Research Article
Volume 2 Issue 1 - 2018
Short Term Anti TB Regime in Management of Pulmonary Tuberculosis
1MBBS (MGIMS): MD (Internal Medicine) DNB (E&M), PhD National Institute of Health & Research Warisaliganj (Nawada); Bihar 805130, India
2BAMS(BRABU): MHA;MPH Director (Hon) Institute of Applied Medicine Aarogyam punarjeevan, Aara Garden, Jagdeo path ,Baily Road Patna 14, Bihar
3BAMS (BRABU): Sr. Research Fellow, Regional Institute of Ayurveda, Tanager (Arunachal) (Under CCRAS, Government of India)
2BAMS(BRABU): MHA;MPH Director (Hon) Institute of Applied Medicine Aarogyam punarjeevan, Aara Garden, Jagdeo path ,Baily Road Patna 14, Bihar
3BAMS (BRABU): Sr. Research Fellow, Regional Institute of Ayurveda, Tanager (Arunachal) (Under CCRAS, Government of India)
*Corresponding Author: Avinash Shankar, MBBS (MGIMS); MD (Internal Medicine) DNB (E&M), PhD National Institute of Health
Research Warisaliganj (Nawada) Bihar 805130, India.
Received: July 14, 2018; Published: August 09, 2018
Abstract
Tuberculosis becoming worse due to increasing incidence of resistant strains of Mycobacterium tuberculosis bacilli to available therapeutics i.e. emergence of TDRTB (Total drug resistance tuberculosis) and progressive increasing prevalence of the diseases. TB statistics in India suggest estimated incidence of 2.79 million in spite of W.H.O supported TB Control program i.e.- Revised National Tuberculosis Program (RNTCP) may be due to increased endemicity Declining nutrition and economic status pose increased threat of tuberculosis even among upper class people and emergence of resistant strain of Mycobacterium Tuberculosis due to increased rate of spontaneous mutation. In addition hepatotoxicity due to commonly prescribed anti TB drugs, a safe potent drug remain the need of time, thus to approve the clinical significance of Levofloxacin and isoniazid in treatment of tuberculosis, an extended study was done.
Objective: To approve and reaffirm the clinic pathological findings of previous study i.e. - Levofloxacin and Isoniazid combination in achieving cure with safety in TB management.
Material and Methods: 1000 cases of clinic pathologically and radiologically established cases of pulmonary tuberculosis attending at Institute of Applied Medicine, Aarogyam Punarjeevan, Ram Bhawan, Ara Garden, Jagdeo path Baily Road Patna 14 and National Institute Of Health & Research, Warisaliganj (Nawada) Bihar were selected for the study .Each patients after investigating for base bio parameters were given trial drug and were evaluated for pre and post therapy vital capacity of lung (Spirometry ), sputum examination, radiological resolution, appetite improvement, gain in body weight and compliance while other haematological, hepatic and renal parameter are repeated to ascertain drug or disease related effects.
Result: Affirm and re approve the clinical outcome i.e. - early sputum conversion, resolution of radiological lesion and alleviation of presenting features in mean duration of 60 ± 5 days and mean weight gain of 6 ± 2Kg, non-recurrence and non-relapse in 2 years of post-therapy follow up, improved drug compliance and utility, improved body physique and 100 % drug compliance without any adversity.
Conclusion: The two drug regime i.e. - Levofloxacin 500mg and Isoniazid 300mg in adult achieves complete sputum sterilization or smear negative by 2 months therapy and 100% recovery with excellent lungs vitality and body weight gain without any drug adversity or disease sequel, thus a worthy prescription to eradicate TB rather to control epidemics of TB.
Keywords: Emergence; TDR TB; endemicity; sputum conversion; Lung vitality; Spirometry; Clinical outcome; Compliance; Disease sequel;
Drug adversity
Introduction
Tuberculosis, a widely spreading infectious disease prevalent in India. As per W.H.O India contributes 1/5th of worldwide tuberculosis infection every year i.e.- 9.4 million or 1.9 million every year and 3.3 million people suffer from one or other form of Tuberculosis and 2,76000 people die every year [1,2].
Average prevalence of all forms of tuberculosis in India is estimated to be 5.05/thousands, prevalence of smear positive cases is 2.27/thousand and average annual incidence of smear positive case is 84/lakh, In India each year approx. 2,20,000 death are reported due to tuberculosis and continue to be the biggest health problem Some epidemiologist forecast a rise of 20% rise in incidence in next 20 years and RNTCP was considered to curb its epidemiological situation, in spite India has highest burden of tuberculosis and as per WHO report India has estimated burden of 2.79 million cases of tuberculosis [3-5].
As per National Tuberculosis control program incidence of Tuberculosis in India is 1.76/lakh population and prevalence is 230/lakh while mortality is 22/lakh population [6].
In spite of W.H.O supported Revised National Tuberculosis Control Program (RNTCP) effective control and cure still remain a continuing major setback. Tuberculosis has become a silent contagious disease with devastating effect on life [7]. India is the higher TB burden country and patients are emerging as dreaded drug resistance form of tuberculosis i.e. - TDR-TB (Total drug resistance TB). Different drugs of the Anti-Tuberculosis regime have different mode of action [8,9] i.e. - INH is bactericidal against replicating bacteria, Rifampicin is bactericidal and possess sterilizing effect, Pyrazinamide weekly bactericidal only in acidic environment but among them Rifampicin and Pyrazinamide are potent hepatotoxic [10-13].
Patients are termed as failed treatment if
- Fails to respond to treatment (Fever persist, cough with sputum persistence throughout the treatment)
- Experience a transient response (patient gets better in the beginning but later become worse)
As per evident clinical response in our previous study , an extensive study been done to reapprove the facts observed to ensure better regime for effective cure , control of the disease and improved quality of life .
Objective of the study
To ascertain the observation of previous study covering large number of patients ie- re approve the short term two drugs regime in management of pulmonary tuberculosis.
To ascertain the observation of previous study covering large number of patients ie- re approve the short term two drugs regime in management of pulmonary tuberculosis.
Duration of Study
January 2015 to April 2018 which also includes 2 years of post-therapy follow up.
January 2015 to April 2018 which also includes 2 years of post-therapy follow up.
Aim of tuberculosis treatment
To ensure the prime objective i.e. to ensure cure with natural repair without drug adversity or disease sequel, the treatment aimed as-
To ensure the prime objective i.e. to ensure cure with natural repair without drug adversity or disease sequel, the treatment aimed as-
- Early elimination of active causative pathogen from body fluids
- Natural repair of damaged tissue to retain normal body biodynamics
- Least drug untoward effect
- Short course therapy
- Minimum drug regime, constitutes no drug of proven drug hazard or dyskinesia
- Drugs must have potent bactericide broad spectrum coverage with high volume distribution and long half-life.
- 100% compliable and dose convenience
Considering the fact two drug regime constituting potent quinolone:
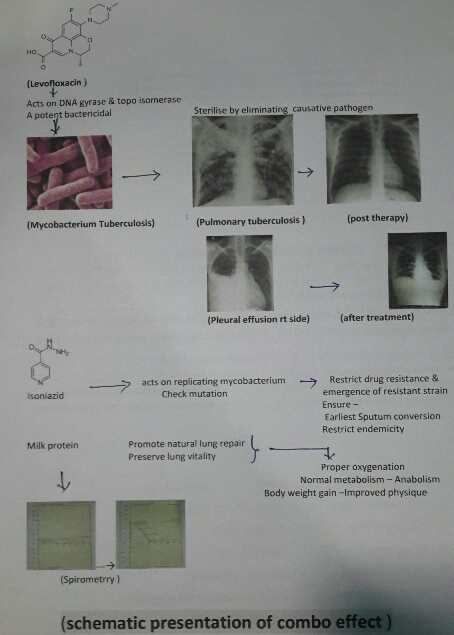
Levofloxacin an optical isomer of Ciprofloxacin having high volume distribution and bactericidal effect on Mycobacterium tuberculosis and Isoniazid, a potent bactericidal having effect on replicating mycobacterium tuberculosis with supplementation of milk protein.
Levofloxacin an optical isomer of Ciprofloxacin having high volume distribution and bactericidal effect on Mycobacterium tuberculosis and Isoniazid, a potent bactericidal having effect on replicating mycobacterium tuberculosis with supplementation of milk protein.
Material and Methods
Material
Patients attending medical OPD of National Institute of Health & Research (RA.Hospital & Research Centre) Warisaliganj and Aarogyam punarjeevan, Ara Garden Road, Jagdeopath, Patna 14 been considered as per following index [14,15] Patients presenting with
Patients attending medical OPD of National Institute of Health & Research (RA.Hospital & Research Centre) Warisaliganj and Aarogyam punarjeevan, Ara Garden Road, Jagdeopath, Patna 14 been considered as per following index [14,15] Patients presenting with
- Evening rise of temperature
- Chronic cough since >2 months duration
- Pain in chest
- Loss of appetite
- Progressive debility and weight loss
- Haemoptysis
- Breathlessness
Patients presenting with above complaints, already consumed multi drug regime or HIV positive or with associated other disease been excluded from the study.
Method
Selected patients were thoroughly interrogated for their clinical presentation, treatment taken and their response. All the patients were clinically examined and investigated for the following [16-29]
Selected patients were thoroughly interrogated for their clinical presentation, treatment taken and their response. All the patients were clinically examined and investigated for the following [16-29]
- Body weight
- Sputum for AFB
- Blood for ESR and CBC patients after due awareness
- X ray chest to explore and asses degree of lesion
- Blood for immunological assessment (IgM &IgG for Tuberculosis)
- Spirometry to asses lung viability
- Haematological, Hepatic and renal status to ascertain safety profile.
Tab Levofloxacin 500mg after food at fixed time 9 AM
Tab Isoniazid 300mg, 1 tab daily at 10 AM
Supportive nutrition: Milk 1 kg in either form daily
Tab Isoniazid 300mg, 1 tab daily at 10 AM
Supportive nutrition: Milk 1 kg in either form daily
Patients were followed weekly for their clinical presentation i.e.- temperature, bout of cough, amount of expectoration, status of appetite, body weight, haematological, hepatic and renal status with pulmonary function In addition Sputum was evaluated every week for sputum conversion, x ray chest every 3 month and spirometry after 6 moth of therapy [30].
Patients were followed up for next 1 year on every 2 months interval for any recurrence, decline if vital function, thus x ray chest, haematological, hepatic and renal para meters were repeated to asses any drug adversity and spirometry to adjudge nature of cure (status of pulmonary function)
Observation
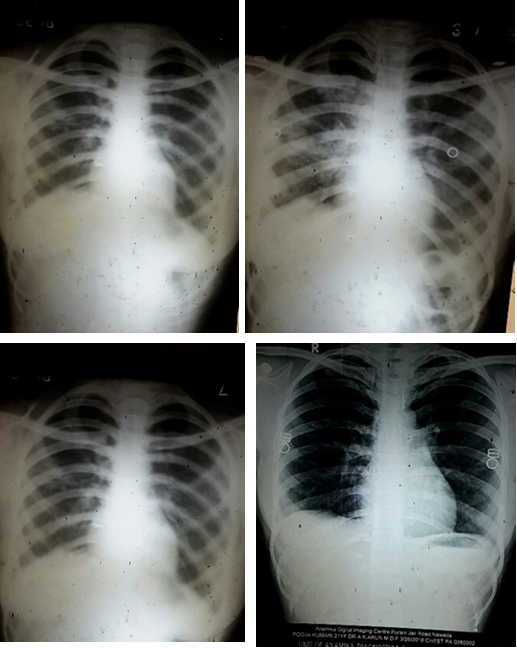
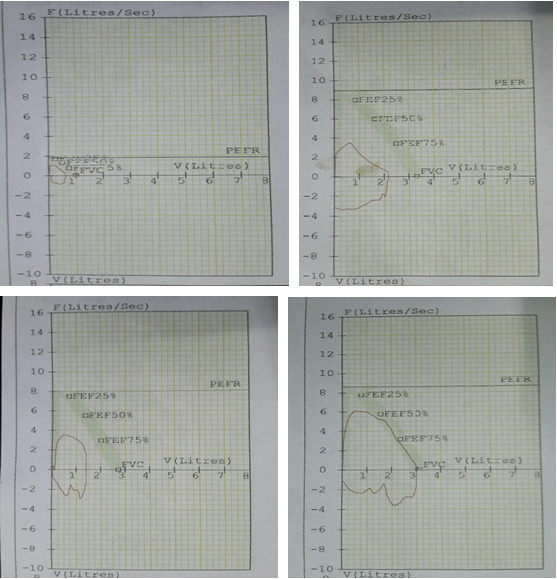
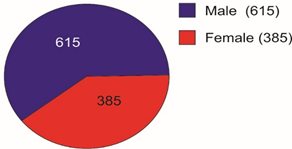
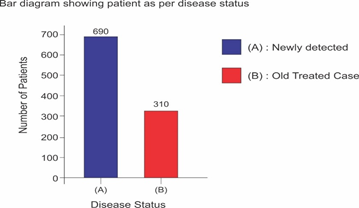
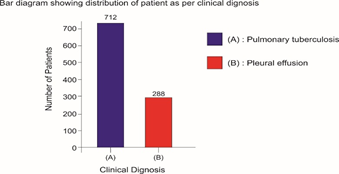
Selected patients were of age group 20-45 years and majority 24.7% were of age group 25-30 years (T-1). Among them 615 were male and 385 were female (figure -1) and 690 were newly detected and rest 310 were old treated cases of tuberculosis (figure -2) Clinically out of all 712 (71.2%) cases were of pulmonary tuberculosis and 288 (28.8%) were of pleural effusion (figure -3) The commonest clinical presentation among them was evening rise of temperature ,chronic and persistent cough ,progressive weight loss ,loss of appetite and general debility, 70% cases presented with haemoptysis, 28.8% with agonising chest pain and 31% with breathlessness (T-2) Among them only 9.5% patients had body weight at par with their Ideal body weight while others lower than IBW, 47.7% with IBW-2SD,37.6% with IBW-3SD (T3) Out of all 72.3% patients were sputum positive while immunological test for tuberculosis was positive in 89% cases, though radiological examination and BCG diagnostic test shown positive for tuberculosis evidence in all the cases (T-4) Assessment after 2 months of therapy all shows sputum conversion ,improved appetite ,weight gain with radiological resolution in 98.2% (Figure A) and improved hepatic function 98.7% without any alter Nance in renal function (T-6) 97.9% shows improved ventilator function as assessed by spirometry (Figure B ) (T-7)Overall clinical response on completion of therapy was indexed as grade I in 98.7% and rest 1.8% grade II clinical response (T-8)
Discussion
Incidence of pulmonary tuberculosis increasing very rapidly in spite of W.H.O intervention and revised national tuberculosis control program and DOTS effort. Tuberculosis bacilli drug resistance also on fast pace and today clinical status TDR-TB (Total drug resistance tuberculosis) has emerged. Considering the outcome in previous study and role of milk protein supplementation in repair of damaged and diseased lung parenchyma, this extensive study also justify the previous outcome. Though sputum conversion, radiological resolution of the lesion are considered as prime index of assessment but even today gain in body weight remain the perfect index of improved lung capacity to enhance gaseous exchange and anabolism, presently adjunct with spirometry to asses lung parenchymal viability and vitality. Achievement of complete recovery, weight gain, early sputum conversion, improved pulmonary bed viability and pulmonary function i.e.- improved physique in all the cases of both fresh old cases are due to [31] Levofloxacin acts as a potent bactericide and sterilizes the lung parenchyma and the body fluids due to its high volume distribution and effect on DNA gyrase & Topo isomerase I-IV [32], ensure early and complete sputum conversion ,prompt natural healing of the pulmonary lesion and ensure better gaseous exchange with weight gain of 7.5 ± 1.2 Kg in 60 days therapy. None show any drug adversity or dyskinesia.
Isoniazid also act as bactericidal on replicating mycobacterium bacilli thus with Levofloxacin completely restrict any chance of drug resistance or drug failure [33] Milk protein supplement ensure natural healing of damaged tissue and prompt anabolism facilitating body weight gain [34].
Early sputum conversion restrict expectoration of active infective mycobacteria bacilli thus also limit endemicity. 3 years post therapy follow up affirms absence of remission, any drug related or disease related untoward effects
Conclusion
The two drug regime i.e. - Levofloxacin 500mg and Isoniazid 300mg in adult achieves complete sterilization or smear negative by 2 months therapy and 100% recovery with excellent lungs vitality and body weight gain without any drug adversity or disease sequel, thus a worthy prescription to eradicate TB rather to control epidemics of TB.
| Age group (in years) |
Number of patients | |||
| Male | Female | Total | % | |
| 20-25 | 99 | 56 | 155 | 15.5 |
| 25-30 | 166 | 81 | 247 | 24.7 |
| 30-35 | 135 | 100 | 235 | 23.5 |
| 35-40 | 102 | 98 | 200 | 20.0 |
| 40-45 | 113 | 50 | 163 | 16.3 |
Table 1: Age and sex wise distribution of patients.
Pie diagram showing Male Female Composition
Bar diagram showing distribution of patient as per clinical dignosis
| Clinical presentation | Number of patients | |||
| Male | Female | Total | % | |
| Evening rise of temperature | 615 | 385 | 1000 | 100.0 |
| Haemoptysis | 410 | 290 | 700 | 70.0 |
| Breathlessness | 206 | 104 | 310 | 31.0 |
| Persistent cough | 615 | 385 | 1000 | 100.0 |
| Progressive weight loss | 615 | 385 | 1000 | 100.0 |
| Pain in chest | 198 | 90 | 288 | 28.8 |
| General debility | 615 | 385 | 1000 | 100.0 |
Table 2: Distribution of patients as per their clinical presentation.
| Body weight (Ideal body weight -SD) | Number of cases | Percentage |
| IBW- 1SD | 52 | 5.2 |
| IBW-2SD | 477 | 47.7 |
| IBW-3SD | 376 | 37.6 |
| IBW | 95 | 9.5 |
| Key: SD = 2.5 kg | ||
Table 3: Distribution of patients as per body weight.
| Pathological status Sputum for Acid Fast Bacilli | Number of patients | % |
| Positive | 723 | 72.3 |
| Negative | 277 | 27.7 |
| Immunological test for Tuberculosis | ||
| Positive | 890 | 89.0 |
| Negative | 110 | 11.0 |
| Radiological | ||
| Present | 1000 | 100.0 |
| Negative | 0000 | None |
Table 4: Distribution of patients as per pathological status.
| Bio parameters Haematology | Number of Patients | ||||
| Male | Female | Total | % | ||
| Haemoglobin | |||||
| <10gm% | 290 | 220 | 510 | 51.0 | |
| >10gm% | 325 | 165 | 490 | 49.0 | |
| Hepatic profile | |||||
| SGOT | |||||
| <35 IU | 435 | 257 | 692 | 69.2 | |
| >35IU | 180 | 108 | 288 | 28.8 | |
| SGPT: | |||||
| <35 IU | 435 | 257 | 692 | 69.2 | |
| >35 IU | 180 | 108 | 288 | 28.8 | |
| Alkaline phosphatase | |||||
| <140 | 615 | 385 | 1000 | 100.0 | |
| >140 | - | - | 0000 | 00 | |
| Renal profile | |||||
| Blood Urea | |||||
| <26 mg% | 615 | 385 | 1000 | 00 | |
| >26mg% | - | - | 0000 | 00 | |
| Serum creatinine | |||||
| <1.5mg% | 615 | 385 | 1000 | 100.0 | |
| >1.5mg% | - | - | 0000 | 00.0 | |
| Urine Protein: | |||||
| Absent | 540 | 367 | 907 | 90.7 | |
| Present | 75 | 18 | 93 | 9.3 | |
Table 5: Distribution of patients as per their basic bio parameters.
| Parameters | Number of patients | |||
| Male | Female | Total | % | |
| Sputum Conversion | ||||
| Smear Positive | None | None | None | 0 |
| Smear Negative | 615 | 385 | 1000 | 100 |
| Radiological status | ||||
| Resolution of lesion | ||||
| Complete | 602 | 380 | 982 | 98.2 |
| Improved | 13 | 05 | 18 | 1.8 |
| Persistent | None | None | None | 0 |
| Body weight | ||||
| Weight gain | 615 | 385 | 1000 | 100 |
| Weight loss | None | None | None | 00 |
| No change | None | None | None | 00 |
| Haematology | ||||
| Haemoglobin | ||||
| Increased | 602 | 380 | 982 | 98.2 |
| Decreased | None | None | None | 00 |
| Unchanged | 13 | 05 | 18 | 1.8 |
| Hepatic profile | ||||
| Improved | 604 | 383 | 987 | 98.7 |
| Unchanged | 11 | 02 | 13 | 1.3 |
| Appetite | ||||
| Improved | 615 | 385 | 1000 | 100 |
| Unchanged | None | None | None | 00 |
| Suppressed | None | None | None | 00 |
| Renal function | ||||
| Altered | None | None | None | 00 |
| Unchanged | 615 | 385 | 1000 | 100 |
| Compliance | ||||
| Rejection | none | None | None | 00 |
| Continuance | 615 | 385 | 1000 | 100 |
Table 6: Status of patients after 2 months therapy.
| Spirometry status | Number of patients | % |
| Restricted ventilatory capacity | none | 00 |
| Unaltered ventilatory capacity | 21 | 2.1 |
| Improved ventilatory capacity | 979 | 97.9 |
Table 7: Distribution of patients as per post therapy spirometry.
| Clinical grade | Number of patients | % |
| Grade I | 987 | 98.7 |
| Grade II | 13 | 1.3 |
| Grade III | none | 00 |
Table 8: Clinical outcome.
References
- “World Health Organization Global tuberculosis control: epidemiology, strategy, financing”. Epidemiology Retrieved 12 (2009): 6-33.
- WHO. Global tuberculosis control. WHO reports? WHO/HTM/TB/2006.362. Geneva: World Health Organization (2006).
- “World Health Organization Global Tuberculosis Control: A Short Update to the 2009 Report”. Geneva, Switzerland: World Health Organization; (2009).
- “World Health Organization Global Tuberculosis Control: Epidemiology, Strategy, Financing: WHO Report 2009”. Geneva, Switzerland: World Health Organization (2009).
- Lonnroth K., et al. “Global epidemiology of tuberculosis: prospects for control”. Seminars in Respiratory and Critical Care Medicine29(2008): 481-491.
- TB Statistics for India. 2012 TB Facts. Retrieved April 3"The Stop TB Strategy, case reports, treatment outcomes and estimates of TB burden" (2013).
- “Global tuberculosis control: epidemiology, strategy, financing”. World Health Organization (2009):187–300. Retrieved (2009): 11-14.
- Rowland K. “Totally drug-resistant TB emerges in India: Nature News & Comment. Nature Publishing Group: science journals, jobs, and information”. Retrieved April 3, 2013, from (2012).
- Udwadia. “Totally drug-resistant tuberculosis (TDR-TB) in India: every dark cloud has a silver lining”. Journal of Epidemiology & Community Health - BMJ Journals Retrieved April 3, 2013, from (2012).
- Tuberculosis - Causes, Symptoms, Treatment, Diagnosis. (2013). C-Health. Retrieved April 3, 2103, from.
- Mishra G., et al. “Tuberculosis Prescription Practices In Private And Public Sector In India”. National Journal of Integrated Research in Medicine 4.2 (2013): 71-78.
- Gyanshankar Mishra., et al. “XDR-TB: An outcome of programmatic management of TB in India”. Indian Journal of Medical Ethics 11.1 (2011): 47-52.
- Lonnroth K., et al. “Tuberculosis control and elimination 2010-50: cure, care, and social development”. The Lancet 375 (2010): 1814-1829.
- Poulsen A. “Some clinical features of tuberculosis; 1: incubation period”. Acta Tuberc et Pneumol Scand, Copenhagen 24.4 (1950): 311-346.
- Poulsen A. “Some clinical features of tuberculosis [concl]”. Acta Tuberc et Pneumol Scand, Copenhagen 33.1 (1957): 37-92.
- Krysl J., et al. “Radiologic features of pulmonary tuberculosis: an assessment of 188 cases”. Canadian Association of Radiologists Journal45 (1994): 101-107.
- Lee KS., et al. “Adult-onset pulmonary tuberculosis: findings on chest radiographs and CT scans”. American Journal of Roentgenology160(1993): 753-758.
- Im JG., et al. “Pulmonary tuberculosis: CT findings–early active disease and sequential change with antituberculous therapy”. Radiology 186(1993): 653-660.
- Marciniuk DD., et al. “Detection of pulmonary tuberculosis in patients with a normal chest radiograph”. Chest 115 (1999):445-452.
- Pepper T., et al. “Normal chest radiography in pulmonary tuberculosis: implications for obtaining respiratory specimen cultures”. International Journal of Tuberculosis and Lung Disease 12 (2008):397-403.
- Berger HW., et al. “Tuberculosis pleurisy”. Chest 63 (1973): 88-92.
- Valdes L., et al. “Tuberculous pleurisy: a study of 254 patients”. Archives of Internal Medicine158 (1998): 2017-2021.
- Gopi A.,et al. “Diagnosis and treatment of tuberculosis pleural effusion in 2006”. Chest 131(2007): 880-889.
- Sharma SK.,et al. “Miliary tuberculosis: new insights into an old disease”. The Lancet Infectious Diseases 5(2005): 415-430.
- Kim JH., et al. “Miliary tuberculosis: epidemiology, clinical manifestations, diagnosis, and outcome”. Reviews of Infectious Diseases 12 (1990): 583-590.
- Piqueras AR., et al. “Miliary tuberculosis and adult respiratory distress syndrome”. Journal of Intensive Care Medicine 13 (1987): 175-182.
- “American Thoracic Society Diagnostic standards and classification of tuberculosis in adults and children”. American Journal of Respiratory and Critical Care Medicine161. 4 (2000): 1376-1395.
- Shinnick TM., et al. “Diagnostic mycobacteriology laboratory practices”. Clinical Infectious Diseases 21.2(1995): 91-299.
- Francis J. “Curry National Tuberculosis Center. Sputum induction. In: Tuberculosis Infection Control: A Practical Manual for Preventing TB”. San Francisco, CA: University of California; 2007:73-86.
- Shankar A., et al. “Clinical evaluation of short term regime in management of Pulmonary tuberculosis”. British Biomedical Bulletin (BBB) 4.3 (2015): 500-507.
- Mitchison DA. “Assessment of new sterilizing drugs for treating pulmonary tuberculosis by culture at 2 months”. The American Review of Respiratory Disease Returns 147(1993): 1062-1063.
- Fish DN., et al. “The clinical pharmacokinetics of levofloxacinClin Pharmacokinetic”. 1997 32.2 (1997): 101-119.
- Timmins GS., et al. “Mechanisms of action of isoniazid”. Molecular Microbiology 62.5 (2006): 1220-1227.
- Shankar A., et al. “effect of milk protein versus non vegetarian food supplement in management of Pulmonary tuberculosis”. Acta Scientific Nutritional Health.
Citation:
Avinash Shankar., et al. “Short Term Anti TB Regime in Management of Pulmonary Tuberculosis”. Pulmonary Research and
Respiratory Care 2.1 (2018): 142-153.
Copyright: © 2018 Avinash Shankar., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.





























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.