Short Communication
Volume 1 Issue 6 - 2017
Computer Graphic Model of Hydroxyapatite Using Windows
Graduate School of Health Care Science, Jikei Institute, Master Course of Management in Health Care Sciences, Japan
*Corresponding Author: Masayuki Okazaki, Graduate School of Health Care Science, Jikei Institute, Master Course of Management in Health Care Sciences, 1-2-8 Miyahara, Yodogawa-ku, Osaka 532-0003, Japan.
Received: November 14, 2017; Published: November 18, 2017
Abstract
Hydroxyapatite is an inorganic component of bone and teeth. Stoichiometric hydroxyapatite is an ionic crystal with Ca2+, PO43-, and OH- as the main components, and the crystal system is hexagonal. In this study, we considered the application of the PC software “PyMOL” to hydroxyapatite. Although this software requires the three- dimensional coordinates of each molecule, we could use the data which were obtained in our previous study. Using this software, we can freely view the stereo structure of any molecules from any direction simply by operating a PC mouse. In addition to the CG of hydroxyapatite, we could show the CG of fluoridated hydroxyapatite with the partial substitution of OH- by F-, and Mg- containing hydroxyapatite with that of Ca2+ by Mg2+.
Introduction
Bone and teeth are composed of an inorganic hydroxyapatite and organic collagen. Tooth enamel contains more than 95% of highly crystallized hydroxyapatite, while bone and tooth dentine contain 60-70% of poorly crystallized hydroxyapatite [1,2]. Strictly speaking, they are carbonate-containing hydroxyapatites, so-called “carbonate apatites” (CO3Ap), which contain several % of CO32- ions [3].
Stoichiometric hydroxyapatite (HAp: Ca10 (PO4)6(OH)2) is an ionic crystal with Ca2+, PO43-, and OH- as the main components, and the crystal system is hexagonal (a = b = 9.432 Å, c = 6.881 Å, α = β = 90º, γ = 120º) [4,5]. It has been reported that almost all elements in the periodic table can adopt the positions of the main components above-mentioned [6]. Therefore, “apatites” has the meaning of thieves because they are very complicated substances from the Greek language. Thus, real apatites contain many trace elements.
The analysis of hydroxyapatite was delayed until 1964 [5], although the crystal structure of fluorapatite (FAp: Ca10 (PO4)6F2) was initially analyzed in 1930 by Náray-Szabó [7]. Because OH- ions in hydroxyapatite shift just 0.3 Å from the stable position, it was difficult to determine the accurate crystal structure solely by X-ray diffraction analysis. Finally, it was determined by combining with neutron diffraction analysis [8]. Hydroxyapatite is sudo-hexagonal (rhombohedral), while FAp is a typical hexagonal crystal. Due to the development of computer science, Okazaki and Sato [9] could successfully construct a crystal model of HAp with a personal computer and the program “PROTGRA” [10]. In their paper, the computer graphics (CG) of HAp and FAP were shown accurately according to the radii of Ca2+ (0.99 Å), P5+ (0.33 Å), O2- (1.40 Å), H+ (negligible), and F- (1.36 Å) based on the data of Pauling [11]. OH- ions in hydroxyapatite shift just 0.3 Å from the stable position, while F- ions in fluorapatite are present at levels of just 1/4 and 3/4 of the height of the crystal unit cell. Lattice dimensions of a = 9.37 Å and c = 6.88 Å were adopted from the data reported by Náray-Szabó [7]. Compared with the structure of hydroxyapatite, that of fluorapatite can readily be seen to be crystallographically more stable. The computer graphics display demonstrated that fluoride ions serve to stabilize the hydroxyapatite crystals and prevent dental caries.
Methods
Recently, the PC software “PyMOL”, which can draw polymer CG on Windows, is often used worldwide. We considered the application of “PyMOL” to hydroxyapatite. At present, “PyMOL” is licensedby Schrödinger. We have already succeeded in the CG drawing of tetrafluoroethylene (PTFE) [12]. Using this software, we can freely view the stereo structure of any molecules. However, this software requires the three- dimensional coordinates of each molecule. Fortunately, we could use the data shown in Table 1, which were obtained in our previous study [9].
The atomic radius of each element is set as the Von der Waals radius by default, and a ball and stick (BS) model is initially drawn. Therefore, each radius was changed into an ionic radius based on Pauling data [11], and then modified slightly to be able to visualize it clearly as follows: Ca2+ (0.99 Å), P5+ (0.33 Å → 0.70 Å), O2- (1.40 Å → 1.20 Å), H+ (negligible → 0.40 Å), F- (1.36 Å → 1.20 Å), and Mg2+ (0.65 Å)
Computer Graphics
Figure 1 shows the original BS model of the hydroxyapatite with the Van der Waals radius displayed from directions perpendicular (Figure 1A) and parallel (Figure 1B) to the c-axis. Figure 2 is an ionic model observed from the same direction as Figure 1. Figure 3 shows the posterior view. We can view the structure freely from any direction simply by operating a PC mouse.
Figure 1 shows the original BS model of the hydroxyapatite with the Van der Waals radius displayed from directions perpendicular (Figure 1A) and parallel (Figure 1B) to the c-axis. Figure 2 is an ionic model observed from the same direction as Figure 1. Figure 3 shows the posterior view. We can view the structure freely from any direction simply by operating a PC mouse.
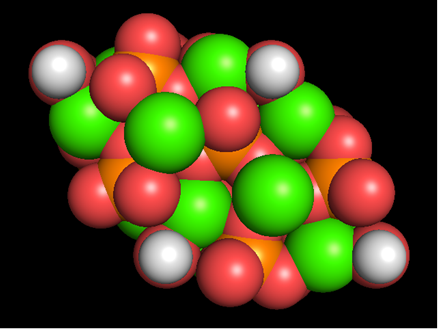
Figure 1: Computer graphics-based Van der Waals model of hydroxyapatite viewed from directions perpendicular (A) and parallel (B) to the c-axis (A), with data obtained by Okazaki and Sato [9] using Windows.
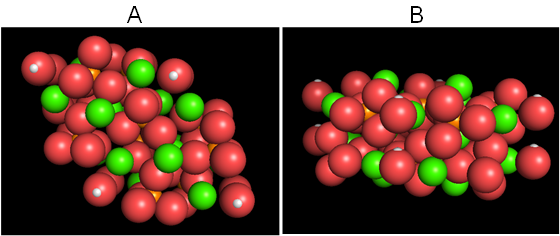
Figure 2: Computer graphics-based ionic model of hydroxyapatite viewed from directions perpendicular (A) and parallel (B) to the c-axis (A). The ionic radii are changed rather than the value of Pauling [11]. Ca2+ (0.99 Å, green), P5+ (0.33 Å → 0.70 Å, orange), O2- (1.40 Å → 1.20 Å, red), H+ (negligible → 0.40 Å, white).
Furthermore, as mentioned above, almost all of the elements in the periodic table can replace for the main components of Ca2+, PO43-, and OH- [4]. Figure 4, as an example, shows the CG of fluoridated hydroxyapatite with the partial substitution of OH- by F- (Figure 4A), and Mg-containing hydroxyapatite with that of Ca2+ by Mg2+ (Figure 4B). Interestingly, some of the crystallographic properties of partially substituted fluoridated hydroxyapatites still remain unknown, although many studies have been conducted [13-16].
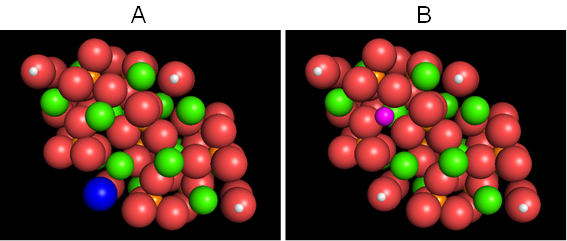
Figure 4: The CG of fluoridated hydroxyapatite partially substituted by F- for OH- (A), and Mg-containing hydroxyapatite partially substituted by Mg2+ for Ca2+ (B). F- (1.36 Å → 1.20 Å, blue), and Mg2+ (0.65 Å, purple).
Future Trends
Bological apatites such as bone and teeth are carbonate-containing hydroxyapatite (CO3Ap). A number of crystallographic studies on CO3Ap have been reported [3,15, 17-20]. Enamel apatite contains 1~3 wt% of CO32- ions, and bone and dentine contain more CO32- ions, 5~6 wt% [2]. It is known that CO32- ions of biological apatites, which are created in aqueous solution, adopt mainly PO43- positions, contrary to CO3Ap synthesized under dry conditions at a high temperature, in which CO32- ions adopt OH- positions. Interestingly, since the molecular weight of hydroxyapatite is 1,000 and that of CO32- ions is 60 (6 wt%), in the case of bone apatite, approximately one CO32- ion can replace six PO43- ions in a unit cell of hydroxyapatite.
Bological apatites such as bone and teeth are carbonate-containing hydroxyapatite (CO3Ap). A number of crystallographic studies on CO3Ap have been reported [3,15, 17-20]. Enamel apatite contains 1~3 wt% of CO32- ions, and bone and dentine contain more CO32- ions, 5~6 wt% [2]. It is known that CO32- ions of biological apatites, which are created in aqueous solution, adopt mainly PO43- positions, contrary to CO3Ap synthesized under dry conditions at a high temperature, in which CO32- ions adopt OH- positions. Interestingly, since the molecular weight of hydroxyapatite is 1,000 and that of CO32- ions is 60 (6 wt%), in the case of bone apatite, approximately one CO32- ion can replace six PO43- ions in a unit cell of hydroxyapatite.
We can also draw the CG of CO3Ap. However, the accurate direction of CO32- is still unknown, because the content of CO32- ions in CO3Ap is small, and the crystallinity of CO3Ap decreases with an increase in the CO32- ion content. At present, we consider the possibility of CO32- existing parallel to the c-axis being strong from the viewpoint of the shortening of the a-axis observed on X-ray diffraction analysis.
References
- Brown WE. “Crystal growth of bone mineral”. Clinical Orthopaedics and Related Research 44 (1966): 205-220.
- Miles AEW. “Structural and Chemical Organization of Teeth. Vol. II”. New York, Academic Press (1967).
- LeGeros RZ. “Calcium Phosphates in Oral Biology and Medicine”. Basel, Karger (1991).
- Cullity BD. “Elements of X-Ray Diffraction. Second Ed”. Reading, Addison-Wesley Publishing Company (1978).
- Kay MI., et al. “Crystal structure of hydroxyapatite”. Nature 204 (1964): 1050-1052.
- Van Wazer JR. “Phosphorus and Its Compounds”. New York, Interscience (1958).
- St Náray-Szabó. “The structure of apatite (CaF)Ca4(PO4)3”. Z Kristallogr 75.1 (1930): 387.
- Sudarsanan K and Young RA. “Significant precision in crystal structure details: Holly springs hydroxyapatite”. Acta Crystallographica Section B25 (1969): 1534-1543.
- Okazaki M and Sato M. “Computer graphics of hydroxyapatite and beta-tricalcium phosphate”. Biomaterials 11.8 (1990): 573-578.
- Nakano H. “Graphics display of protein by personal computer”. Information 5.10 (1986): 65
- Pauling L. “The Nature of the Chemical Bond. 3rd edn”. Ithaca, Cornell University Press (1960).
- Furuta M. et al. “Molecular level analyses of mechanical properties of PTFE sterilized by Co-60 γ-ray irradiation for clinical use”. Radiation Physics and Chemistry 139 (2017): 126-131.
- Moreno EC., et al. “Physicochemical aspects of fluoride apatite systems relevant to the study of dental caries”. Caries Research 11 (Suppl. 1) (1977): 142-171.
- Okazaki M., et al. “Solubility and crystallinity in relation to fluoride content of fluoridated hydroxyapatites”. Journal of Dental Research 60 (1981): 845-849.
- Elliott JC. “Structure and Chemistry of the Apatites and Other Calcium Orthophosphates”. Amsterdam, Elsevier (1994).
- Tohda H., et al. “Transmission electron microscopic observation of heterogeneous fluoridated hydroxyapatites”. Biomaterials 16.12 (1995): 945-950.
- LeGeros RZ., et al. “Apatite crystallites: Effects of carbonate on morphology”. Science 155.3768 (1967): 1409-1411.
- Okazaki M., et al. “Solubility behavior of CO3 apatites in relation to crystallinity”. Caries Research 15.6 (1981): 477-483.
- Okazaki M., et al. “Insolubilized properties of UV-irradiated CO3 apatite-collagen composites”. Biomaterials 11.8 (1990): 568-572.
- Yamasaki Y., et al. “Action of FGMgCO3Ap-collagen composite in promoting bone formation”. Biomaterials 24.27 (2003): 4913-4920.
Citation:
Masayuki Okazaki. “Computer Graphic Model of Hydroxyapatite Using Windows”. Orthopaedic Surgery and Traumatology
1.6 (2017): 237-241.
Copyright: © 2017 Masayuki Okazaki. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.




































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.