Research Article
Volume 1 Issue 2 - 2017
Qualitative Analysis of Phytocompounds and Synthesis of Silver Nanoparticles from Centella Asiatica
Plant Biotechnology Laboratory, Department of Biotechnology, Delhi Technological University, New Delhi
*Corresponding Author: Navneeta Bharadvaja, Plant Biotechnology Laboratory, Department of Biotechnology, Delhi Technological
University, New Delhi-110042.
Received: June 12, 2017; Published: June 30, 2017
Abstract
In the Ayurvedic system of medicine, Centella Asiatica (gotu kola) is one of the important rejuvenating herbs for nerve and brain cells
and is believed to be capable of increasing intelligence, longevity, and memory. It contains several active constituents and the most
important bioactive compounds are triterpenoid saponins, including asiaticoside, centelloside, madecassoside, and asiatic acid. In
the present investigation, qualitative analysis of phytocompounds was done in five accessions of Centella Asiatica using ethanolic,
methanolic and aqueous extract of the whole plant. In the case of ethanolic and methanolic extract, it showed positive results for
terpenoid, steroid, flavonoid and coumarins whereas in the case of aqueous extract it showed positive results for terpenoid, steroid,
flavonoid, coumarins and tannin. Further synthesis of silver nanoparticles was done and characterization of synthesized nanoparticles
was done by using UV-VIS spectroscopy, FT-IR and SEM.
Keywords: Phytocompounds; Plant extract; Silver nanoparticles; UV-VIS spectroscopy; FT-IT; SEM
Introduction
Centella Asiatica is one of the important traditional medicinal plant belonging to family Apiaceae and commonly known as ‘Gotu kola’, ‘Indian Pennywort’ or ‘Mandookaparni’ in India. It is an important perennial medicinal herb found in the tropical and subtropical countries like India, Sri Lanka and Bangladesh. C. Asiatica contains several triterpene, saponins like asiaticoside, asiatic acid, sapogenins, madecassic acid, vellarin, adecassoside, glycosides and centelloside (Glasby, 1991). Phytochemicals are natural bioactive compounds found in plants. These work with nutrients and fiber to form an integral part of defence system against various diseases. The most important bioactive constituents of this plant are saponin (asiaticoside, medecassic acid, asiatic acid and madecassoside), terpenoid, steroid, flavanoids, and tannins. These compounds are responsible for the wide therapeutic activity. However there is a significant difference in the active constituent content among samples from different locations.
Qualitative analysis reveals the presence of different phytocompounds in this plant. In recent times nanotechnology is one of the fast growing fields. It mainly concern to the synthesis and designing of nanomaterials within the range of 1-100 nm. Nanoparticles exhibit new properties based on specific characteristics such as, size, morphology and distribution, if compared to with the large particles of bulk material. Nanoparticles present a high surface to volume ration with decreasing size. Generally nanomaterials synthesized by using physical and chemical methods but the by-products from these methods are toxic in nature and the process is also costly. To overcome this problem there is a need of environment friendly methods of synthesis. Synthestis of nanoparticles using plant extract is an important branch (Logeswari., et al. 2015).Several nanoparticles have been synthesized by this plant like silver, gold, copper oxide, etc. Using plant for nanoparticles synthesis can be an advantageous over other biological processes by eliminating the elaborate process of maintaining cell culture. Among several metal nanoparticles, silver nanoparticles have attained a special focus due to its stability, good conductivity and antimicrobial activity.
Materials and Methods
Five accessions i.e. 281374, 383913, 342109, 347492, 331514 of Centella Asiatica belonging to different regions of India were
collected from NBPGR, New Delhi and were maintained at the plant tissue culture laboratory of Department of Biotechnology, Delhi
Technological University.
Inoculation and incubation of explants
The laminar air flow chamber was properly surface sterilized with alcohol and UV lights for 30 minutes. The explants were trimmed to a suitable size of about 2 cm by keeping it in sterile Petri-dishes. Then a cut was given on both basal and top portion to remove the undesirable or dead portion. The forceps were rinsed in 70% ethanol and were flamed and then kept for some time to get cool. Then the lid from the culture tube was removed and mouth of it was flamed to avoid further chances of contamination. Each explant was then aseptically inoculated on the MS media containing 1 mg/l BAP in an erect position with long forceps without touching the rim of the culture tube. Then the lid was finally replaced carefully and sealed with the parafilm. The inoculated culture tubes were incubated in the culture room at 25 ± 2°C, with a light intensity of 2500 lux and a photoperiod of 16 hour light, 8 hour dark and 65% humidity.
The laminar air flow chamber was properly surface sterilized with alcohol and UV lights for 30 minutes. The explants were trimmed to a suitable size of about 2 cm by keeping it in sterile Petri-dishes. Then a cut was given on both basal and top portion to remove the undesirable or dead portion. The forceps were rinsed in 70% ethanol and were flamed and then kept for some time to get cool. Then the lid from the culture tube was removed and mouth of it was flamed to avoid further chances of contamination. Each explant was then aseptically inoculated on the MS media containing 1 mg/l BAP in an erect position with long forceps without touching the rim of the culture tube. Then the lid was finally replaced carefully and sealed with the parafilm. The inoculated culture tubes were incubated in the culture room at 25 ± 2°C, with a light intensity of 2500 lux and a photoperiod of 16 hour light, 8 hour dark and 65% humidity.
Phytochemicals Analysis
Ethanolic extract preparation: Fresh in-vitro grown plant material was ground with mechanical grinder. One gram of plant material was then macerated in 10 ml of absolute ethanol for 48 hours and covered properly with aluminium foil and labelled. After 48 hours of extraction, each extract was filtered through the Whatman’s filter paper. The filtrate was stored at 4°C temperature.
Ethanolic extract preparation: Fresh in-vitro grown plant material was ground with mechanical grinder. One gram of plant material was then macerated in 10 ml of absolute ethanol for 48 hours and covered properly with aluminium foil and labelled. After 48 hours of extraction, each extract was filtered through the Whatman’s filter paper. The filtrate was stored at 4°C temperature.
Methanolic extract preparation: Fresh in-vitro grown plant material was ground with mechanical grinder. One gram of plant material was then macerated in 10 ml of methanol for 48 hours and covered properly with aluminium foil and labelled. After 48 hours of extraction, each extract was filtered through the Whatman’s filter paper. The filtrate was stored at 4°C temperature.
Aqueous extract preparation: About 100 mg of powdered plant materials was mixed with 10 ml of miliQ water and boiled for 20 minutes for the formation of plant extract. Obtained plant extract was filtered through Whatman No.-1 filter paper. Then transferred into autoclaved vials and stored at 4°C for further analysis.
Terpenoid Test (Salkowski Test): 100 μl of plant extract was mixed with 2 ml of chloroform and 3 ml of concentrated sulphuric acid to form a layer. A reddish brown color at interface is formed to show positive result of the presence of terpenoid and triterpenoids.
Steroid test(Salkowski Test): 100 μl of plant extract was dissolved in 2 ml of chloroform. Sulphuric acid was carefully added to form a lower layer. A reddish brown color at the interface is an indicative of the presence of steroidal ring.
Saponin test (Foam test): Add 100 mg of powdered plant material to 10 ml of distilled water. Heat the mixture and observe for persistent froth. Formation of froth indicates the presence of saponins.
Flavonoid test (NaOH test): 100 μl of plant extract was treated with few drops of sodium hydroxide solution. Formation of intense yellow color, which becomes color less on addition of dilute acid, indicates the presence of flavanoids.
Tannin test (FeCl3 test): - 100 μl of plant extract was treated with few drops of freshly prepared 6% FeCl3. Formation of green color indicates the presence of tannin.
Glycosides test (Fehling’s Test): - Equal volume of Fehling A and Fehling B reagents were mixed together and 2 ml of it was added to the 100 μl of plant extract and genteelly boiled. A brick red precipitate appeared at the bottom of the test tube indicates the presence of reducing sugars.
Coumarins: 200 μl of plant extract was mixed with few drops of 10% NaOH. Presence of yellow coloration indicates the presence of coumarins.
Alkaloids(Mayer’s Reagent):One milliliter ethanolic filtrate was taken in test tube and 2 ml of 2N HCL was added to it then solution was shaken vigorously to mix and kept for 5 minutes. Few drops of Mayer’s reagent (HgCl2+ KI in water) was added to it. Formation of creamy color precipitation indicates the presence of alkaloids.
Synthesis of silver nanoparticles
Preparation of aqueous plant extract: In-vitro grown plant materials were dried for seven days. Powdered plant materials were used for the extract preparation. About 100 mg of powder was mixed with 10 ml of miliQ water and boiled for 20 minutes for the formation of plant extract. Obtained plant extract was filtered through Whatman No.-1 filter paper. Then transferred into autoclaved vials and stored at 4°C for further analysis.
Preparation of aqueous plant extract: In-vitro grown plant materials were dried for seven days. Powdered plant materials were used for the extract preparation. About 100 mg of powder was mixed with 10 ml of miliQ water and boiled for 20 minutes for the formation of plant extract. Obtained plant extract was filtered through Whatman No.-1 filter paper. Then transferred into autoclaved vials and stored at 4°C for further analysis.
Preparation of silver nitrate solution: Silver nitrate (AgNO3) was collected from Fisher Scientific. Molecular weight of AgNO3 is 169.87 g/mol.
For the preparation of 1 mM AgNO3 solution, 16.987 mg of AgNO3 was added to 100 ml of mili-Q water and mixed thoroughly. Solution was stored in flask covered with aluminium foil.
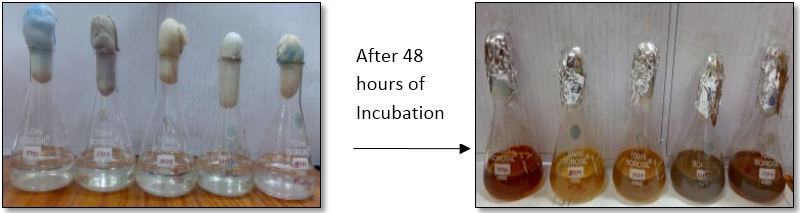
Synthesis of silver nanoparticles:For the synthesis of silver nanoparticles, 90 ml of 1 mM AgNO3 solution was taken in an autoclaved flask and 10 ml of aqueous plant extract was added to it. Solution was mixed well and kept in incubator shaker at 37°C, 150 rpm for 48 hours. As a result, formation of dark yellowish brown colour indicates the formation of silver nanoparticles.
Characterization of silver nanoparticles
UV-Vis spectroscopy: Silver nanoparticles exhibits dark yellowish brown color in aqueous solution due to the Surface Plasmon Resonance phenomenon. Thus silver nanoparticles formed were separated from the residual and characterized by UV-VIS absorption spectroscopy. Bio-reduction of pure silver ion was monitored. Deionised water was used as a blank. The wavelength range for the silver nanoparticles detection was 300 to 700 nm and the presence of reduced silver ions was highlighted by a peak of absorption in the range of 350 to 500 nm.
UV-Vis spectroscopy: Silver nanoparticles exhibits dark yellowish brown color in aqueous solution due to the Surface Plasmon Resonance phenomenon. Thus silver nanoparticles formed were separated from the residual and characterized by UV-VIS absorption spectroscopy. Bio-reduction of pure silver ion was monitored. Deionised water was used as a blank. The wavelength range for the silver nanoparticles detection was 300 to 700 nm and the presence of reduced silver ions was highlighted by a peak of absorption in the range of 350 to 500 nm.
Scanning Electron Microscope (SEM) Analysis:Scanning Electron Microscope analysis was done using Hitachi 3700N SEM machine. Thin films of samples were prepared on a carbon coated copper grid by dropping very small amount of sample on the grid, extra solution was removed using a blotting paper and then film on the SEM grid were allowed to dry by putting it under a mercury lamp for 5 minutes.
Fourier Transmission Infrared Spectroscopy (FT-IR) Analysis: FT-IR was used to identify the possible functional groups responsible for the reduction of Ag ions and capping of the bio reduced silver nanoparticles synthesized. In order to determine the functional groups and their possible involvement in the synthesis of silver nanoparticles, FT-IR analysis was carried out. Liquid samples were used for the analysis. Samples were analyzed using Thermo Scientific Nicolet 380 FT-IR spectrophotometer. Spectrum was recorded in FT-IR in the range of 4000-500 cm-1 at a resolution of 4 cm-1. Peaks obtained were plotted as % Transmittance in Y axis and wave number (cm-1) in X axis.
Results and Discussion
Qualitative analysis of phytochemicals: Three different extracts i.e. ethanolic, methanolic and aqueous extract of Centella asiatica (whole plant) were used in qualitative analysis of phytochemicals. This study revealed the presence of terpenoid, saponin, steroid, flavonoid, tannin and coumarins. In case of ethanolic and methanolic extract it showed positive results for terpenoid, steroid, flavonoid and coumarins whereas in case of aqueous extract it showed positive results for terpenoid, steroid, flavonoid, coumarins and tannin. These photochemical compounds are major compounds which impart medicinal value of this plant. However all the three extracts showed negative result for glycoside and alkaloids test. Singh., et al. 2012 used methanolic extract for the phytochemicals test in C. Asiatica.
| Extract | Phytoconstituents | ||||||||
| Accessi on | Terpenoid | Steroid | Saponin | Flavonoid | Tannin | Glycosides | Coumarins | Alkaloids | |
| Ethanolic | 342109 | + | + | + | + | - | - | + | - |
| 347492 | + | + | + | + | - | - | + | - | |
| 331514 | + | + | + | + | - | - | + | - | |
| 383913 | + | + | + | + | - | - | + | - | |
| 281374 | + | + | + | + | - | - | + | - | |
| Methanolic | 342109 | + | + | + | + | - | - | + | - |
| 347492 | + | + | + | + | - | - | + | - | |
| 331514 | + | + | + | + | - | - | + | - | |
| 383913 | + | + | + | + | - | - | + | - | |
| 281374 | + | + | + | + | - | - | + | - | |
| Aqueous | 342109 | + | + | + | + | + | - | + | - |
| 347492 | + | + | + | + | + | - | + | - | |
| 331514 | + | + | + | + | + | - | + | - | |
| 383913 | + | + | + | + | + | - | + | - | |
| 281374 | + | + | + | + | + | - | + | - | |
+ = Positive - = Negative
Table 1: Phytochemicals analysis of different accessions of Centella asiatica using ethanolic, methanolic and aqueous extract.
Table 1: Phytochemicals analysis of different accessions of Centella asiatica using ethanolic, methanolic and aqueous extract.
Silver nanoparticles synthesis
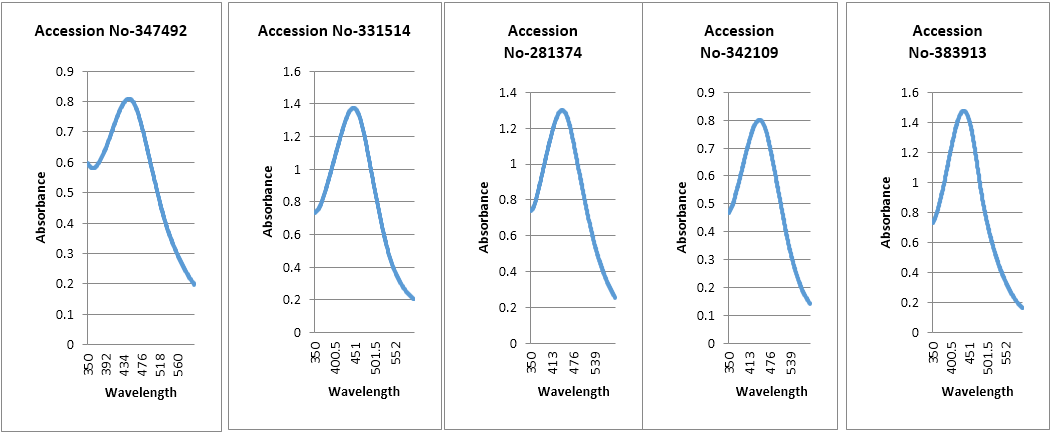
UV-Vis spectroscopy: Reduction of silver ions into silver nanoparticles during exposure to plant extracts was observed as a result of change in colour. Change in colour is due to the Surface Plasmon Resonance phenomenon. Metal nanoparticles have free electrons, which give the SPR absorption band due to the combine vibration of electrons of metal nanoparticles in resonance with light wave. Peak of silver nanoparticles were observed at 446.5 nm, 447.0 nm, 444.0 nm, 443.0 nm and 437 nm in case of accession no- 347492, 331514, 342109, 281374 and 383913. From literature review it was found that silver nanoparticles show peak in a range of 350 nm to 500 nm. Broadening of peak indicates that the particles are poly dispersed. From this study we found the SPR peak is nearly 437-447 nm for different accessions. The metal particles were observed to be stable in solution even four weeks after synthesis that means the optical properties of nanoparticles in solution with time. Maximum absorbance was observed in the accession no-383913 which is 1.477.
Scanning Electron Microscope (SEM) Analysis: SEM provides the details about the morphology of the silver nanoparticles. SEM
images were taken at different resolutions i.e. 500 nm, 2 μm, 5 μm, 2 μm and 10 μm in case of accession no-347492, 331514, 342109,
281374 and 383913. Size of synthesized nanoparticles was more than the size of nanoparticles which should be between 1-100 nm.
Size was more than the desired size due to the protein which were bound in the surface of nanoparticles. Result shows that the particles
were of relatively spherical shape in all the accessions i.e. no- 347492, 331514342109, 281374 and 383913 [Figure 11].
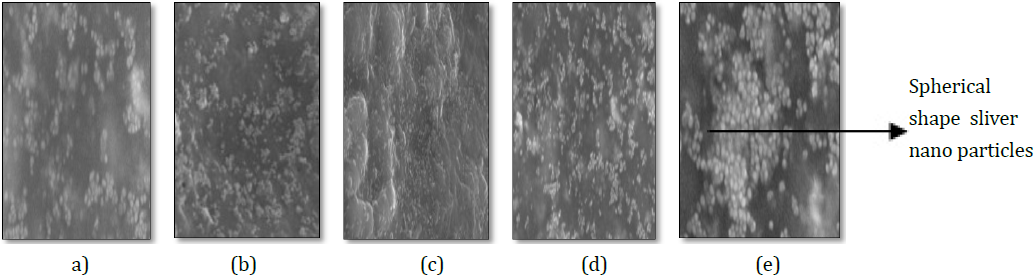
Figure 3: SEM images of silver nanoparticles. Accession number.
(a) 281374, (b) 331514, (c) 342109, (d) 347492 and (e) 383913
(a) 281374, (b) 331514, (c) 342109, (d) 347492 and (e) 383913
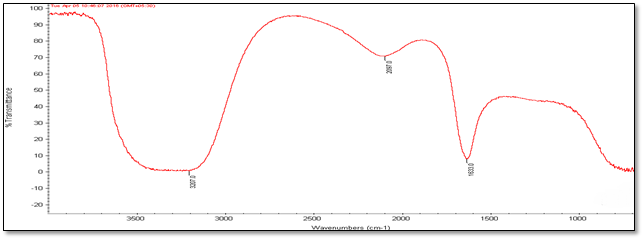
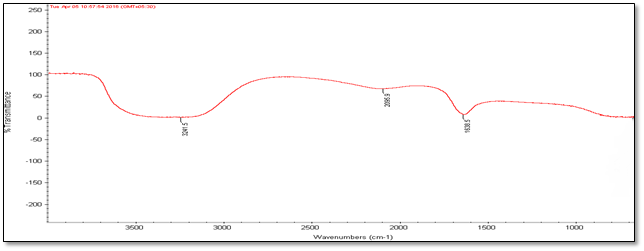
Fourier Transmission Infrared Spectroscopy (FT-IR) Analysis: FT-IR measurements were carried out to identify the biomolecules
for capping and efficient stabilization of metal nanoparticles synthesised. FT-IR spectrum of silver nanoparticles shows the band between
3207-3288 cm-1 corresponds to O-H stretch H-bonded alcohols and phenol. Peaks found around 1633-1639 cm-1 corresponds
to carbonyl stretch vibrations from carboxylic acid and phenols and N-H identified as amide and arise due to a carbonyl stretch in the
amide linkages of the proteins and peaks around 2095-2118 corresponds to C≡C stretch. Therefore synthesised nanoparticles were
surrounded by proteins and metabolites such as flavanoids and terpenoids (Singh, 2016). Based on the physical state of the extracts
and the characteristic features of the infrared vibrational peaks in the spectra, terpenoids, long chain fatty acids and secondary amide
derivatives were the possible compounds in the obtained nanoparticles. Form FT-IR analysis we confirmed that the carbonyl groups
from amino acid residues and proteins has stronger ability to bind metal which indicating that the proteins involve in capping of silver
nanoparticles and prevents from agglomeration and stabilize the medium.
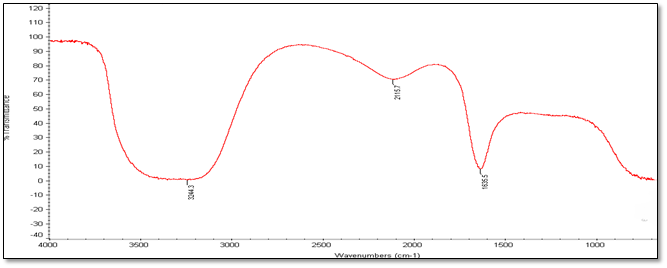
(a) Accession Number-281374
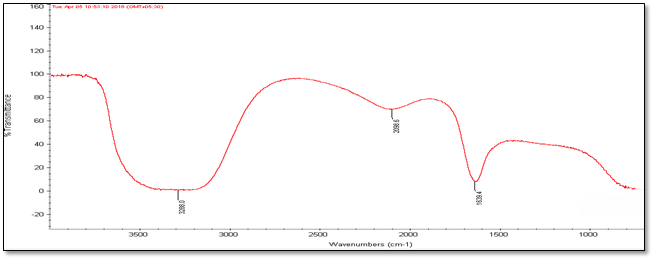
(b) Accession Number- 331514
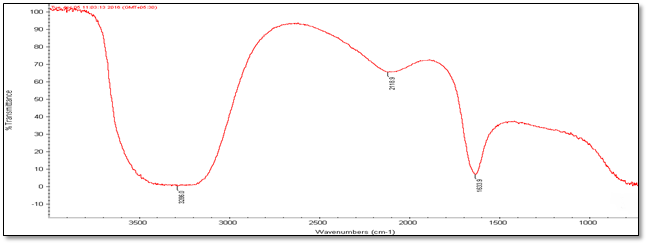
(c) Accession Number- 342109
(d) Accession Number- 347492
(e) Accession Number- 383913
Figure 4: FT-IR Analysis of silver nanoparticles of five different accessions (a) to (e).
Figure 4: FT-IR Analysis of silver nanoparticles of five different accessions (a) to (e).
| Accession No. | FT-IR | |||||
| 347492 | 331514 | 342109 | 281374 | 383913 | Vibrational frequencies | |
| Vibrational peak (cm-1) | 1635.5 | 1639.4 | 1633.9 | 1633 | 1638.5 | C–N and C–C stretching indicating the presence of proteins (Prakash., et al. 2013), N-H (Logeswari., et al. 2013) |
| 2115.7 | 2098.6 | 2118.9 | 2097 | 2095.9 | Could be C≡C (Devaraj., et al. 2013) | |
| 3244.3 | 3288 | 3286 | 3207 | 3241.5 | Free O-H group present in Phenols (Kumari., et al. 2016) | |
Table 2: FT-IR Vibrational peak of silver nanoparticles.
Conclusions
Qualitative analysis of phytochemicals revealed the presence of terpenoids, steroids, saponin flavonoids, tannins and coumarins in Centella Asiatica. All the five accession i.e. accession no.-342109, 281374, 331514, 383913 and 347492 showed the presence of these phytocompounds.Presence of these compounds suggests the medicinal properties of this plant. There is a need of further studies to know their biological effects which could be beneficial in the treatment of various diseases.
Rapid biological synthesis of silver nanoparticles using Centella Asiatica aqueous extract provides a simple, efficient and environment friendly method for the synthesis of nanoparticles. All the five accession i.e. accession no. 342109, 281374, 331514, 383913 and 347492 has potential for the silver nanoparticles synthesis. Synthesized nanoparticles were relatively spherical in shape. Nanoparticles were surrounded by a thin layer of proteins and metabolites such as terpenoids having functional groups of alcohols, amines, aldehydes etc., which were found from the characterization using UV-VIS spectroscopy, SEM and FT-IR techniques. It was observed that accession no.-383913 has more potential to synthesized silver nanoparticles as compared to the other accessions. From technological point of view these silver nanoparticles have potential application in biomedical field and this simple procedure has several advantages such as cost effective, compatibility for medical and pharmaceutical applications as we as large scale production.
Acknowledgment
Sincere thanks to the Department of Biotechnology, Delhi Technological University for making necessary facilities during this study, National Bureau of Plant Genetic Resources, New Delhi for providing the plant materials and Department of Applied Physics, Delhi Technological University for providing the FT-IR and SEM facility.
Sincere thanks to the Department of Biotechnology, Delhi Technological University for making necessary facilities during this study, National Bureau of Plant Genetic Resources, New Delhi for providing the plant materials and Department of Applied Physics, Delhi Technological University for providing the FT-IR and SEM facility.
References
- Devaraj P., et al. “Synthesis and Characterization of Silver Nanoparticles Using Cannonball Leaves and Their Cytotoxic Activity against MCF-7 Cell Line”. Journal of Nanotechnology(2013):
- Glasby JS. “Dictionary of Plants Containing Secondary Metabolites”. Flavour and Fragrance Journal 7.3 (1992): 512.
- Kumari J., et al. “Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics”. Journal of radiation Research and Applied Sciences 9.3 (2016): 217-227.
- Logeswari P., et al. “Ecofriendly synthesis of silver nanoparticles from commercially aviable plant powders and their antibacterial properties”. Scientia Iranica20.3 (2013): 1049-1054.
- Logeswari P., et al. “Synthesis of silver nanoparticles using plants extract and analysis of their antimicrobial property”. Journal of Saudi Chemical Society 19.3 (2015): 311-317.
- Prakash P., et al. “Green synthesis of silver nanoparticles from leaf extract of Mimusops elengi, Linn. for enhanced antibacterial activity against multi drug resistant clinical isolates”. Colloids and Surface B: Biointerfaces108 (2013): 255-259.
- Singh D., et al. “Qualitative estimation of presence of bioactive compounds in Centella asiatica: an important medicinal plant”. International Journal of Life Scienece and Medical Science2.1 (2012): 5-7.
- Singh S. “Green nanobiotechnology - an overview of synthesis, chracterisation and applications”. World Journal of Pharmacy and Pharmaceutical Sciences 5.8 (2016): 501-531.
Citation:
Arpita Roy and Navneeta Bharadvaja. “Qualitative analysis of phytocompounds and synthesis of silver nanoparticles from
Centella asiatica”. Innovative Techniques in Agriculture 1.2 (2017): 88-95.
Copyright: © 2017 Arpita Roy and Navneeta Bharadvaja. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.





























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.