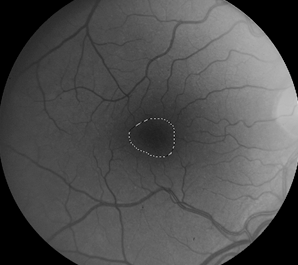
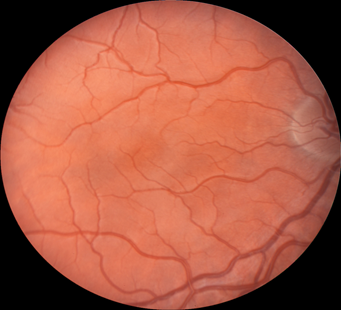
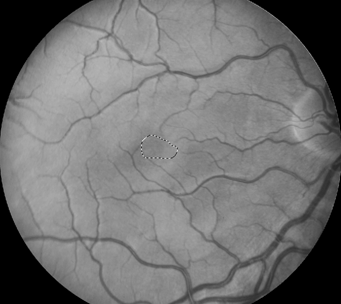
Retinal images were obtained with a Zeiss FF4 fundus camera (Oberkochen, Germany) in 46 individuals with albinism at enrollment in a prospective clinical trial [www.ClinicalTrials.gov (NCT01176435)] and were analyzed for the current study. Images using the same parameters were also obtained in 46 controls with no retinal disease. The 30-degree field included the optic nerve and FAZ. The camera has a telecentric optical design that adjusts the image based on refractive error when the image is in good focus. Only one eye was evaluated for each study participant. The right eye was used in 41 of the 46 patients with albinism. The left eye was chosen when the photograph if the right eye did not include sufficient optic nerve to accurately provide study measurements accurately. Images from the right eye were evaluated in all of the healthy control group of adults. Retinal images for both groups were optimized for vessel visualization by using Adobe Photoshop. The green channel image was initially extracted because the blood vessels are relatively darker compared to the normal intensity image. Contrast was then adjusted accordingly to better visualize the macular vasculature. In the FAZ determinations, vessel end points were determined and manually connected [Figure]. Then the FAZ center was determined along the horizontal and vertical dimensions. The images were evaluated for the height in the 90-degree meridian, greatest width, and the overall area of the FAZ; distance from the temporal optic nerve (ON) border (excluding a peripapillary halo or scleral crescent, if present) to the center of the FAZ; distance from the temporal ON to the first vessel to traverse the horizontal meridian; and the distance between the major macular vascular arcades at the FAZ center. These different macular parameters were calculated by using a percentage of the pixels found in a normal optic nerve (# pixels = 100%) to express it as a pixel ratio (PR).
Figure 1: Fundus photograph of a control subject (above),
enhanced image with outline of FAZ (below).
BCVA was measured in all subjects with albinism with linear letters using an electronic visual acuity tester (EVA) designed by JAEB Center for Health Research, Tampa, FL [5]. ETDRS letters or HOTV letters with crowding bars were used at a distance of 118 cm. If the patient was too young for letter matching or identification, LEA symbols were used. Preferred head posture was permitted during vision testing. Binocular BCVA was used as the most reliable estimate of vision due to the frequent degradation of monocular acuity measurements associated with monocular fogging or occlusion. All patients had no fixation preference for the right or left eye and only 2 patients with albinism had spherical equivalents that differed by > 1.5D.
Cycloplegic refraction was performed following instillation of a proprietary mixture of 1.3% cyclopentolate, 0.17% tropicamide and 1.7% phenylephrine for individuals less than 10 years old, and 1% tropicamide and 2.5% phenylephrine for those who were 10 and older. Refractive errors were recorded in conventional form using positive cylinder and were then converted into spherical equivalent.
A comparison of the macular parameters between the group with albinism and the control group was performed by using an unpaired Student t-test. BCVA was converted to LogMAR acuity for statistical analysis. A regression analysis with Spearman correlation was performed to determine the correlation between the macular parameters and binocular BCVA in the group with albinism. Pearson correlation was also performed to determine the correlation between the macular parameters and the spherical equivalent of the refractive errors.
Results
There were 18 females among a total of 46 individuals in the group with albinism. Data for type of albinism, refractive error (spherical equivalent), and BCVA are given in Table 1. Mean age for the group with albinism was 10.4 years (range: 4 to 44 years). Mean age for the control group was 30 years (range: 20 to 50 years).
| Patient # | Type of albinism | BCVA (Snellen) | Spherical equivalent refractive error |
| 1 | OCA2 | 20/80 | +4.25 |
| 2 | OCA1B | 20/100 | +4.00 |
| 3 | OCA2 | 20/40 | +3.75 |
| 4 | OA1 | 20/125 | +2.00 |
| 5 | OCA1A | 20/100 | +3.25 |
| 6 | OCA1A | 20/80 | +3.50 |
| 7 | OCA1A | 20/160 | +3.50 |
| 8 | OCA1B | 20/80 | +3.50 |
| 9 | OCA1B | 20/125 | +2.00 |
| 10 | OCA1B | 20/100 | +3.25 |
| 11 | OCA1B | 20/80 | +3.37 |
| 12 | OCA2 | 20/60 | +2.75 |
| 13 | OCA2 | 20/40 | +3.00 |
| 14 | OCA2 | 20/100 | +6.25 |
| 15 | OCA1A | 20/80 | +4.25 |
| 16 | OCA2 | 20/60 | +5.25 |
| 17 | OCA1B | 20/80 | -3.00 |
| 18 | OCA1B | 20/160 | -1.75 |
| 19 | OCA1A | 20/80 | -0.50 |
| 20 | OCA1B | 20/70 | +1.62 |
| 21 | OCA1A | 20/60 | plano |
| 22 | OCA1B | 20/125 | +2.50 |
| 23 | OCA1B | 20/125 | +2.87 |
| 24 | OCA2 | 20/63 | -3.25 |
| 25 | OCA1A | 20/125 | +6.25 |
| 26 | OA1 | 20/70 | +0.50 |
| 27 | OCA1A | 20/150 | +2.00 |
| 28 | OCA1B | 20/25 | +4.00 |
| 29 | OCA1A | 20/100 | +5.00 |
| 30 | OCA2 | 20/80 | +3.00 |
| 31 | OCA2 | 20/125 | +3.00 |
| 32 | OCA1A | 20/125 | +6.25 |
| 33 | OCA1B | 20/50 | -4.00 |
| 34 | OCA2 | 20/60 | +1.50 |
| 35 | OCA2 | 20/50 | +5.50 |
| 36 | OA1 | 20/100 | +2.50 |
| 37 | OCA1B | 20/40 | +2.25 |
| 38 | OA1 | 20/60 | -6.00 |
| 39 | OCA2 | 20/200 | -1.75 |
| 40 | OCA2 | 20/260 | +1.75 |
| 41 | OCA1B | 20/30 | +2.50 |
| 42 | OCA1A | 20/125 | +3.25 |
| 43 | OCA2 | 20/100 | +2.00 |
| 44 | OCA2 | 20/30 | +0.25 |
| 45 | OCA2 | 20/20 | -1.25 |
| 46 | OCA1B | 20/60 | +2.25 |
Table 1: Characteristics of group with albinism.
Table 2 shows the comparison between macular parameters for the two groups. A significant difference (p < 0.001) between the
group with albinism and the control group was found for four of the macular parameters (FAZ height, width, and area, and distance
from the temporal ON to the center of the FAZ). However, the distance from the ON temporal border to the first vessel to cross the
horizontal meridian, and the maximum distance between the superior and inferior vascular arcades around the macula were not significantly
different between the groups.
| Macular Parameter | Group with Albinism (PR) | Control Group (PR) |
P value |
| FAZ height-mean (range) | 0.74 (0.4-1.26) |
0.99 (0.71-1.26) |
< 0.001 |
| FAZ width-mean (range) | 1.12 (0.53-1.72) |
1.85 (1.14-2.39) |
< 0.001 |
| FAZ area-mean (range) | 0.63 (0.31-1) |
1.38 (0.82-2.02) |
< 0.001 |
| Distance from temporal ON to FAZ center-mean (range) | 4.04 (2.38-5.43) |
3.47 (2.95-3.93) |
< 0.001 |
| Distance from temporal ON to first vessel to cross horizontal meridian-mean (range) | 2.70 (0.9-6.02) |
2.78 (0.53-5.15) |
NS |
| Distance between superior and inferior macular arcades-mean (range) | 2.69 (2.31-4.89) |
2.78 (2.15-4.71) |
NS |
FAZ = foveal avascular zone; ON = optic nerve; PR = pixel ratio; NS = not significant
Table 2: Comparison of Macular Parameters for the Two Studied Groups.
Table 2: Comparison of Macular Parameters for the Two Studied Groups.
Data for BCVA and refractive error in subjects with albinism are given in Table 3. Mean visual acuity in OCA1A was worse than OCA1B and OCA2 (p = 0.041 and 0.064, respectively). Visual acuity in patients with albinism correlated poorly with FAZ height, width
and area (r < 0.076, 0.010 and 0.080, respectively). The correlation of the distance from the temporal edge of the ON to the center of
the FAZ and BCVA, and the distance from the temporal edge of the ON to the first vessel to cross the horizontal meridian was also poor
(r < -0.0397 and -0.0815, respectively). Lastly, the correlation between the distance from the superior to the inferior temporal arcades
at the level of the FAZ center and BCVA was poor (r ≤ -0.0261).
| Type of albinism | Binocular BCVA logMAR mean (Snellen equivalent) (range) |
Spherical equivalent Diopters (range) |
| All subjects (n = 46) | 0.59 (20/77) (20/20 to 20/200) |
+2.03 (-8.50 to +6.75) |
| OCA1A (n = 11) |
0.709 (20/102) (20/60 to 20/160) |
+3.34 (-0.50 to +6.25) |
| OCA1B (n = 16) |
0.550 (20/70) (20/25 to 20/120) |
+0.96 (-8.50 to +4.00) |
| OCA2 (n = 16) |
0.534 (20/68) (20/20 to 20/200) |
+2.28 (-3.75 to +6.75) |
| OA1 (n = 4) |
0.636 (20/86) (20/60 to 20/120) |
-0.25 (-6.00 to +0.50) |
BCVA = Best corrected visual acuity; OCA=Oculocutaneous albinism; OA=Ocular albinism
logMAR = logarithm of minimal angle of resolution.
Table 3: BCVA and Refraction for the types of Albinism in the study cohort.
Table 3: BCVA and Refraction for the types of Albinism in the study cohort.
The correlations between all the macular parameters and spherical equivalent of the refractive error were also poor (area of the
FAZ r < 0.2719; distance from the ON border to the center of the FAZ r < 0.2771; distance from the temporal ON to the first vessel to traverse
the horizontal meridian r < -0.1405; and the distance between the major macular vascular arcades at the FAZ center r < 0.2493).
Discussion
The normal macula is a highly specialized region of the human retina characterized by a foveal avascular zone, in addition to complete excavation of inner retinal neurons creating a pit, increased cone packing, and an absence of rod photoreceptors [5,7]. Although the macula represents a small area of the retina, it drives the majority of the visual function. The macula in albinism has been shown to lack a foveal pit, referred to as foveal hypoplasia, and to have a more loosely packed cone mosaic, indicative of an arrest in development [4]. In this study, we have shown that the height, width, and area of the FAZ are smaller in albinism, but despite quantifying these additional anatomic changes and comparing them to a control group, we are unable to find a relationship between these vascular abnormalities in the macula and BCVA to explain reduced visual function in albinism. Our study also showed that the distance from the temporal edge of the optic nerve to the center of the FAZ is significantly increased in albinism when compared to controls. Such corroborates the finding of a more temporally located preferred retinal focus in subjects with albinism [4]. The more temporal location of the center of the FAZ likely explains the clinical finding of positive angle kappa (visual axis located nasal to pupillary axis) in this population [8,9]. Merrill et al examined angle kappa in 178 patients with albinism and found that 99.6% had a positive angle kappa and 88% of the patients with angle kappa had at least a 2 mm nasal displacement of the light reflex detected with monocular fixation on a small light at near. Nasal displacement of the light reflex is also noted in individuals with cicatricial retinopathy of prematurity [9,10] due to temporal displacement of the macula (“temporal dragging”). A study based on OCT in 14 individuals with albinism showed that a larger angle kappa was associated with more subnormal visual acuity, but a temporally displaced preferred retinal locus was not consistently present [11]. In albinism, the more temporal location of the center of the FAZ, a positive angle kappa, and a temporal preferred retinal focus appear to be related to the more temporal line of decussation of the retinostriate fibers that has been demonstrated with visual evoked potentials and fMRI in this disorder [12,13].
The anatomy of the macula in albinism has been a long term interest in ophthalmology, as it provides an important characteristic for diagnosis and has been considered to be linked to the reduced BCVA in albinism. In 1908, Fritsch reported on the fovea of a Negro albino as having an unusual structure, while Elschnig, in 1913, described the complete absence of the fovea in patients with albinism [14,15]. Usher concluded that the main cause of defective vision and nystagmus in this population was the imperfectly developed or absent fovea [16]. Ultrastructural examination of the fovea in OCA showed absence of melanosomes in the retinal pigment epithelium [5].
With improved imaging, in vivo studies of the macula in albinism are possible. In 1978, Gregor, demonstrated abnormal retinal pigment epithelium and a direct anastomosis between the superior and inferior macular arcades by using fluorescein angiography [17]. Spedick and Beauchamp, in 1986, studied eighteen eyes of ten patients with albinism who had prominent retinal vessels coursing through the putative macular area instead of arching around it [18].
Advances in high-resolution ophthalmic imaging make it possible to quantitatively assess foveal morphology in the retina. Optical coherence tomography (OCT) offers high axial resolution and enables visualization of retinal lamination. Harvey et al studied 11 patients with albinism and a BCVA of at least 20/60 with the purpose of evaluating macular architecture and to determine if Stratus (time-domain) OCT was a more sensitive method of detecting rudimentary foveal development than indirect ophthalmoscopy. They found that indirect ophthalmoscopy was a more sensitive method of detection than OCT. In this study, even with multiple OCT images, detection of a rudimentary fovea was found in only four eyes of 11 patients [1]. Problems in detecting a central depression with OCT likely reflect imaging difficulty related to unsteady fixation when nystagmus is present. More recently, spectral domain optical coherence tomography (SD-OCT) has shown that there is some variation in foveal morphology among those with albinism [19-22], from a shallow foveal pit, and outer segment lengthening to fovea plana and persistent of multiple layers of inner retina that are normally absent in the fovea. A study of 6 patients with albinism (4 with OCA1B and 2 with OA1) and 167 controls with fundus photography and volumetric images of the macula using the HD-OCT showed a continuum of foveal maturity in albinism [4]. This spectrum of foveal development was nicely demonstrated with adaptive optics fundus photography by McAllister., et al. in 2007 [4]. They showed that foveal cone specialization is variable in individuals with albinism and concluded that while the appearance of in vivo imaging seems to correlate with BCVA, there are likely several features to account for reduced BCVA in an individual with albinism. Our study of several different parameters of the macular vasculature in albinism is, to our knowledge, the first study to provide quantitative measurement of macular vascular abnormalities in albinism, but it appears that these abnormalities are not a contributing factor to subnormal vision. Even though certain measurements of the FAZ were significantly different from controls, there was overlap among the two studied groups, as noted in the range for the measurements in Table 2.
The presumed relationship between high refractive errors and foveal abnormalities in patients with albinism suggests that emmetropization is abnormal [23]. Healey., et al. studied 50 patients with nystagmus, 33 of whom had albinism and found statistically significant differences when comparing the extent of foveal hypoplasia measured by OCT (foveal pit depth, widening of outer nuclear layer, lengthening of outer segments) to the spherical equivalent of the refractive error [24]. A study of 14 patients with albinism by McCafferty., et al. noted that better vision was associated with a defined FAZ and annular reflex in the macula, and that there is a spectrum of foveal morphology in albinism [11]. Our study did not include the extent of macular hypoplasia but when comparing the vascular characteristics in the macula to the spherical equivalent of refractive errors in albinism, we failed to find a significant relationship. The FAZ metrics might not have a direct relationship to described foveal morphology. OCT angiography might more accurately define the perifoveal vascular anatomy in albinism in the future [25]. Two patients with albinism have been recently studied with OCT angiography and showed no foveal avascular zone [26].
Strengths of this study include the number of subjects in the group with albinism and the comparison to a normal control group. All fundus photographs were of excellent quality. The software within the camera with telecentric optics corrected images for refractive error. In addition, BCVA was measured with standard testing procedures. We used digital fundus images to assess the FAZ and others have used fluorescein angiogram (FA) to better visualize the retinal vasculature [27]. However, angiography is more invasive due to the injection of the contrast agent into a peripheral vein, and cooperation among our pediatric patients would have been difficult. In addition, digital fundus images have proven to be an effective, non-invasive method of analyzing the foveal vasculature [28]. We are aware that some the microvasculature could be missed in a fundus photo; however, we applied same rigor in defining the edge of the FAZ in both groups. All the measurements were done by the same investigator and the technique was reviewed with a co-author who corroborated the extent of the FAZ. Although ideally we would mask the two groups, this could not be performed due to foveal dysmorphology characteristics of albinism being obvious to the observer.
Being a retrospective study carries the limitation of digital imaging not being available for all subjects with albinism seen at this institution. Therefore, consecutive patients enrolled in the aforementioned clinical trial were sampled. A potential limitation could also be the controls not being age matched; however, the youngest patient with albinism was three years old and we are unaware of any data suggesting that the retinal vasculature normally changes at age 3 or greater. Using age-matched controls was not feasible.
Conclusions
Despite finding significant differences in the FAZ of individuals with albinism compared to controls, this study failed to find a relationship between various macular parameters and reduced visual acuity in albinism, perhaps due to a multifactorial cause for reduced visual acuity. However, the finding of a more temporally located center of the FAZ in those with albinism compared to the control group points to a preferred retinal focus in albinism being modulated by the neural retina temporal to the site of normal (the fovea in normal eyes), demarcating the extent of fibers that cross to the contralateral occipital cortex. Such a temporal location of the FAZ also explains the finding of a positive angle kappa in albinism.
Acknowledgements
We thank Pat S. Harvey, CRA and Mark Cohen for their assistance obtaining fundus photographs.
We thank Pat S. Harvey, CRA and Mark Cohen for their assistance obtaining fundus photographs.
Conflict of Interest
None of the authors have a conflict of interest with the material in this manuscript.
None of the authors have a conflict of interest with the material in this manuscript.
References
- Harvey., et al. “Spectrum of foveal development in albinism detected with optical coherence tomography”. Journal of American Association for Pediatric Ophthalmology 10.3 (2006): 237-242.
- O’Donnell., et al. “X-linked ocular albinism in blacks”. Archives Ophthalmology 96.7 (1978): 1189-1192.
- Nettleship E. “Note on the retinal blood vessels of the yellow spot region”. Ophthalmic Hospital Report 8 (1876): 260-263.
- McAllister., et al. “Arrested development: High-resolution imaging of foveal morphology in albinism”. Vision Research 50.8 (2010): 810-817.
- Mietz., et al. “Foveal hypoplasia in complete oculocutaneous albinism, a histopathologic study”. Retina 12.3 (1992): 254-260.
- Summers CG. “Vision in albinism”. Transactions of the American Ophthalmological Society 94 (1996): 1095-1155.
- Dubis., et al. “Relationship between foveal avascular zone and foveal pit morphology”. Investigative ophthalmology & visual science 53.3 (2012): 1628-1636.
- Merrill., et al. “Positive angle kappa in albinism”. Journal of American Association for Pediatric Ophthalmology 8.3 (2004): 237-239.
- Brodsky and M Fray K. “Positive angle kappa: a sign of albinism in patients with congenital nystagmus”. American Journal of Ophthalmology 137.4 (2004): 625-629.
- Wu WC., et al. “Visual acuity, optical components, and macular abnormalities in patients with a history of retinopathy of prematurity”. Ophthalmology 119.9 (2012): 1907-1916.
- McCafferty BK., et al. “Clinical insights into foveal morphology in albinism”. Journal of Pediatric Ophthalmology and Strabismus 52.3 (2015): 167-172.
- Creel DJ., et al. “Visual anomalies associated with albinism”. Ophthalmic Paediatrics and Genetics 11.3 (1990): 193-200.
- Schmitz B., et al. “Morphology of the optic chiasm in albinism”. Der Ophthalmologe 104.8 (2007): 662-665.
- Fritsch GT. “Uber Bau und Bedeutung der Area centralis des Menschen”. Reimer (Berlin) (1906): 22.
- Elschnig A. “Zur Anatomie des menschlichen Albinoauges”. Archiv fur Ophthalmologie 84 (1913): 401-419.
- Usher C. “Histological examination of an adult human albino’s eyeball with a note of mesoblastic pigmentation in foetal eyes”. Biometrika 13 (1920): 46-56.
- Gregor Z. “The perifoveal vasculature in albinism”. British Journal of Ophthalmology 62.8 (1978): 554-557.
- Spedick M and Beauchamp G. “Retinal vascular and optic nerve abnormalities in albinism”. Journal of Pediatric Ophthalmology and Strabismus 23.2 (1986): 58-63.
- Chong GT., et al. “Abnormal foveal morphology in ocular albinism imaged with spectral-domain optical coherence tomography”. Archives ophthalmology 127.1 (2009): 37-44.
- Cronin TH., et al. “Spectral domain optical coherence tomography for detection of foveal morphology in patients with nystagmus”. Journal of American Association for Pediatric Ophthalmology 13.6 (2009): 563-566.
- Thomas MG.,et al. “Structural grading of foveal hypoplasia using spectral-domain optical coherence tomography”. Ophthalmology 118.8 (2011): 1653-60.
- Mohammad S., et al. “The functional significance of foveal abnormalities in albinism measured using spectral-domain optical coherence tomography”.Ophthalmology 118.8 (2011): 1645-1652.
- Wildsoet CF., et al. “Albinism: its implications for refractive development”. Investigative ophthalmology & visual science 41.1 (2000): 1-7.
- Healey N., et al. “Investigating the relationship between foveal morphology and refractive error in a population with infantile nystagmus syndrome”. Investigative ophthalmology & visual science 54.4 (2013): 2934-2939.
- Guo J., et al. “Repeatability and reproducibility of foveal avascular zone measurements using Angio Plex spectral domain optical coherence tomography angiography in healthy subjects”. Ophthalmologica 237.1 (2017): 21-28.
- Pakzad-Vaezi.,et al. “Optical coherence tomography angiography of foveal hypoplasia”. British Journal of Ophthalmology (2016).
- Alipour SH., et al. “Analysis of foveal avascular zone for grading of diabetic retinopathy severity based on curvelet transform”. Graefe's Archive for Clinical and Experimental Ophthalmology 250.11 (2012): 1607-1614.
- Fadzil A., et al. “Determination of foveal avascular zone in diabetic retinopathy digital fundus images”. Computers in Biology and Medicine 40.7 (2010): 657-664.
Citation:
C Gail Summers MD., et al. “Digital Image Analysis of the Foveal Avascular Zone in Albinism”. Ophthalmology and Vision Science
1.1 (2017): 46-54.
Copyright: © 2017 C Gail Summers MD., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.