Research Article
Volume 1 Issue 1 - 2017
Ventricular Partitioning Using the Parachute® Device: Challenging Access Routes and Device Retrieval
1Division of Cardiology, Asklepios Clinic St. Georg Hospital, Hamburg, Germany
2Asklepios proresearch, Clinical Research and Development, Hamburg, Germany
2Asklepios proresearch, Clinical Research and Development, Hamburg, Germany
*Corresponding Author: Tobias Schmidt, Asklepios Clinic St. Georg, Department of Cardiology, Hamburg, Germany.
Received: May 10, 2017; Published: May 26, 2017
Abstract
Aims: 17 patients with left ventricular aneurysm underwent a Parachute® device implantation procedure at our single centre. Aim of this report is to describe various first-in-man implantation techniques and a percutaneous Parachute® removal as well as peri-procedural complications.
Methods: ‘Standard’ procedures retrogradely over the aortic valve as well as through two bioprostheses (29 mm Sapien XT, 23 mm Perimount) and in one case after prior MitraClip were performed. Parachute® implantation was combined with a MitraClip due to severe mitral valve regurgitation in two patients using a transseptal and transmitral access. Peri-procedural events were analyzed in all patients.
Results: Procedural success rate was 94.1% (16/17). One percutaneous removal of the device after dislocation from the proper landing zone was necessary. Uncomplicated implantation through a valvular aortic bioprosthesis in two patients as well as a transvenous, transseptal and transmitral implantation in combination with MitraClip procedures were done without any difficulties. Periprocedural mortality (≤ 30 days) was 0%. Peri-/post-interventional monitoring showed a common rate of ventricular tachycardia after implantation (6/17, 35.3%). The risk of ventricular tachycardia, bleeding complications and thrombus formation are seen.
Conclusions: This report of 17 patients treated at a single centre includes various first-in-man implantation techniques. Despite the small sample size, this study contains some value since it comprises the largest series of patients being treated within a single centre with a comprehensive analysis of complications. In addition, re-evaluation of various access techniques for this device might be of interest in the future. The management and knowledge of an increased risk for ventricular arrhythmias after implantation needs to be confirmed in a larger study cohort, but careful patient selection and previous EP examination or ICD implantation should be considered.
Keywords: Left ventricular aneurysm; Anterior myocardial infarction; Percutaneous heart failure therapy
Abbreviations: MI: Myocardial Infarction; LV: Left Ventricle; CT: Computer Tomography; TTE: Transthoracic Echocardiography; ICD: Implantable Cardioverter/Defibrillator; LVEDV: Left Ventricular End-Diastolic Volume; LVESV: Left Ventricular End-Systolic Volume; SV: Stroke Volume; LVEDD: Left Ventricular End-Diastolic Diameter; VT: Ventricular Tachycardia; TAVI: Transcatheter Aortic Valve Implantation; CRT: Cardiac Resynchronization Therapy; EP: Electrophysiological Examination
Introduction
Ischemic dilated heart failure after myocardial infarction (MI) appears in 24% of all patients [1]. More than 20 million people worldwide are affected with heart failure and their symptoms [2]. Data from Germany shows that anterior myocardial infarction occurs in 17% of all MI [3]. Left ventricular (LV) remodelling with abnormal wall motion, myocardial thinning of the affected region, geometrical changes with increased wall stress and, as a late consequence, increased left ventricular volumes with elevated filling pressure and stiffening of the ventricular wall can be the consequence [4].
In the past, surgical aneurysmectomy with the need of a heart-lung machine was the only treatment option for symptomatic patients with left ventricular aneurysm. Surgical restoration therapy has unacceptable high mortality rates [5-6], without reliable clinical and technical success [7]. Nowadays, a percutaneous treatment option with the Parachute® device exists for these high surgical risk patients. New data from the Parachute III trial showed the feasibility, safety and improvement in quality of life with reduced rates of mortality and heart failure hospitalization [8]. Reports from our centre as well as from Lee., et al. described acute haemodynamic benefits with reduction of the left ventricle diameter as well as impact on left ventricular contractility [9-10].
For surgical aneurysmectomy, thoracotomy, cardiopulmonary bypass and cardioplegia are needed, while the Parachute® Ventricular Partitioning Device (CardioKinetix, Inc., Menlo Park, CA, USA) can be implanted via a percutaneous catheter approach in a beating heart situation under conscious sedation. The device is placed in the apex/aneurysm of the LV coming retrograde over the aortic valve.
The guidelines of the European Society of Cardiology recommend optimal medical treatment (OMT) and the consideration of cardiac resynchronization therapy (CRT) in patients with symptoms of heart failure secondary to prior myocardial infarction, left ventricular aneurysm and decreased ejection fraction [11]. Surgical treatment of an LV aneurysm is still rare. The need for an additional percutaneous device therapy is persistent for this very sick patient population. Patients with existence of heart failure for a longer time also had previous interventions or operations (e.g. previous surgical aortic valve replacement) or even have present secondary heart failure problems such as mitral valve regurgitation (MR). These barriers are seen generally in these patients and can limit new devices for their use.
Standard procedures for a Parachute® device implantation are described in great numbers. [12-16]. The aim of this report was to describe different access routes, special indications and a percutaneous Parachute® device retrieval for opening the indication of this device for a larger patient selection and showing the possibility of a percutaneous retrieval without conversion to open heart surgery.
Methods
Patients: Between July 2012 and April 2014, 32 patients with heart failure due to an anterior myocardial infarction with following anterior aneurysm were screened for a percutaneous left ventricular reduction therapy at our institution; screening included a clinical examination, transthoracic echocardiography (TTE) and cardiac computed tomography (CT) to evaluate the geometry of the left ventricle. Out of these 32 patients, implantation of a Parachute® device was judged feasible in 17 patients due to their clinical status with chronic symptoms of heart failure (New York Heart Association (NYHA) functional class II to IV) and as well as possible CT and TTE criteria. All of these patients received a Parachute® device.
The first 8 patients were treated in the context of the PercutAneous Ventricular RestorAtion in Chronic Heart failUre PaTiEnts (PARACHUTE) III trial, whereas the other 9 patients were treated in the context of an institutional post-market release Parachute Registry. Complete coronary revascularization if possible as well as implantation of an implantable cardioverter/defibrillator (ICD) with or without cardiac resynchronization modality was performed before Parachute implantation. For these 9 patients outside of the PARCHUTE III trial the inclusion criteria were extend in patients with prior surgical aortic valve implantation, prior MitraClip implantation, patients with an LV aneurysm and present MR. These patients were admitted to our hospital frequently due to decompensation of heart failure.
Parachute® device: The Parachute® device is comprised of a fluoropolymer (expanded polytetrafluoroethylene) membrane stretched over a self-expanding nitinol frame consisting of 16 struts attached to the foot of the implant. The struts, which carry 2-mm anchors at their distal ends, open up like struts of an umbrella. The device is delivered by way of a guide catheter (available in 14 Fr and 16 Fr). The delivery system is 125 cm in length and has a threaded tip where a 20 cc balloon (for deployment of the device) and the Parachute® implant is attached.
The device is currently available in four diameters (65 mm, 75 mm, 85 mm, and 95 mm) and two foot heights (standard [3.8 mm] and short [0.9 mm]).
Procedural details and device implantation: Standard Parachute® device implantations are described in the literature numerous times [12-16]. All our procedures were performed under transoesophageal echocardiographic guiding in a hybrid operating room. Most procedures (82.4%) were performed under general anaesthesia. In all procedures haemodynamic monitoring was performed, as previous described [9], using a pigtail catheter in the left ventricle (LV) and a Swan-Ganz catheter in the pulmonary artery. Most of the procedures (15/17) were done via a percutaneous retrograde transfemoral arterial approach, through a 14 or 16Fr guide catheter according to the instructions for use of the device. Immediately after access site puncture/preparation, 100 IU/kg of unfractionated heparin were administered to achieve an activated clotting time of 250-300 seconds. Two pigtail catheters (5 and 6F) were advanced retrogradely over the aortic valve into the LV, one for haemodynamic measurements and later for visualizing the landing zone, and the second one to introduce an Amplatz Super stiff guide wire (0.035 in, 260 cm, Boston Scientific, Marlborough, MA, USA) wire in the potential landing zone. Then, the delivery system with the prepared and collapsed Parachute® device was advanced over the guide catheter to aneurysmatic apex of the left ventricle (intended landing zone).
Once the landing zone was successful targeted, the guide catheter was pulled back and the radiopaque flexible foot of the device was released into the apex of the LV with the Parachute® being still attached to the delivery system. Careful attention is necessary to obtain the optimal Parachute® device position with the foot of the device being in the aneurysm at the spot where the new apex will be and where the end of the nitinol struts are in position of the borderline of the scar and normal myocardium. After decision of the interventionalist for the exact device position, the attached 20 cc balloon gets inflated and deflated (“point of no return”). After balloon inflation an angiogram of the LV is performed to evaluate the final position. We aimed to obtain a device anchoring within the LV with as few as possible residual leaks diagnosed by angiogram and TTE between the left ventricular walls and the implanted device. To prevent dislodgement or migration of the implant, the optimal size is pre-interventional performed by CT and TTE measurements by the company (height of the aneurysm, distance of the papillary muscle and the left ventricular end diastolic diameter are important) and a push and pull maneuver is performed before full release of the device. Once the device is in its final position the delivery system gets unscrewed and withdrawn into the guide catheter. Then the guide catheter is pulled back and removed. Both ipsilateral and contralateral arterial access sites were closed by percutaneous closure devices (large-bore access site: 14/16F, ProGlide or Prostar; contralateral access site: 6F, Starclose, all Abbott Vascular, Inc., Menlo Park, CA, USA). An additional pressure bandage was dressed for at least 6 hours to prevent bleeding complications.
Peri- and post-procedural management: Post-procedure, all patients were ECG-monitored for at least 48 hours regardless of the presence or absence of an ICD. Laboratory diagnostics were performed on the next day as well as during clinical follow-up of the femoral puncture side. Before discharge, TTE was performed for screening of the device position and potential para-device leaks. Other haemodynamic measurements apart from blood pressure and heart rate were not taken during the stay at the hospital.
Results
Patients and procedures: Between August 2012 and April 2014, 17 patients received a Parachute® device at our centre; mean patient age was 70 ± 9 years and 12 patients (70.6%) were male. All patients were at least NYHA class II or higher, with 15 (88%) in NYHA class III (1 in NYHA class II, 1 in NYHA class IV). Detailed patient characteristics are shown in Table 1. In the 12 months before Parachute implantation, 15 patients (88%) were hospitalized due to heart failure. Ten patients (77%) had an ICD, 3 had a CRT-ICD device [Table 1]. All patients had a prior myocardial infarction due to a history of left anterior descending coronary artery (LAD) occlusion. According to the study inclusion criteria, all patients had akinesia or dyskinesia of the antero-apical left ventricular region.
| N | 17 |
| Age, years (mean ± SD) | 70 ± 9 |
| Male gender, n (%) | 12 (70.6) |
| NYHA functional class, n (%) | |
| II | 1 (5.9) |
| III | 15 (88.2) |
| IV | 1 (5.9) |
| Hypertension, n (%) | 16 (94.1) |
| Diabetes, n (%) | 8 (47.1) |
| Smoking, n (%) | 7 (41.2) |
| Dyslipidaemia, n (%) | 16 (94.1) |
| Hospitalization (12 months prior), n (%) | 15 (88.2) |
| ICD, n (%) | |
| CRT-ICD | 3 (17.7) |
| Single ICD | 10 (58.8) |
| No ICD | 4 (23.5) |
CRT: Cardiac Resynchronization Therapy; ICD: Implantable Cardioverter/Defibrillator; NYHA: New York Heart Association
Table 1: Baseline patient characteristics.
Table 1: Baseline patient characteristics.
Mean left ventricular (LV) ejection fraction (EF) was 26 ± 8% measured by TTE and 30 ± 9% on CT. Mean LV end-diastolic diameter on TTE was enlarged at 63 ± 5 mm. On baseline CT a mean LV end-diastolic volume (LVEDV) of 270 ± 107 ml and a mean LV end-systolic volume (LVESV) of 192 ± 87 ml were measured. Mean stroke volume was 79 ± 33 ml [Table 2].
| TTE | |
| LV ejection fraction, % | 26 ± 8 |
| Mitral regurgitation, n (%) | |
| None | 2 (11.8) |
| Mild | 5 (29.4) |
| Moderate | 7 (41.2) |
| Moderate to severe/severe | 3 (17.7) |
| Akinesia/dyskinesia of anteroapical wall, n (%) | 17 (100) |
| LVEDD, mm | 63 ± 5 |
| CT | |
| LV ejection fraction, % | 30 ± 9 |
| LVEDV, ml | 270 ± 107 |
| LVESV, ml | 192 ± 87 |
| Stroke volume, ml | 79 ± 33 |
CT: Computed Tomography; LV: Left Ventricular; LVEDD: Left Ventricular End-Diastolic Diameter; LVEDV: Left Ventricular End-Diastolic Volume; LVESV: Left Ventricular End-Systolic Volume; TTE: Transthoracic Echocardiography.
Table 2: Transthoracic echocardiography and computed tomography data.
Table 2: Transthoracic echocardiography and computed tomography data.
Fourteen procedures (82%) were performed with the patients in general anaesthesia; local anaesthesia with slight analgosedation was performed in the 3 remaining patients. Anaesthesiologic or haemodynamic complications did not occur in any procedure.
Procedural success, defined as device positioning in the proper landing zone was achieved in 16 patients (94%). In these patients, the Parachute® device was positioned in the intended landing zone with insignificant para-prosthetic leaks in 13 patients. Larger leaks were present in 3 patients.
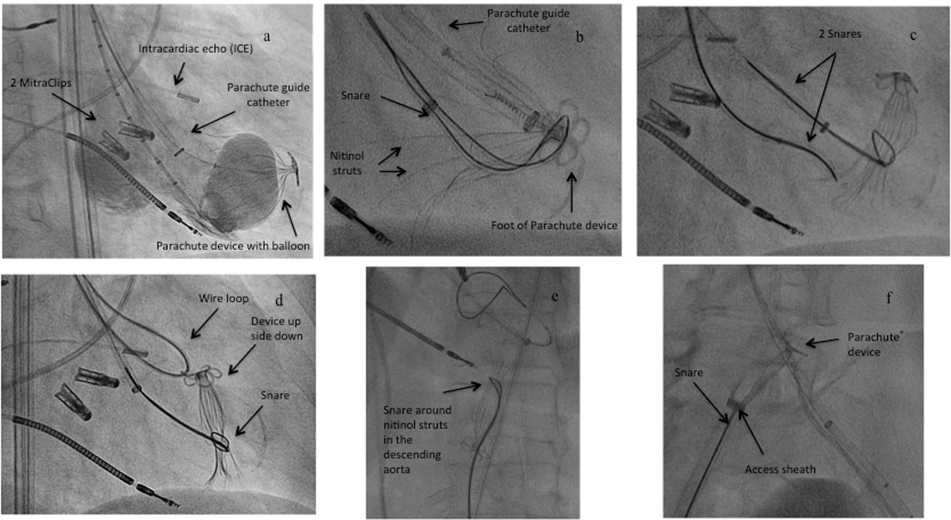
Removal of a dislocated device: Due to dislocation from the proper landing zone one device had to be retrieved from the left ventricle with a complex snare maneuver [Figure 1]. During balloon inflation (“point of no return”) the foot of the Parachute® device dislocated from its intended landing zone to a more cranial and anterior position with flattening of the device in the LV. Since the device was still attached to the delivery system, further balloon inflations with more than 20 cc fluid were performed for possible rescuing of the device position with no success [Figure 1a]. Release of the device in the LV was not possible due to the not intended landing zone. Since stable hemodynamic conditions in this high-risk patient were present, percutaneous removal of the device was tried instead of conversion to open heart surgery for removal of the device. Removal of the device was started with a snare (ONE Snare®, Merit Medical Systems, Inc., South Jordan, UT, USA) entangled around the foot of the device to removed it from the apex [Figure 1b]. Then the device was released from the delivery system and tried to relieve from the apex. Since the device was partly positioned, a second snare for more tensile force was necessary and looped around the device struts, while the first snare was positioned around the umbrella to collapse the device [Figure 1c]. This maneuver released the device into the LV. For recovery of the device, the second snare was removed, the first snare collapsed the umbrella completely and a wire loop through the wholes of the foot was placed to turn the device upside down for better removal antegrade across the aortic valve [Figure 1d]. Removal from the LV into the descending aorta and also the retrieval into the 16F access sheath was performed without any complaints [Figure 1e, 1f]. Postinterventional angiography and echocardiography showed no trauma in the LV, on the aortic valve and the previous implanted MitraClips.
Parachute® implantation through aortic bioprostheses: As stated above, the standard procedure was done retrogradely across the aortic valve, via the femoral artery. In two cases, Parachute implantation was performed through an aortic bioprosthesis (29-mm Sapien XT; 23-mm Carpentier-Edwards Perimount, both Edwards Life sciences Corp., Irvine, CA, USA) [Figure 2]. Same approach as used for valve-in-valve implantations or “normal” Parachute® implantations with pigtail insertion into the LV and switch to an Amplatz super stiff guide wire were performed. During assertion of the guide catheter including the delivery system attention need to be paid especially when crossing the aortic bioprothesis. Protection of the sharp edge of the guide catheter can be performed with look out of a balloon inserted in the guide catheter when crossing the aortic bioprothesis. Central positioning of the guide is another issue to minimize the risk of injury on the bioprothesis. During implantation of the Parachute® device the guide should stay in a central position over the aortic valve.
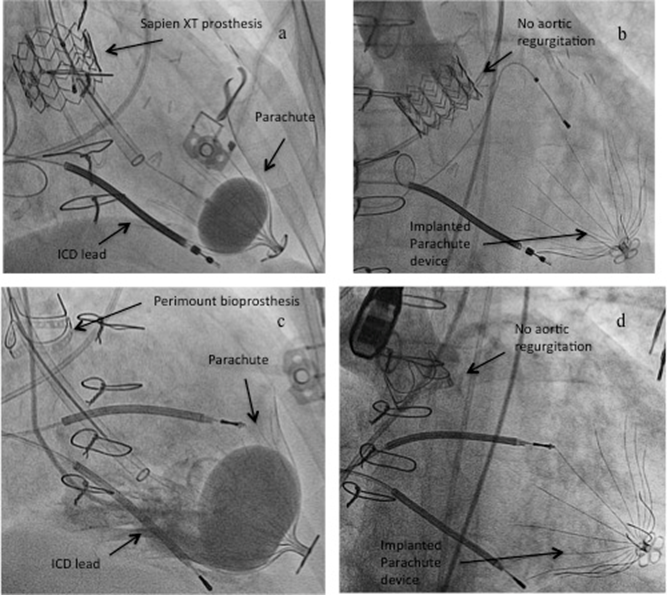
Figure 2:
a) Retrograde Parachute® implantation across a 29-mm Sapien XT prosthesis and c) across a 23-mm Perimount bioprosthesis showing no injury/regurgitation of the two bioprostheses after implantation (b and d)
a) Retrograde Parachute® implantation across a 29-mm Sapien XT prosthesis and c) across a 23-mm Perimount bioprosthesis showing no injury/regurgitation of the two bioprostheses after implantation (b and d)
Parachute® implantation in the presence of a MitraClip or in conjunction with MitraClip implantation: 6 months prior to Parachute® implantation 1 patient had received a single MitraClip (Abbott Vascular, Inc.) for severe mitral regurgitation (MR). Two other patients were treated in combination with a MitraClip procedure. In both patients, the Parachute device was introduced transseptally by way of the 24 Fr MitraClip guide catheter. Due to the steerability of that guide catheter, antegrade and transmitral access to the landing zone and implantation of the Parachute® device was achieved without any problems prior to the MitraClip procedure [Figure 3]. Crossing of the mitral valve antegrade in this combined procedure is not as dangerous as in aortic bioprotheses where the crossing of the valve is retrograde. Attention need to be paid more during the MitraClip procedure to not get caught with the nitinol struts of the Parachute®. Changing of the geometry of the MR was not seen after Parachute® implantation. Subsequently, standard MitraClip implantation, as previously described [17-18], was successfully performed; MR was reduced to < grade 1+ at discharge.
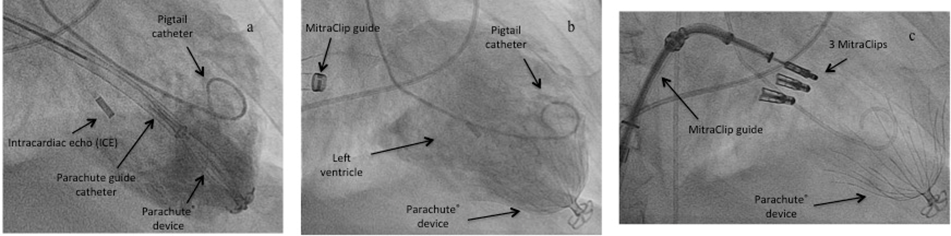
Figure 3: Transseptal Parachute® implantation in a combined procedure with a MitraClip.
a) the Parachute® device was introduced transseptally by way of the 24 Fr MitraClip guide catheter, antegrade and transmitral access to the landing zone and implantation of the Parachute® device was achieved without any problems, b) angiography of the implanted Parachute® device, c) MitraClip procedure with the same transseptal access and implantation of three MitraClips
a) the Parachute® device was introduced transseptally by way of the 24 Fr MitraClip guide catheter, antegrade and transmitral access to the landing zone and implantation of the Parachute® device was achieved without any problems, b) angiography of the implanted Parachute® device, c) MitraClip procedure with the same transseptal access and implantation of three MitraClips
Post-procedural observations and management: Three patients had episodes of sustained ventricular tachycardia (VT)/ventricular fibrillation within 24 hours of the procedure; all received shocks from their ICDs. These three patients were the only ones in our cohort with a history of electrophysiological examination due to VT. Two patients had been treated with VT ablation therapy within the LV apex (Parachute® landing zone) 4 weeks and 9 years prior to Parachute® implantation. The third patient had undergone electrophysiological stimulation for polymorphic VT 7 years earlier without ablation [Table 3]. After medical stabilization with amiodarone and magnesium, VTs were no longer observed in any of the 3 patients during the ensuing 48 hours. Three additional patients showed asymptomatic non-sustained VTs within 48 hours after Parachute® implantation. In these as well as the remaining patients no more VTs occurred after 48 hours.
| Prior electrophysiological study, n (%) | 3 (17.7)* |
| VT origin, n (%) | |
| LV apex/anteroapical wall | 2 (11.8) |
| Polymorphic | 1 (5.9) |
| ICD check prior to Parachute® implantation, n (%) | 11/13 (84.6) |
| no ICD implanted | 4 (23.5) |
| no prior check | 2/13 (15.4) |
| History of VT before Parachute® implantation, n (%) | 3 (17.7)* |
| VT after Parachute® implantation, n (%) | 6 (35.3) |
| sustained VT/ICD shock | 3 (17.7)* |
| non-sustained VT | 3 (17.7)* |
*Same Patients
ICD: Implantable Cardioverter/Defibrillator; LV: Left Ventricular; VT: Ventricular Tachycardia
Table 2: Data of ventricular tachycardia and electrophysiological examination.
ICD: Implantable Cardioverter/Defibrillator; LV: Left Ventricular; VT: Ventricular Tachycardia
Table 2: Data of ventricular tachycardia and electrophysiological examination.
Three patients had slight haematoma (Bleeding Academic Research Consortium type 2). No other bleeding complications were recorded. One patient with severe peripheral arterial disease and very small peripheral vessels (5 mm diameter) had a minor vascular complication according to Valve Academic Research Consortium-2 (VARC-2) with occlusion of the right femoral artery one day after the procedure; the condition was successfully treated by stenting.
Before discharge, TTE demonstrated that all implanted devices were still in their intended landing positions. All patients were discharged on phenprocoumon or new oral anticoagulants.
Blood pressure and heart rate showed no significant changes during hospital stay.
The median hospital stay was 6 days (minimum 3, maximum 46 days; 5 patients [31%] stayed for only 3-5 days), including pre-interventional screening in some cases.
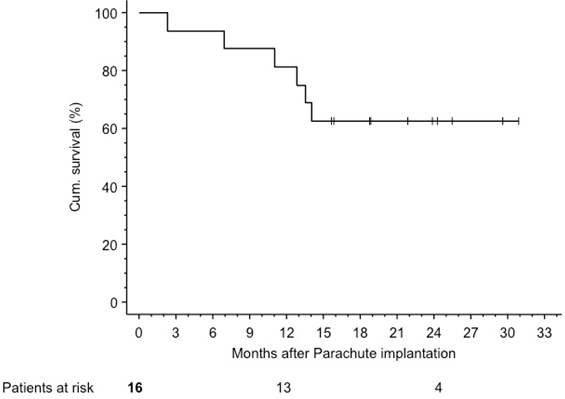
Follow up: There was no acute and 30-day mortality. Patients were followed for a median of 17.3 (IQR 13.2-24.1) months. Kaplan-Meier estimated survival was 81.2% (95% CI 62.1-100%) at 1 year and 62.5% (95% CI 38.8-86.2%) at 2 years [Figure 4]. Three patients died within 12 months. One patient sustained a fatal stroke 2.3 months after device implantation, another patient died from lung oedema (7 months) and the third patient died from peritonitis due to a known colon carcinoma (11 months).
Eleven months after implantation, a thrombus at the center of the device was seen in one patient. TTE had not shown thrombus at the device at 4 weeks, 7 months and 9.5 months after implantation. At the time the thrombus was discovered, the patient was treated with dabigatran alone (110 mg b.i.d.), whereas he had additionally been treated with clopidogrel (due to prior stent implantation) during the initial seven months after Parachute® implantation. After switching to phenprocoumon the thrombus was regressive and had disappeared by 14 months after implantation.
Discussion
Parachute® device implantation is mainly performed in settings of trials (e.g. PARACHUTE Trial, US Feasibility Trial, III Trial, IV Trial). Several exclusion criteria (e.g. MR > 2+, previous aortic valve replacement) are named for these trials. Patients with the need for an additional therapy to CRT and OMT with heart failure exist. The Parachute® device can also be implanted in patients with an aortic bioprotheses (independent of surgical or transcatheter), in patients with concomitant MR or after mechanical aortic valve replacement (with a transseptal access) and in patients with a prior MitraClip therapy. For these special patients we can show that these procedures can be performed without injury of any structure of the heart. Interactions with the Parachute® device and a prior MitraClip was not seen. Very interesting and helpful can be the described strategy of the device removal if the intended landing zone cannot be achieved. The possibility of a percutaneous removal of the device is really important in this very sick patient collection, in which a conversion to open heart surgery is of high risk for mortality.
The guidelines of the European Society of Cardiology recommend OMT and the consideration of CRT in patients with symptoms of heart failure secondary to prior myocardial infarction, left ventricular aneurysm and decreased ejection fraction [11]. The Parachute® Ventricular Partitioning Device has been designed to improve symptoms and quality of life in patients on OMT and/or CRT. The safety profile of Parachute® implantation has previously been described, most recently in the PARACHUTE III trial [8]. Haemodynamic benefits, left ventricular improvement, and clinical outcomes in humans, animal models and in a CT simulated model have also been published [8-10,14-15,19]. The proof of an additive effect of device implantation in conjunction with optimal medical treatment is currently being investigated (e.g. in the PARACHUTE IV trial) [20].
Procedural safety, feasibility, and expanding indications: A small number of patients being treated globally so far with this device, we could treat 17 patients at our centre. Procedural details for a ‘standard’ Parachute® device implantation are known and described, mainly in case reports of first implant procedures [14,16,19]. This report reveal the largest population treated at a single centre with this device in such a sick patient population who are for example pre-treated by cardiac surgeons (Perimount prosthesis) or cardiologists (previous MitraClip or Sapien XT prosthesis). The absence of damage on these devices/prostheses might open up the indication if more reports will confirm these results. Despite the small number of very sick patients in this registry the procedural mortality, as well as 30-day mortality was 0%.
The described retrieval of a dislocated device from the left ventricle was a challenging intervention that should only be performed as a bail-out measure. Questioning our procedure in retrospect, our patient was a suboptimal candidate due to a prominent pseudo cord close to the targeted landing zone. Accurate pre-interventional screening, especially for pseudo-cords or heavy calcification in the landing zone is mandatory to minimize the need for this manoeuvre. Before pulling back the guide catheter to release the Parachute® in the LV (“point of no return”) the radiopaque foot needs to be in position of the landing zone. Minute attention is necessary to prevent dislocation of the device.
Transseptal Parachute® implantation in combination with a MitraClip procedure is an elegant way to treat heart failure patients with severe mitral valve regurgitation. Currently, severe mitral regurgitation is considered an absolute contraindication for Parachute trials. Commercial Parachute® implantation in patients with concomitant MR can be performed. In our small cohort of patients MR grade was not significant better after Parachute implantation. Instead of improvement of the MR, two patients received a MitraClip during the follow up. For a simultaneous MitraClip procedure, the steerable MitraClip guide catheter is also suitable for Parachute® implantation allowing accurate positioning due to the distinctive control of the guide. This also offers the option for patients with mechanical aortic valves in whom retrograde aortic access is not possible.
Vascular complications: In general, access-site bleeding complications have an important clinical impact, resulting in higher mortality rates. Statistical comparison of the bleeding complications due to the small number in this report is not meaningful. Major vascular bleeding rates of transcatheter aortic valve implantation (TAVI) are approximately 7.1% [21]. According to the VARC-2 criteria, only 1 patient in our cohort had a minor vascular complication and another 3 patients had minor hematomas without need for blood transfusion. These are acceptable rates when comparing to reports of TAVI vascular access-site complication rates [21].
Arrhythmias: The high incidence of VTs after Parachute® implantation (3/17 [17.6%]) is cause for concern. To date, VTs after Parachute® device implantation have rarely been reported. Lauschke., et al. described in 2013 a patient with recurrent VTs after Parachute® implantation [22]. This patient most likely had an endocardial VT substrate behind the Parachute® ‘umbrella’ in the aneurysmatic apex of the LV, since epicardial visualization of diastolic potentials was not feasible. Thus, patients with recurrent episodes of VT after anterior myocardial infarction and apical aneurysm formation should undergo electrophysiological examination and possibly VT ablation before Parachute implantation is considered. A minimum requirement should be the implantation of an ICD before Parachute® device implantation.
Since we have seen arrhythmias after Parachute® implantation in our patients, all patients were transferred to the intensive care unit for at least 24 hours direct after the procedure for better monitoring and faster potentially medical intervention. Interestingly, all patients with sustained VTs and ICD shocks had a history of electrophysiological examination due to VTs. Possible mechanical forces caused by the device were liable for these VTs, since they occurred directly after the procedure and medical treatment stabilized these VTs without any more VTs in the in-hospital stay. Also the one year follow up (ICD check) of these 3 patients did not show more VTs as documented before.
Four patients had no ICD before Parachute® implantation. The need for ICD implantation before Parachute® implantation in patients with no indication for an ICD, LV EF > 35% and no VT in their medical history cannot be resumed from these data. More data are necessary and a prior careful screening might help to minimize the arrhythmogenic risk for these patients. In addition, all patients who obtained a previous ICD treatment, prior accurate ICD check-up for VT should be performed. In patients with documented VTs in the check up or a prior history of EP testing or ablation of VT, Parachute® implantation should be discussed carefully as well as a prior EP examination/ablation.
Anticoagulation: Thrombus formation 11 months after Parachute® implantation was observed in a single patient despite treatment for 7 months with the vitamin K antagonist phenprocoumon followed by monotherapy with the direct thrombin inhibitor dabigatran. Luckily, the thrombus dissolved after reconstitution of phenprocoumon without any sequelae. Subsequently, all patients were kept on phenprocoumon for at least 12 months after discharge. There is no clear recommendation at present on the best anticoagulation regimen after Parachute® implantation. However, there appears to be an increased risk of thrombus formation due to the large thrombogenic surface area of the device. In fact, one patient discharged on aspirin, clopidogrel and phenprocoumon because of prior stenting of the left main coronary artery and persisting atrial fibrillation died of a stroke with aspiration pneumonia.
Histological data of 7 Parachute® devices retrieved at autopsy or during transplantation showed early adherent thrombus formation with focal inflammatory response and an organizing thrombus layer with neoendocardial thickening; moreover, devices that were removed more than 300 days after implantation demonstrated the greatest organization of thrombus as well as endocardial intima [23]. Hence, prolonged anticoagulation after Parachute® implantation should be considered even in patients without a clear indication for anticoagulation (no atrial fibrillation with a CHA2DS2-VASc Score ≥ 2, no mechanical valves). More data from larger studies are necessary to understand the role of the new anticoagulants after Parachute® device implantation.
Conclusion
This report of 17 patients treated at a single centre includes various first-in-man implantation techniques. Despite the small sample size, this study contains some value since it comprises the largest series of patients being treated within a single centre with a comprehensive analysis of complications. In addition, re-evaluation of various access techniques for this device might be of interest in the future. The management and knowledge of an increased risk for ventricular arrhythmias after implantation needs to be confirmed in a larger study cohort, but careful patient selection and previous EP examination or ICD implantation should be considered.
References
- Jhund PS and McMurray JJV. “Heart failure after acute myocardial infarction: a lost battle in the war on heart failure?”. Circulation 118.20 (2008): 2019-2021.
- Go AS., et al. “Heart disease and stroke statistics--2013 update: a report from the American Heart Association”. Circulation 127.1 (2013): e6-e245.
- Biermann J., et al. “Economic burden of patients with various etiologies of chronic systolic heart failure analyzed by resource use and costs”. International Journal of Cardiology 156.3 (2012): 323-325.
- Sutton MGSJ and Sharpe N. “Left ventricular remodeling after myocardial infarction: pathophysiology and therapy”. Circulation 101.25 (2000): 2981-2988.
- Burton NA., et al. “Left ventricular aneurysm. Preoperative risk factors and long-term postoperative results”. The Journal of Thoracic and Cardiovascular Surgery77.1 (1979): 65-75.
- Olearchyk AS., et al. “Left ventricular aneurysm. Ten years' experience in surgical treatment of 244 cases. Improved clinical status, hemodynamics, and long-term longevity”. The Journal of Thoracic and Cardiovascular Surgery 88.4 (1984): 544–553.
- Buckberg GD., et al. “The STICH trial unravelled”. European Journal of Heart Failure 12.10 (2010): 1024-1027.
- Schäfer U and Abraham W. “Twelve-month clinical data from PARACHUTE III, a study of 100 post-market European patients with ischemic heart failure treated consecutively between 2011 and 2013. In: 2014”.
- Schmidt T., et al. “New evidence for favourable effects on haemodynamics and ventricular performance after Parachute® implantation in humans”. European Journal of Heart Failure 16.10 (2014): 1112-1119.
- Lee LC., et al. “Patient-specific finite element modeling of the Cardiokinetix Parachute® device: effects on left ventricular wall stress and function”. Medical & Biological Engineering & Computing 52.6 (2014): 557-566.
- McMurray JJ., et al. “ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC”. European Journal of Heart Failure 33.14 (2012): 1787-1847.
- Nikolic SD., et al. “Percutaneous implantation of an intraventricular device for the treatment of heart failure: experimental results and proof of concept”. Journal of Cardiac Failure 15.9 (2009): 790–797.
- Sharkey H., et al. “Left ventricular apex occluder. Description of a Ventricular Partitioning Device”. EuroIntervention 2.1 (2006): 125-127.
- Sagic D., et al.“Percutaneous implantation of the left ventricular partitioning device for chronic heart failure: a pilot study with 1-year follow-up”. European Journal of Heart Failure 12.6 (2010): 600-606.
- Mazzaferri EL., et al. “Percutaneous left ventricular partitioning in patients with chronic heart failure and a prior anterior myocardial infarction: Results of the PercutAneous Ventricular RestorAtion in Chronic Heart failUre PaTiEnts Trial”. American Heart Journal163.5 (2012): 812-820.
- Boerlage-van Dijk K., et al. “Percutaneous left ventricular partitioning device for chronic heart failure”. Netherlands Heart Journal 20.12 (2012): 513-515.
- Maisano F., et al. “The evolution from surgery to percutaneous mitral valve interventions: the role of the edge-to-edge technique”. Journal of the American College of Cardiology 58.21 (2011): 2174-2182.
- Condado JA., et al. “Percutaneous edge-to-edge mitral valve repair: 2-year follow-up in the first human case”. Catheterization and Cardiovascular Interventions 67.2 (2006): 323–325.
- Skowasch M., et al. “Percutaneous ventricular restoration in a chronic heart failure patient”. EuroIntervention 2.1 (2006): 128-131.
- Costa MA., et al. “The PARACHUTE IV trial design and rationale: percutaneous ventricular restoration using the parachute device in patients with ischemic heart failure and dilated left ventricles”. American Heart Journal 165.4 (2013): 531-536.
- Frerker C., et al. “Ipsilateral arterial access for management of vascular complication in transcatheter aortic valve implantation”. Catheterization and Cardiovascular Interventions 81.4 (2013): 592-602.
- Lauschke J., et al. “Ventricular tachycardia ablation in a patient with a parachute device: a decent word of warning”. Europace16.2 (2014): 207.
- Ladich E., et al. “A pathologic study of explanted parachute devices from seven heart failure patients following percutaneous ventricular restoration”. Catheterization and Cardiovascular Interventions 83.4 (2014): 619-630.
Citation:
Tobias Schmidt., et al. “Ventricular Partitioning using the Parachute® Device: Challenging Access Routes and Device Retrieval”.
Therapeutic Advances in Cardiology 1.1 (2017): 19-30.
Copyright: © 2017 Tobias Schmidt., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.





























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.