Research Article
Volume 2 Issue 1 - 2018
Epidemiological, Clinical, Paraclinical, Etiological and Evolutionary Profile of Bronchiectasis
1Pneumology Department CHNU de Fann Dakar- Sénégal
2Radiology Department CHNU de Fann Dakar-Sénégal
2Radiology Department CHNU de Fann Dakar-Sénégal
*Corresponding Author: Dia S, Pneumology Department CHNU de Fann Dakar- Sénégal.
Received: August 07, 2018; Published: August 18, 2018
Introduction
In tuberculosis endemic areas, bronchiectasis is one of the aftereffect that alter and disrupt the quality of life of patients. The aim of our study is to determine the epidemiological, clinical, paraclinical, etiological and evolutionary profile of bronchiectasis.
Methods
We report a descriptive retrospective study over a period of 12 months (December 2015 to November 2016) on 131 patients with bronchiectasis.
Results
Epidemiology
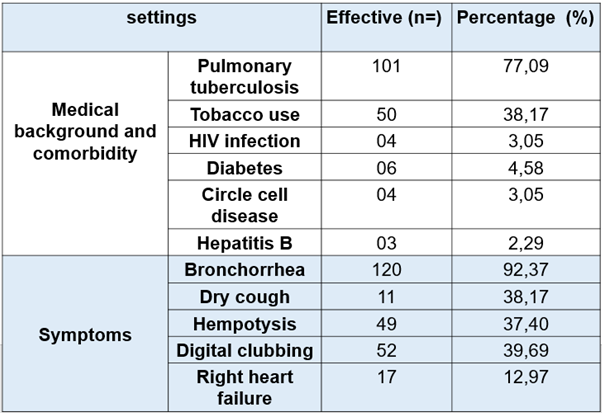
Our study included 131 patients with a relatively young population (mean age 44.87 years and extremes ranging from 16 to 83 years) and a clear male predominance (sex ratio was 1.85 including 85 men and 46 women). In the medical background, smoking was 38.16% (n = 50), pulmonary tuberculosis 77.09% (n = 101), and 4 patients living with HIV. (See Table 1)
Our study included 131 patients with a relatively young population (mean age 44.87 years and extremes ranging from 16 to 83 years) and a clear male predominance (sex ratio was 1.85 including 85 men and 46 women). In the medical background, smoking was 38.16% (n = 50), pulmonary tuberculosis 77.09% (n = 101), and 4 patients living with HIV. (See Table 1)
Clinic
The symptoms were dominated by mucosal bronchorrhea (50.38%), muco-purulent (41.22%), exercise dyspnea (83.96%), hemoptysis (37.40%) and dry cough (8.4%). Fifty-two (39.69%) patients had digital clubbing and 17 (12.97%) patients had signs of right heart failure. (See Table 1)
The symptoms were dominated by mucosal bronchorrhea (50.38%), muco-purulent (41.22%), exercise dyspnea (83.96%), hemoptysis (37.40%) and dry cough (8.4%). Fifty-two (39.69%) patients had digital clubbing and 17 (12.97%) patients had signs of right heart failure. (See Table 1)
Paraclinic
Chest imaging, in particular the thoracic CT scan, revealed more diffuse bronchiectasis (56.49% n = 74) than localized (43.51% n = 57) with a clear predominance of pure cystic forms (83.21% n = 57). 109) followed by mixed forms (14.50% n = 19) and pure cylindrical shapes (2, 29% n = 3). Associated signs were dominated by atelectasis in 53% of cases.
Chest imaging, in particular the thoracic CT scan, revealed more diffuse bronchiectasis (56.49% n = 74) than localized (43.51% n = 57) with a clear predominance of pure cystic forms (83.21% n = 57). 109) followed by mixed forms (14.50% n = 19) and pure cylindrical shapes (2, 29% n = 3). Associated signs were dominated by atelectasis in 53% of cases.
Cytobacteriological explorations allowed the isolation of one germ in 8 out of 10 patients, including 6 colonizations (4 pneumococci, 1klebsiella pneumoniae enterobacter) and 2 Escherichia coli infections sensitive to amoxicillin-clavulanic acid and resistant to ampicillin, ticarcillin and bactrim. Twenty-seven patients had carry out cardiac ultrasound with 48.15% of chronic pulmonary heart and 14.81% of isolated PAH. (see table 2)
Etiology
Etiologies were respectively post-tuberculosis in 77.86% of cases (n = 102) indeterminate (12.98% n = 17), occupational PID (3.05% n = 4), active pulmonary tuberculosis (2.29% n = 3), rheumatoid arthritis (1.53% n = 2), ciliary dyskinesia (1.53%) including Kartagener's syndrome and Young's syndrome and post-infectious non-tuberculosis (0.76%) (see Table 2)
Etiologies were respectively post-tuberculosis in 77.86% of cases (n = 102) indeterminate (12.98% n = 17), occupational PID (3.05% n = 4), active pulmonary tuberculosis (2.29% n = 3), rheumatoid arthritis (1.53% n = 2), ciliary dyskinesia (1.53%) including Kartagener's syndrome and Young's syndrome and post-infectious non-tuberculosis (0.76%) (see Table 2)
Evolution
Two (2) patients underwent left lobectomy for an associated aspergillary transplant, 1 patient was on long-term oxygen therapy, and 15 deaths were noted. (See Table 2)
Two (2) patients underwent left lobectomy for an associated aspergillary transplant, 1 patient was on long-term oxygen therapy, and 15 deaths were noted. (See Table 2)
Comments
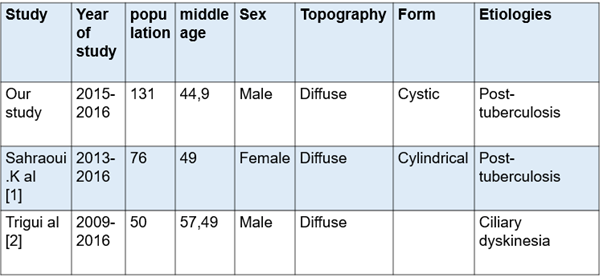
Our study, compared to the series made in Africa, particularly the Maghreb countries, with the series of Sahraoui K., et al. [1] in Algeria and Trigui., et al. [2] in Tunisia. We find that our study has a larger workforce with a male predominance. This could be explained by the capacity and the recruitment method of our service where we have 84 beds including 24 beds for women. Compared to the series of Sahraoui K., et al. [1], we notice the same diffuse topography with practically an average age that approaches with the same etiological profiles, the difference is in the forms of bronchiectasis (see table 3) Regarding the study by Trigui., et al. [2], we have the same profiles concerning gender and topography. Its etiological profile is closer to the European profile with ciliary dyskinesias as its main etiology.
Our study, compared to the series made in Africa, particularly the Maghreb countries, with the series of Sahraoui K., et al. [1] in Algeria and Trigui., et al. [2] in Tunisia. We find that our study has a larger workforce with a male predominance. This could be explained by the capacity and the recruitment method of our service where we have 84 beds including 24 beds for women. Compared to the series of Sahraoui K., et al. [1], we notice the same diffuse topography with practically an average age that approaches with the same etiological profiles, the difference is in the forms of bronchiectasis (see table 3) Regarding the study by Trigui., et al. [2], we have the same profiles concerning gender and topography. Its etiological profile is closer to the European profile with ciliary dyskinesias as its main etiology.
Conclusion
In Senegal, bronchial dilatations are post-tuberculous, diffuse and a source of respiratory disability.
Annex
| Paraclinical examinations | Effective (n=) | Percentage (%) | ||
| Thoracic CT scan | Diffuse bronchiectasis | 74 | 56,49 | |
| Localized bronchiectasis | 57 | 43,51 | ||
| Cystic bronchiectasis | 109 | 83,21 | ||
| Cylindrical bronchiectasis | 3 | 2,29 | ||
| Mixed bronchiectasis | 19 | 14,50 | ||
| Cytobacter Iological examination of sputum | Colonization | 6 | 60 | |
| Pneumococcal | 4 | 40 | ||
| Infection | Escherichia coli | 2 | 20 | |
| Echocardiography | Chronic pulmonary heart | 13 | 48,15 | |
| Pulmonary arterial hypertension | 4 | 14,81 | ||
| Normal | 10 | 37,04 | ||
| Etiologies | Post- tuberculosis | 102 | 77,86 | |
| Active tuberculosis | 3 | 2,28 | ||
| Occupational diffuse infiltrative pneumopathy | 4 | 3,05 | ||
| Rheumatoid arthritis | 2 | 1,53 | ||
| post- infectious non-tuberculosis | 1 | 0,76 | ||
| Ciliary dyskinesia | 2 | 1,53 | ||
| Indeterminate | 17 | 12,98 | ||
| Evolution | Lobectomy | 2 | ||
| Long-term oxygenation at home | 1 | |||
| Death | 15 | |||
Table 2: Paraclinical etiological and evolutionary profile of bronchiectasis Dakar-Sénégal (n = 131).
References
- Sahraoui K., et al. "Etiological and therapeutic radiological clinical profile of bronchiectasis in Oran. About 76 cases". Revue des Maladies Respiratoires 35 (2018): 236.
- Trigui G., et al. "Current profile of bronhiectasis". Revue des Maladies Respiratoires 34 (2017): 252.
Citation:
Dia S., et al. “Epidemiological, Clinical, Paraclinical, Etiological and Evolutionary Profile of Bronchiectasis”. Pulmonary Research
and Respiratory Care 2.1 (2018): 154-157.
Copyright: © 2018 Dia S., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.





























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.