Editorial
Volume 1 Issue 2 - 2017
Underground Mystery: The Role of Chemotactic Attractants in Plant Root and Phytonematode Interactions
Department of Entomology and Plant Pathology, Auburn University, AL 36849
*Corresponding Author: Sang-Wook Park, Department of Entomology and Plant Pathology, Auburn University, AL 36849.
Received: June 08, 2017; Published: June 21, 2017
Phytonematodes are microscopic roundworms that develop an obligate parasitic relationship with plant hosts. Once they reached the root surface, they slowly insert a stylet, needle-like structure, and feed cytosolic nutrients from root hairs, which cause cell and tissue motility (Mitchum., et al. 2013, Fous-Nyarko and Jones 2016). In the modern agriculture, the phytonematode diseases have become of great economic importance causing an estimated annual loss of 10 percent of world crop production (Nicol., et al. 2011), thus needing an urgent breakthrough in developing effective and sustainable disease management programs such as new resistance cultivars. It is however not necessarily forthcoming, largely due to our little knowledge of the pathophysiology of phytonematodes. Hence this editorial will briefly revisit current information gaps, and introduce our new studies in the mode of interactions between plant roots and phytonematodes, which help revamp unique and alternative prospective in future studies.
Recent increases in Agronomic burden by Phytonematodes
Plant parasitic nematodes, belonging to the phylum Nematoda, are microscopic animals that have evolved to over 4,000 species and adapted to a broad range of environment from forests to oceans (Nicol., et al. 2011, Hodda 2011, Zhang 2013). Previously, many - if not most - of them were viewed as benign or non-damaging, but recent reports have recognized that selective species such as Rotylenchulusspp. Meloidogynespp. and Heteroderaspp., are agronomical important pests, attributing the annual losses of crop production at ~14 % in worldwide (Nicol 2002, Nicol., et al. 2011). For instance, R. reniformis(reniform nematodes) have become a major threat over the last decade towards cotton farming in the southern regions of the U.S., leading to an estimated yield loss of over $100 million annually. Cotton is the most important fiber producing crop of which its production in the U.S. accounts for about one quarter of the world supply (~ $25 billion values, Koenning., et al. 2004,), and creates over 200,000 jobs (NCCA 2015). However the currently available integrated pest management method against phytonematodes (IPM-N) is limited to the casual application of toxic pesticides, which in turn has caused numerous unexpected ecological, economic and social drawbacks. Hence in order to develop more efficacious and sustainable IPM-N, a large number of efforts have made over the past 10 years to understand the pathophysiology of plant-nematode interactions, but our knowledge regarding i) the pathogenicity of phytonematodes and ii) the defense responses of host plants against phytonematodes are still rudimentary, compared to other plant-microbial pathogen interactions.
Plant parasitic nematodes, belonging to the phylum Nematoda, are microscopic animals that have evolved to over 4,000 species and adapted to a broad range of environment from forests to oceans (Nicol., et al. 2011, Hodda 2011, Zhang 2013). Previously, many - if not most - of them were viewed as benign or non-damaging, but recent reports have recognized that selective species such as Rotylenchulusspp. Meloidogynespp. and Heteroderaspp., are agronomical important pests, attributing the annual losses of crop production at ~14 % in worldwide (Nicol 2002, Nicol., et al. 2011). For instance, R. reniformis(reniform nematodes) have become a major threat over the last decade towards cotton farming in the southern regions of the U.S., leading to an estimated yield loss of over $100 million annually. Cotton is the most important fiber producing crop of which its production in the U.S. accounts for about one quarter of the world supply (~ $25 billion values, Koenning., et al. 2004,), and creates over 200,000 jobs (NCCA 2015). However the currently available integrated pest management method against phytonematodes (IPM-N) is limited to the casual application of toxic pesticides, which in turn has caused numerous unexpected ecological, economic and social drawbacks. Hence in order to develop more efficacious and sustainable IPM-N, a large number of efforts have made over the past 10 years to understand the pathophysiology of plant-nematode interactions, but our knowledge regarding i) the pathogenicity of phytonematodes and ii) the defense responses of host plants against phytonematodes are still rudimentary, compared to other plant-microbial pathogen interactions.
Current update on plant-nematode interactions
The current working model of plantnematode interactions is built on the basis of two major hypotheses that i) phytonematodes use chemotaxis to sense and direct towards host plant roots, and ii) plant roots operate essentially similar - if not the same - defense mechanisms against phytonematodes as do plant leaves against other microbial and herbivore pathogens. Indeed, a single dominant gene (Mi-1) conferring resistance against the root-knot nematode Meloidogynespp. was isolated over half a century ago from a tomato relative (Lycopersicon peruvianum, Bailey 1941). Since then, the major research goals of plant-nematode interactions have focused on espying phytonematode-derived avirulence (avr)-genes (also called effectors) that bind and trigger resistance (R)-gene (i.e. Mi-1)-mediated resistance (also called effector-triggered immunity, ETI). However, the identity of phytonematode-derived avr-gene is - if it is present - still elusive. Instead, several studies have proposed a pivotal role of phytonematode-derived cell wall degrading enzymes (CWDE, sugar hydrolases) in host plant defense responses, although their modes of action are not yet understood (Mitchum., et al. 2013, Fosu-Nyarko and Jones 2016). On the other hand, a recent study has underpinned that phytonematodes secrete conserved molecules, so called ascarosides that are capable of eliciting PAMP (pathogen-associated molecular pattern) responses (referred to PAMP-triggered immunity, PTI, or basal resistance) in various plants (Manosalva., et al. 2015). Although the cognate pattern recognition receptors (PRRs) of ascarosides are yet to be identified, this finding reveal the perception of PAMPs and other molecular patterns converges on triggering plant immunity. In addition, these results perhaps shed new light on an actual role of phytonematode-derived CWDE which could activate the production of damage-associated molecular patterns (DAMP, Gillet 2017), instead of targeting to nucleotide binding domain leucine rich repeat (NB-LRR) proteins leading to ETI. DAMP then target PRRs and induce several downstream signaling events during plant immune responses (Seong and Matzinger, 2004).
The current working model of plantnematode interactions is built on the basis of two major hypotheses that i) phytonematodes use chemotaxis to sense and direct towards host plant roots, and ii) plant roots operate essentially similar - if not the same - defense mechanisms against phytonematodes as do plant leaves against other microbial and herbivore pathogens. Indeed, a single dominant gene (Mi-1) conferring resistance against the root-knot nematode Meloidogynespp. was isolated over half a century ago from a tomato relative (Lycopersicon peruvianum, Bailey 1941). Since then, the major research goals of plant-nematode interactions have focused on espying phytonematode-derived avirulence (avr)-genes (also called effectors) that bind and trigger resistance (R)-gene (i.e. Mi-1)-mediated resistance (also called effector-triggered immunity, ETI). However, the identity of phytonematode-derived avr-gene is - if it is present - still elusive. Instead, several studies have proposed a pivotal role of phytonematode-derived cell wall degrading enzymes (CWDE, sugar hydrolases) in host plant defense responses, although their modes of action are not yet understood (Mitchum., et al. 2013, Fosu-Nyarko and Jones 2016). On the other hand, a recent study has underpinned that phytonematodes secrete conserved molecules, so called ascarosides that are capable of eliciting PAMP (pathogen-associated molecular pattern) responses (referred to PAMP-triggered immunity, PTI, or basal resistance) in various plants (Manosalva., et al. 2015). Although the cognate pattern recognition receptors (PRRs) of ascarosides are yet to be identified, this finding reveal the perception of PAMPs and other molecular patterns converges on triggering plant immunity. In addition, these results perhaps shed new light on an actual role of phytonematode-derived CWDE which could activate the production of damage-associated molecular patterns (DAMP, Gillet 2017), instead of targeting to nucleotide binding domain leucine rich repeat (NB-LRR) proteins leading to ETI. DAMP then target PRRs and induce several downstream signaling events during plant immune responses (Seong and Matzinger, 2004).
Underground talks between plant roots and Phytonematodes
It has long been speculated that chemotaxis is primary means by which Phytonematodes locate host plants (Curtis 2008), as they are motile animals undulating in the dorsal ventral direction (snake-like motion, Backholm., et al. 2013). Phytonematodes develop longitudinal muscles, and a thick cuticle that molts which serves as a hydrostatic skeleton used for locomotion, commonly referred to move ~1 meter through the soil within their lifetime (Davis and MacGuidwin 2000, Moore., et al. 2010). However, it is unclear if the movement of phytonematodes i) is autonomous or needs environmental matrices such as water, wind, insects and/or animals, or ii) targets towards specific chemical attractants (host plants) or reach host plants opportunistically via environmental matrices.
It has long been speculated that chemotaxis is primary means by which Phytonematodes locate host plants (Curtis 2008), as they are motile animals undulating in the dorsal ventral direction (snake-like motion, Backholm., et al. 2013). Phytonematodes develop longitudinal muscles, and a thick cuticle that molts which serves as a hydrostatic skeleton used for locomotion, commonly referred to move ~1 meter through the soil within their lifetime (Davis and MacGuidwin 2000, Moore., et al. 2010). However, it is unclear if the movement of phytonematodes i) is autonomous or needs environmental matrices such as water, wind, insects and/or animals, or ii) targets towards specific chemical attractants (host plants) or reach host plants opportunistically via environmental matrices.
Thus far, at least 50 different nematode motility or chemotaxis assays have been carried out via employing agar gel, pluronic F-127 gel, natural sand and soil as migration matrices, which displayed that phytonematodes are responsive to CO2, pH and electrical gradients (Fosu-Nyarko and Jones 2016). However, considering that each phytonematode species only targets a selective group of host, but not non-host, plant species (Nicol., et al. 2011), it is quite feasible to hypothesize that phytonematodes are able to perceive and locate chemotactic compounds originated from root cap slime or cells sloughed from the roots. One well-studied example of an attractant is the volatile (E)-β-caryophyllene emitted by the maize roots in response to feeding by the larvae of the Western corn rootworm (WCR) (Rasmann., et al. 2005, Degenhardt., et al. 2009). This volatile is highly attractive to an entomopathogenic nematode, H. megidis, which parasitizes and kills WCR within a few days (Degen., et al. 2004, Rasmann., et al. 2005). These studies illustrate the signaling role of root- derived allelochemicals which are likely to be involved in plant-nematode interactions. Therefore, to further substantiate the hypothesis, we recently have developed a novel nematode chemotaxis assay using an agar assay plate of which surface a) is hydrophilic (0.02% agar, Figure 1A) enough to evade the surface tension of nematodes (adhesion, shown in e.g. 0.1% agar; Figure 1B), and b) forms the shape of a volcano in the middle [Figure 1C]. Phytonematodes (e.g. reniform nematodes) are then placed around the slope of volcano, and their motilities are monitored in response to plant-derived compounds introduced on the top of volcano [Figure 1D]. Note that nematodes and plant compounds are closely positioned, but yet not directly contact each other.
Exudates from Cotton Roots Signal and Attract Reniform Nematodes.
As shown in Figure 1E as a control experiment, reniform nematodes were gradually slid away from the top once water is placed on there, because of gravity on the slope. In contrast, reniform nematodes stayed on the slope, and/or crawled up to the top upon the application of cotton root exudates (Figure 2B), underpinning that cotton roots secret underground chemotactic attractants seduce reniform nematodes. On the other hand, reniform nematodes exhibited little if any response to the extracts and exudates prepared from non-host plants such as peanut (data not shown), concurring with the conclusion that phytonematodes are able to i) recognize chemotactic attractants, ii) specific allelochemicals released from host plant roots to rhizosphere, and iii) travel autonomously towards the origins.
As shown in Figure 1E as a control experiment, reniform nematodes were gradually slid away from the top once water is placed on there, because of gravity on the slope. In contrast, reniform nematodes stayed on the slope, and/or crawled up to the top upon the application of cotton root exudates (Figure 2B), underpinning that cotton roots secret underground chemotactic attractants seduce reniform nematodes. On the other hand, reniform nematodes exhibited little if any response to the extracts and exudates prepared from non-host plants such as peanut (data not shown), concurring with the conclusion that phytonematodes are able to i) recognize chemotactic attractants, ii) specific allelochemicals released from host plant roots to rhizosphere, and iii) travel autonomously towards the origins.
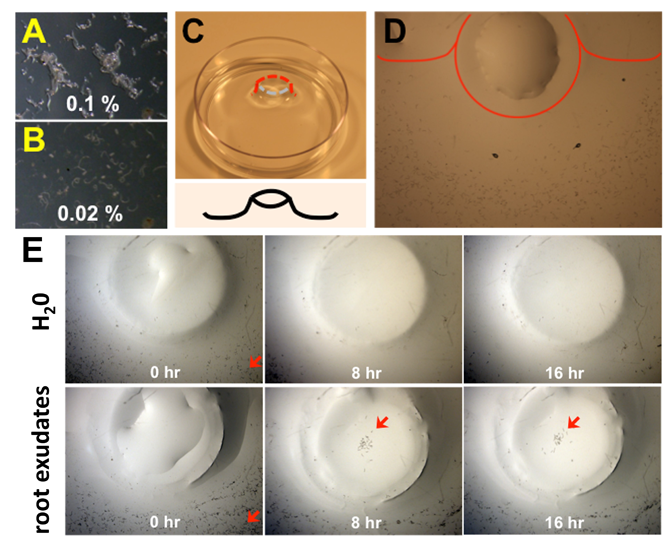
Figure 1: Exudates from cotton roots signal and attract reniform nematodes. (A to E).
Establishment and optimization of novel nematode chemotaxis assay. Since nematodes formed a cluster on a normal agar concentration plate (A), agar concentrations were lowered to 0.02 % (w/v) maintain a plate surface hydrophylic (B) with the shape of a volcano in the middle (C) where the chemical of interests were applied on the top, while nematodes were placed around the slope of a volcano mountain (D). Red line outlines the shape of a volcano. (E) Following the application of H2O (upper panel) and root exudates prepared from 2 wk grown cottons (lower panel), the motility and movement of reniform nematodes were monitored every hr, and representative photographs were taken at 8 and 16 hr via the high-definition color camera (Nikon DS-Fi1) attached to the Zoom Stereomicroscope system (Nikon SMZ1500). Red arrows indicate reniform nematodes.
Establishment and optimization of novel nematode chemotaxis assay. Since nematodes formed a cluster on a normal agar concentration plate (A), agar concentrations were lowered to 0.02 % (w/v) maintain a plate surface hydrophylic (B) with the shape of a volcano in the middle (C) where the chemical of interests were applied on the top, while nematodes were placed around the slope of a volcano mountain (D). Red line outlines the shape of a volcano. (E) Following the application of H2O (upper panel) and root exudates prepared from 2 wk grown cottons (lower panel), the motility and movement of reniform nematodes were monitored every hr, and representative photographs were taken at 8 and 16 hr via the high-definition color camera (Nikon DS-Fi1) attached to the Zoom Stereomicroscope system (Nikon SMZ1500). Red arrows indicate reniform nematodes.
Exudates from cotton roots signal and attract reniform nematodes
As shown in Figure 1E as a control experiment, reniform nematodes were gradually slid away from the top once water is placed on there, because of gravity on the slope. In contrast, reniform nematodes stayed on the slope, and/or crawled up to the top upon the application of cotton root extracts or exudates [Figure 2B], underpinning that cotton roots secret underground chemotactic attractants seduce reniform nematodes. On the other hand, reniform nematodes exhibited little if any response to the extracts and exudates prepared from non-host plants such as peanut (data not shown), concurring with the conclusion that phytonematodes are able to i) recognize chemotactic attractants, ii) specific llelochemicals released from host plant roots to rhizosphere, and iii) travel autonomously towards the origins.
As shown in Figure 1E as a control experiment, reniform nematodes were gradually slid away from the top once water is placed on there, because of gravity on the slope. In contrast, reniform nematodes stayed on the slope, and/or crawled up to the top upon the application of cotton root extracts or exudates [Figure 2B], underpinning that cotton roots secret underground chemotactic attractants seduce reniform nematodes. On the other hand, reniform nematodes exhibited little if any response to the extracts and exudates prepared from non-host plants such as peanut (data not shown), concurring with the conclusion that phytonematodes are able to i) recognize chemotactic attractants, ii) specific llelochemicals released from host plant roots to rhizosphere, and iii) travel autonomously towards the origins.
Concluding Remarks
Given the considerable economic impact of phytonematodes on global crop yields, the development of unique and effective IMP for disease control requires particular attention (Gillet., et al. 2017). In line with this scenario, discovery of the chemotactic attractant(s) will not only increase our basic understanding on plant-nematode interactions, but also provide key resources in genetic engineering or molecular breeding approaches to upgrade the plants’ own defense capacities, which in turn maximize the yield and survival for food, fiber or biofuel crops. Recent studies of ours [Figure 1] and other groups (Reynolds., et al. 2011 Hinda., et al. 2015) have finally started to corroborate a half-century old hypothesis that “phytonematodes recognize and infect target plants through hijacking root-released allelochemicals in perhaps rootrhizosphere interactions”. In particular, the results obtained from the studies of cotton root reniform nematode interactions [Figure 1] will serve as an outset to finally reveal the chemical identity of chemotactic attractant(s) as e.g.) our following studies have employed the preparatory high-performance liquid chromatography analysis to profile the reniform nematode attractant activity of metabolic compounds separated from cotton root exudates. Information collected from these studies will develop a protocol to disrupt or neutralize plant root-phytonematode (e.g. cotton root-reniform nematode) interactions by i) further delineating the biosynthetic pathways of chemotactic attractant(s), ii) which then allows us to generate transgenic GM plants knocking down the biosynthetic pathways, or alternatively iii) screening chemical antidotes to the attractant(s); together help improve the economic and environmental sustainability of agriculture.
Acknowledgement
We thank William Groover for the steady supply of R. reniformis. This work was supported in part by the Alabama Agricultural Experiment Station (S.W.P.), the Hatch program of the National Institute of Food and Agriculture, USDA (S.W.P.), and the Alabama Cotton Commission (S.W.P.).
We thank William Groover for the steady supply of R. reniformis. This work was supported in part by the Alabama Agricultural Experiment Station (S.W.P.), the Hatch program of the National Institute of Food and Agriculture, USDA (S.W.P.), and the Alabama Cotton Commission (S.W.P.).
References
- Backholm M., et al. “Viscoelastic properties of the nematode Caenorhabditis elegans, a self-similar, shear-thinning work”. Proceedings of the National Academy of Sciences of the USA 110.12 (2013): 4528-4533.
- Bailey DM. “The seedling method for root-knot nematode resistance”. Proceedings of the American Society for Horticultural Science 38 (1941): 573-575.
- Curtis RHC. “Plant-nematode interactions: environmental signals detected by the nematode’s chemosensory organs control changes in the surface cuticle and behaviours”. Parasite 15.3 (2008): 310-316.
- Davis EL and MacGuidwin AE. “Lesion nematode disease”. The Plant Health Instructor (2005):
- Degen T., et al. “High genetic variability of herbivoreinduced volatile emission within a broad range of maize inbred lines”. Plant Physiology 135.4 (2004): 1928-1938.
- Degenhardt J., et al. “Restoring a maize root signal that attracts insect-killing nematodes to control a major pest”. Proceedings of the National Academy of Sciences of the USA 106.32 (2009): 13213-13218.
- Fosu-Nyarko J and Jones MGK. “Advances in understanding the molecular mechanisms of root lesion nematode host interactions”. Annual Review of Phytopathology 54 (2016): 253-278.
- Gillet FX., et al. “Plant-parasitic nematodes: towards understanding molecular players in stress responses”. Annals of Botany 119.5 (2017): 775-789.
- Hida H., et al. “Chemotaxis assay of plantparasitic nematodes on a gel-filled microchannel device”. Sensors and Actuators B: Chemical 221 (2015): 1483-1491.
- Zhang ZQ. “Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness (Addenda 2013)”. Zootaxa 3703 (2013): 1-82.
- Koenning SR., et al. “Plantparasitic nematodes attacking cotton in the United States: Old and emerging production challenges”. Plant Disease 88.2 (2004): 100-113.
- Manosalva P.,et al. “Conserved nematode signaling molecules elicit plant defenses and pathogen resistance”. Nature Communications 6 (2013): 7795.
- Mitchum MG., et al. “Nematode effector proteins: an emerging paradigm of parasitism”. New Phytologist Journal 199.4 (2013): 879-894.
- Moore SR.,et al. “Natural migration of Rotylenchulus reniformis in a No-Till cotton system”. Journal of Nematology 42.4 (2010): 307-312.
- National Cotton Council of America (NCCA) (2015) NCC Comments on Pollinator Proposal.
- Nicol JM. “Important nematode pests In: Curtis BC, Rajaram S, Gómez M (eds) Bread wheat improvement and production”. FAO Plant production and Protection Series (2002): 567.
- Nicol JM., et al. “Current nematode threats to world agriculture”. Genomics and Molecular Genetics of Plant- Nematode Interactions(2011):21-43.
- Rasmann S., et al. “Recruitment of entomopathogenic nematodes by insect- damaged maize roots”. Nature 434 (2005): 732-737.
- Reynolds AM., et al. “Chemotaxis can take plant-parasitic nematodes to the source of a chemo-attractant via the shortest possible routes”. Journal of the Royal Society Interface 8.57 (2011): 568-577.
- Seong SY and Matzinger P. “Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses”. Nature Reviews Immunology 4 (2004): 469-478.
- Zhang Z. “Animal biodiversity: An update of classification and diversity in 2013, In: Zhang ZQ (eds) Animal Biodiversity: An Outline of Higher-level Classification and Survey of Taxonomic Richness (Addenda 2013)”. Zootaxa3703.1 (2013): 5-11.
Citation:
Sang-Wook Park., et al. “Underground Mystery: The Role of Chemotactic Attractants in Plant Root and Phytonematode Interactions”.
Innovative Techniques in Agriculture 1.2 (2017): 83-87.
Copyright: © 2017 Sang-Wook Park., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.





























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.