Research Article
Volume 1 Issue 3 - 2017
Macro Invertebrate Communities in the spring and Stream Sites of Upper Awash River at Chilimo, Ethiopia
1Ambo University, Department of Biology, Ambo, Ethiopia
2Ethiopian Institute of Agricultural Research, National Fishery and Aquatic Life Research Centre, Sebeta, Ethiopia
3University of Natural Resources and Life Sciences, Department of Water, Atmosphere and Environment, Institute of Hydrobiology and Aquatic Ecosystem Management, Vienna, Austria
2Ethiopian Institute of Agricultural Research, National Fishery and Aquatic Life Research Centre, Sebeta, Ethiopia
3University of Natural Resources and Life Sciences, Department of Water, Atmosphere and Environment, Institute of Hydrobiology and Aquatic Ecosystem Management, Vienna, Austria
*Corresponding Author: Prabha Devi, Ambo University, Department of Biology, Ambo, Ethiopia.
Received: June 27, 2017; Published: August 31, 2017
Abstract
The aim of this study was to determine the abundance, distribution, composition and diversity of benthic macro invertebrate community at the different spring and stream habitats of upper Awash River at Chilimo, Dendi district, Ethiopia. Monthly benthic macro invertebrate samples were collected from the five selected stations between February 2016 and April 2016. Benthic fauna were collected from the pool and riffle habitats using standard net 25 cm x 25 cm respectively. Benthic macro invertebrates were identified and their abundance, distribution, composition and diversity were reported in relation to presence of natural forest vegetation covers and anthropogenic impact. The abundance of the fauna was high in station II and the lowest at station IV. The number of families of benthic fauna was high at stations III (23) and V (28) than the other sites. Ephemeroptera was the most abundant group at all sampling stations especially at station IV ranging between 400 and 592 No/m2. The EPT index value 78.29% was recorded from the less impacted station II. The highest Hilsenhoff Family Biotic index (H-FBI = 3.75) was noticed at station V. Dissolved oxygen varied between 6.39 mg/l in station I and 8.62 mg/l in station V. Relatively higher values of PH, temperature, electrical conductivity and BOD were observed in station V which is highly impacted by human activities.
Keywords: Biotic index; Richness; Insecta; Pool; Riffle
Introduction
Springs and streams are the most important freshwater resources for the people residing in the highlands of Ethiopia. The River Awash originates from the Chilimo forest, at elevations ranging from 2000 to 3200 m a.s.l. The study area is lying between latitude 40028’-E and 40059’-E and longitudes 9099’N and 10003’N. There are many springs that supply water to the streams which join together to form the head water of Awash river found inside the state protected forest dominated by trees such as Junipers procera, Podocarpus falcatus, Prunus africana, Olea europaea subspecies cuspidata, Hagenia abyssinica, Apodytes dimidiata, Ficus spp., Erythrina brucei, and Croton macrosytachus [21]. In recent years, however, the increased population growth, industrialization, land use modification, removal of riparian vegetation and poor implementation of environmental regulations have resulted in the degradation of the water quality [34,4] of the major rivers in Ethiopia. The level of degradation of lotic systems are assessed by physico-chemical and biological methods. The macro benthic macro invertebrates (BMI) are often the taxa group of choice for bio monitoring in streams and rivers as they are ubiquitous, sensitive to several anthropogenic pressures such as water pollution and hydro-morphological alterations [8,7]. Each macro invertebrate has particular requirements with respect to the physical, chemical and biological conditions of its habitat.
Changes in these conditions can result in the reduction in taxa richness and change in community structure. Among biotic factors, presence or absence of aquatic vegetation, predation and competition also limit the distribution of benthic macro organisms causing variation in community composition [12]. Water level or depth fluctuation also affect indirectly by influencing the appearance and growth patterns of aquatic vegetation [16,10]. The studies conducted in Ethiopia demonstrated that benthic macro invertebrates are effective tools for evaluating the ecological status of lotic systems [22,3]. The springs and streams in the Chilimo forest are utilized by the local community for rearing cattle, growing food crops and several domestic purposes. No attempt has been made to study the nature of the water and benthic fauna of upper Awash River spring and stream habitats and the present study is the first report on the environmental status of the this part of the river.
Materials and Methods
Five sampling stations [Figure 1] were selected based on topographic features, nature of bottom substratum habitat structure, exposure to vegetation cover and various human activities, following the rapid bio assessment protocol criteria [7] besides considering the major human activities in the spring and stream proper and the surrounding areas as stressors.
Station 1- Warabo spring- located at the origin of Warabo stream and where human activity is less
Station 2- Arera spring - at the origin of the Arera stream with less human intervention
Station 3- Awash1 located downstream after the confluence of the Warabo and Arera streams with high human activities
Station 4- Dabo spring- located at the origin of Dabo stream and less human activities
Station 5- located downstream of Dabo stream where much human activities and with no vegetation canopy
Station 2- Arera spring - at the origin of the Arera stream with less human intervention
Station 3- Awash1 located downstream after the confluence of the Warabo and Arera streams with high human activities
Station 4- Dabo spring- located at the origin of Dabo stream and less human activities
Station 5- located downstream of Dabo stream where much human activities and with no vegetation canopy
The spring stations 1 and 2 were surrounded by natural forest and shrubs and there was large quantity of dry and partially decomposed leaves accumulated on the soil. At station 3 there is forest and riparian vegetation with human intervention. Station 4 has sparse vegetation on the buffer zone alone the stream. At station 5 human intervention in the form of washing, watering cattle and other activities.
Sampling of macro invertebrates
For the collection of macro invertebrates Multi-Habitat Sampling (MHS) scheme [23] using a standard hand net with frame width of 25*25 cm2 and mesh size 500 μm. A composite sample consisting of 20 sampling units were taken from all habitat types each with a share of at least 5% habitat coverage. Samplings were done starting from the downstream end of the reach and proceeds upstream against the current. Megalithic stones were sampled by brushing their surfaces approximately equal to the size of the sampling net. Macrolithal stones were picked by hand and their surfaces were brushed to dislodge clingers and sessile organisms. After every 3 sampling, the net was rinsed by running clean stream water to avoid clogging which could interfere in obtaining an appropriate sample. Samples were initially preserved in 4% formalin in the field and after final identification preserved in 70% alcohol.
For the collection of macro invertebrates Multi-Habitat Sampling (MHS) scheme [23] using a standard hand net with frame width of 25*25 cm2 and mesh size 500 μm. A composite sample consisting of 20 sampling units were taken from all habitat types each with a share of at least 5% habitat coverage. Samplings were done starting from the downstream end of the reach and proceeds upstream against the current. Megalithic stones were sampled by brushing their surfaces approximately equal to the size of the sampling net. Macrolithal stones were picked by hand and their surfaces were brushed to dislodge clingers and sessile organisms. After every 3 sampling, the net was rinsed by running clean stream water to avoid clogging which could interfere in obtaining an appropriate sample. Samples were initially preserved in 4% formalin in the field and after final identification preserved in 70% alcohol.
Physico-chemical parameters
Water quality parameters such as temperature, pH, dissolved oxygen and conductivity were measured in-situ using a portable multi-parameter probe before sampling the macro invertebrates. Water samples collected were in 2L polyethylene bottles for the analysis of Total phosphorus (TP), Nitrate (NO3), Ammonia (NH4) and five day biochemical oxygen demand (BOD) following standard methods [2].
Water quality parameters such as temperature, pH, dissolved oxygen and conductivity were measured in-situ using a portable multi-parameter probe before sampling the macro invertebrates. Water samples collected were in 2L polyethylene bottles for the analysis of Total phosphorus (TP), Nitrate (NO3), Ammonia (NH4) and five day biochemical oxygen demand (BOD) following standard methods [2].
The preserved macro invertebrate samples were passed through a set of sieves (5000, 3000, 2000, 1000 and 500 µm mesh size) in order to separate size classes [3]. When density of some organisms was high, sub sampling was applied according to (Barbour., et al. 1999). Identification of the organisms was performed based on the South African aquatic invertebrate’s identification key [17]. Benthic macro invertebrate indices (BMI) such as Hilsenhoff Family-Level Biotic Index (H-FBI), Margalef Family-Level richness index, Percentage of EPT index, Percent of Chironomidae, Percentage of dominant taxa, ETHbios [26,4,5] and Average Score Per Taxon (ASPT) were calculated.
Results
Composition and abundance of invertebrate families
During the study period, a total of one subclass (Oligochaeta), 10 orders and 37 families of macro invertebrates were identified from the five stations [Table 1]. The nymph and larval stages of the insects identified belongs to 33 families, while Gastropoda and Oligochaeta were the non-insect macro invertebrates representing 4 families. There were 7 orders of the class Insecta namely Plecoptera, Ephemeroptera, Odonata, Hemiptera, Trichoptera, Coleoptera and Diptera collected from the study sites.
During the study period, a total of one subclass (Oligochaeta), 10 orders and 37 families of macro invertebrates were identified from the five stations [Table 1]. The nymph and larval stages of the insects identified belongs to 33 families, while Gastropoda and Oligochaeta were the non-insect macro invertebrates representing 4 families. There were 7 orders of the class Insecta namely Plecoptera, Ephemeroptera, Odonata, Hemiptera, Trichoptera, Coleoptera and Diptera collected from the study sites.
In the Warabo spring (St I) 13 families of macro invertebrates were identified from the pool and riffle part [Table 1]. At this station two families of gastropods, Planariidae and Planorbidae were found. The insect groups represented are Plecoptera, with one family (Perlidae), Ephemeroptera, two families (Baetidae and Caenidae), Odonata two families (Aeshnidae and Libellulidae), Hemiptera one family (Coroxidae), Coleoptera with three families (Gyrinidae, Elmidae and Dytiscidae) and Diptera with two families (Tipulidae and Chironomidae).
In the riffle section two families of Tricoptera, (Hydropschidae and Ecnomidae), two families of Coleoptera (Gyrinidae and Elmidae) and three Diptera families (Tipulidae, Simulidae and Chironomidae) were observed. Among the insect families Baetidae was numerically abundant followed by Caenidae. At station II (Arera Spring) pool and riffle habitats showed 11 and 12 families of invertebrates. In the pool habitat Baetidae and Caenidae were dominant whereas in the riffle section Simulidae members were abundant.
At station III the pool habitat showed 12 and riffle part 21 families. The Gastropoda was represented with two families (Planariidae and Planirbidae). The insect families recorded were Perlidae (Plecoptera), Baetidae and Caenidae (Ephemeroptera), Coenagrionidae (Odonata), Coroxidae (Hemiptera) Gyrinidae and Elmidae (Coleoptera), as well as Psychodidae, Simulidae and Chironomidae (Diptera).
| Sub class/Order | Family/Taxa | Site 1 | Site 2 | Site 3 | Site 4 | Site 5 |
| Ind/m2 | Ind/m2 | Ind/m2 | Ind/m2 | Ind/m2 | ||
| Turbellaria | Planariidae | 539 | 419 | 63 | 2 | 6 |
| Gastropoda | Physidae | 3 | 6 | 177 | ||
| Planorbidae | 148 | 11 | 6 | 269 | ||
| Oligochaeta | Oligochaeta | 4 | ||||
| Plecoptera | Perlidae | 41 | 64 | |||
| Ephemeroptera | Baetidae | 965 | 917 | 992 | 11 | 407 |
| Caenidae | 420 | 303 | 439 | 3 | 92 | |
| Heptageniidae | 1 | 8 | ||||
| Odonata | Aeshnidae | 2 | 6 | 14 | ||
| Libellulidae | 2 | 1 | 7 | 18 | ||
| Coenagrionidae | 4 | 5 | 18 | |||
| Calopterygidae | 15 | |||||
| Hemiptera | Gerridae | 6 | 2 | 18 | ||
| Notonectidae | 26 | |||||
| Corixidae | 81 | 57 | 17 | 3 | ||
| Nepidae | 1 | |||||
| Trichoptera | Hydropsychidae | 24 | 4 | 51 | 3 | 927 |
| Philopotamidae | 1 | 3 | ||||
| Lepidostomatidae | 2 | 5 | ||||
| Ecnomidae | 5 | |||||
| Leptoceridae | 1 | |||||
| Sericostomatidae | 13 | |||||
| Coleoptera | Gyrinidae | 40 | 3 | 2 | 8 | 73 |
| Elmidae | 6 | 8 | 1 | |||
| Hydrophilidae | 2 | |||||
| Psephenidae | 6 | 32 | ||||
| Dytiscidae | 37 | 2 | 5 | 79 | ||
| Diptera | Tipulidae | 196 | 280 | 170 | 6 | |
| Psychodidae | 9 | 1 | 1 | 7 | ||
| Simuliidae | 11 | 1022 | 56 | |||
| Chironomidae | 78 | 66 | 277 | 91 | 117 | |
| Ceratapogonidae | 4 | |||||
| Muscidae | 1 | 3 | ||||
| Tabanidae | 1 | 18 | ||||
| Lepidoptera | Pyralidae | 2 |
Table 1: Taxa identified from the five sampling sites.
The gastropod families, Planariidae and Physidae were found of which Planariidae. In the riffle site 21 families of insects were observed with more representatives the orders Trichoptera, Coleptera and Diptera.
In Dabo spring (station IV), pool site three families (Planariidae, Physidae and Planorbidae) of gastropods and 8 families of insects were present. A total of 12 families were found in the riffle. The number of insect families belonging to the order Ephemeroptera and Diptera were reduced. In Awash Dabo stream (station V) the pool habitat showed the presence of 19 families of invertebrates of which 16 were insect families. The family Baetidae was the most dominant at all stations the highest percentage followed by Caenidae and Simulidae among the insect families.
Numerical abundance of major orders of class Insecta
The benthic macro invertebrate population density showed marked variations in the riffle and pool sections within each sampling sites [Table2]. There were members of the class Insecta occurred in the samples falls under 7 orders. The different life stages of these insects were observed in all the sampling sites. The population density of the order Plecoptera was the least ranging from (0.95% to 3.81%) when present.
The benthic macro invertebrate population density showed marked variations in the riffle and pool sections within each sampling sites [Table2]. There were members of the class Insecta occurred in the samples falls under 7 orders. The different life stages of these insects were observed in all the sampling sites. The population density of the order Plecoptera was the least ranging from (0.95% to 3.81%) when present.
| Order | StationI | StationII | Station III | StationIV | StationV | |||||
| pool | Riffle | pool | Riffle | pool | Riffle | pool | Riffle | pool | Riffle | |
| % | %. | %. | % | % | % | % | % | % | % | |
| Plecoptera | 0.95 | 3.34 | 3.81 | 2.14 | ||||||
| Ephemeroptera | 75 | 70.51 | 86.28 | 21.19 | 71.42 | 67.08 | 18.2 | 6.98 | 21.98 | 28.13 |
| Odonata | 0.32 | 0.4 | 0.54 | 20.5 | 4.44 | 13.92 | 0.79 | |||
| Hemipetra | 6.46 | 2.09 | 6.06 | 0.18 | 1.49 | 0.14 | 6.81 | 1.59 | 11.72 | 0.06 |
| Trichoptera | 3.03 | 2.37 | 22.18 | 4.51 | 2.27 | 1.27 | 9.89 | 59.43 | ||
| Coleoptera | 7.76 | 0.8 | 0.16 | 1.32 | 0.74 | 29.6 | 5.4 | 31.78 | 4.4 | |
| Diptera | 9.49 | 20.22 | 5.29 | 56.29 | 21.54 | 24.84 | 22.7 | 80.32 | 10.71 | 7.19 |
Table 2: Percentage composition (mean) of insect orders at the five stations.
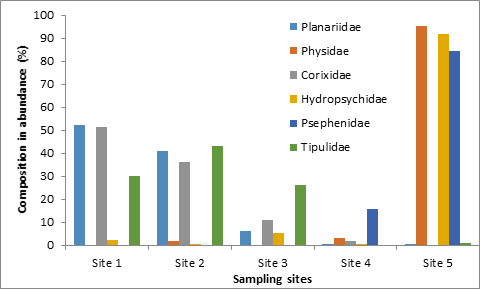
The orders Ephemeroptera, Diptera and Hemiptera were found throughout the study period at all stations and habitat types [Table 2]. Ephemeroptera was highly significant in terms of their percentage composition at stations I, II, III and V. The highest 86.28% was found in the pool at station II. The order Coleptera was more abundant in the pools of stations IV (29.6%) and V (31.78%) while their number was very less in other stations. The percentage composition of the Order Odonata was high at station IV (20.5%) and V (13.92%) pool sites and order Tricoptera at station V (59.43%) and II (22.18%) riffle section. The families of insects Corixidae, Planariidae and Tipulidae decrease dramatically with increasing anthropogenic influence, while the beetles Psephenidae, the caddisfly family Hydropsychidae and the snails Physidae showed increasing densities (Station V).
The Hilsenhoff family biotic index is a weighed measure of individuals in a population. During the period of study the number of individuals in family (family richness) showed considerable variation between the different habitats [Table 3]. Station IV had relatively higher H –FBI index (5.76) followed by station III (4.96), station II (3.99), station V (3.75) and station I (3.2).
| Metrics | Station I | Station II | Station III | Station IV | Station V |
| Taxa richness | 17 | 13 | 23 | 17 | 25 |
| Abundance(individuals/m2 | 2597 | 3093 | 2179 | 161 | 2349 |
| Hilsenhoff Family Biotic Index (H-FBI) | 3.2 | 3.99 | 4.96 | 5.76 | 3.75 |
| % EPT | 56.1 | 39.6 | 71.3 | 10.6 | 61.1 |
| % Chironomidae | 3 | 2.1 | 12.7 | 56.5 | 4.9 |
| ETHbios | 80 | 57 | 120 | 68 | 102 |
| ASPT (ETHbios) | 6.7 | 5.2 | 6.3 | 4.53 | 4.86 |
Table 3: Biotic indices calculated from sampling sites.
The Percentage of Ephemeroptera, Plecoptera, Trichoptera index (% EPT) was maximum at station III (71.3%) and lowest at station IV (10.6%) indicating the level impact of human interventions. The percentage of Chironomidae was very high at station IV (56.5%) followed by station III (12.7%).In the other sites the population of chironomids was very much reduced.
The results on the ETHbios index indicated that Station III had the highest sensitivity score (120) followed by station V (102).The lowest score was obtained for station II (57). The ASPT value was comparatively high for station I (6.67) and III (6.31) and least for station IV (4.53).
Environmental parameters
The water quality parameters measured (mean) in the field and laboratory during the sampling period from February to April are presented in [Table 4].The mean atmospheric temperature was high (21.07 ± 1.81°C and 21.02 ± 0.43°C) at stations I and V. The water temperature was in general less than the atmospheric temperature. However, the temperature recoded at station IV was (22.73 ± 2.05°C) high and the lowest (18.7 ± 1.35°C) were recorded at station II (Arera spring). The pH of water at all stations was alkaline in nature ranging from 8.1 ± 0.45 at Dabo spring and 8.53 ± 0.08 at Station V. There was very little variation in pH at stations II and III. The lowest mean dissolved oxygen value (6.32 ± 0.4 mg/l) was observed at Awash I stream and the highest (8.62 ± 0.28 mg/l) at station IV.
The water quality parameters measured (mean) in the field and laboratory during the sampling period from February to April are presented in [Table 4].The mean atmospheric temperature was high (21.07 ± 1.81°C and 21.02 ± 0.43°C) at stations I and V. The water temperature was in general less than the atmospheric temperature. However, the temperature recoded at station IV was (22.73 ± 2.05°C) high and the lowest (18.7 ± 1.35°C) were recorded at station II (Arera spring). The pH of water at all stations was alkaline in nature ranging from 8.1 ± 0.45 at Dabo spring and 8.53 ± 0.08 at Station V. There was very little variation in pH at stations II and III. The lowest mean dissolved oxygen value (6.32 ± 0.4 mg/l) was observed at Awash I stream and the highest (8.62 ± 0.28 mg/l) at station IV.
The dissolved oxygen saturation was comparatively low at Awash I stream (93.92 ± 5.95%) and maximum (128.6 ± 0.89%) at Dabo spring. The highest dissolved oxygen concentration (DO) was observed at station V (8.62 ± 0.28) and the lowest dissolved oxygen concentration (DO) was observed at station III (6.32 ± 0.4). The electrical conductivity of water was a useful indicator of its salinity or total salt content. The maximum conductivity value (561.19 ± 4.01) was observed at station IV and the minimum (175.45 ± 4.87) at Station II. The biological oxygen demand of the water was carried out for 5 days incubation .The highest BOD5 level of the water (4.91 ± 0.04) was recorded at station V and the least value (0.51 ± 0.03) for station III.
| Parameters | Site 1 | Site 2 | Site 3 | Site 4 | Site 5 |
| Temperature (°C) | 19.23 ± 0.93 | 18.7 ± 1.35 | 19.7 ± 1.37 | 22.73 ± 2.05 | 19.4 ± 0.52 |
| pH | 8.38 ± 0.15 | 8.51 ± 0.34 | 8.52 ± 0.1 | 8.1 ± 0.45 | 8.53 ± 0.08 |
| Dissolved Oxygen(mg/l) | 6.39 ± 0.18 | 7.37 ± 0.39 | 6.32 ± 0.4 | 8.04 ± 0.06 | 8.62 ± 0.28 |
| Dissolved oxygen (%) | 98.86 ± 2.71 | 104.17 ± 5.49 | 93.92 ± 5.95 | 128.6 ± 0.89 | 121.55 ± 3.95 |
| Conductivity (µS/cm) | 175.45 ± 4.87 | 230.74 ± 12.43 | 281.9 ± 25.78 | 561.19 ± 4.01 | 348.33 ± 37.1 |
| BOD5(mg/l) | 0.91 ± 0.03 | 1.26 ± 0.07 | 0.51 ± 0.03 | 4.91 ± 0.04 | 1.52 ± 0.05 |
| NH4-N(mg/l) | 0.49 ± 0.21 | 0.21 ± 0.07 | 0.35 ± 0.07 | 0.35 ± 0.21 | 0.14 ± 0 |
| NO3-N(mg/l) | 0.77 ± 0.07 | 0.7 ± 0.14 | 0.56 ± 0.28 | 5.09 ± 0.16 | 0.21 ± 0.07 |
| Total Phosphorus(mg/l) | 0.03 ± 0.01 | 0.05 ± 0.02 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.02 |
Table 4: Mean and standard deviation of environmental parameters in the five sampling sites.
The ammonia nitrogen (NH4+N) content was high (0.49 ± 0.21 mg/l) at station I (Warabo spring) and lowest (0.14 ± 0 mg/l) at Station V (Awash Dabo stream). The nitrate nitrogen (NO3-N) in the water was very high at station IV (5.09 ± 0.16mg/l) and low at station V (0.21 ± 0.07mg/l).The total phosphorus concentration in the water varied from (0.03 ± 0.01 to 0.05 ± 0.02) in the study sites. However, the variations are not significant. The slightly highest value was observed from Station II (0.05 ± 0.02) and the lowest value from stations I, III and IV.
Discussion
The benthic fauna in the spring and stream habitats of upper Awash River showed significant variations in the abundance and composition in the selected study sites during the sampling months. In general, pool habitats showed less population density and family richness than riffle. In the riffle section microlithal, mesolithal and macrolithal habitat combined with organic habitats such as plant roots and fallen leaves were dominant which facilitates the suitable substratum for the macro benthos to inhabit. In the pool section the stream bed is dominated by sand and mud habitats where few taxa with special adaption features can survive. Station II had the highest abundance of benthic macro invertebrates followed by station I. The factors that influence the macro benthos include environmental variables, anthropogenic impact, habitat condition, tolerance value of the benthic macro invertebrates and climatic factors. Further, human activities such as cattle watering, washing, domestic waste disposal, agricultural activities could also affects the species composition and abundance of benthos in the rivers and streams [4,5]. Unexpected rain accompanied with short term flooding would may cause drifting of benthic macro invertebrates and only those taxa having ability to attach with different stone type may survive