Research Article
Volume 2 Issue 1 - 2017
Studies on the Influence of Water Quality on Periphyton and Benthic Fauna along the River Gora A Tributary of Blue Nile, Ethiopia
Department of biology, Ambo University, Ambo, West shoa zone, Oromia region, Ethiopia
*Corresponding Author: Prabhadevi L, Department of biology, Ambo University, Ambo, West shoa zone, Oromia region, Ethiopia.
Received: October 09, 2017; Published: October 30, 2017
Abstract
The water quality in relation to benthic macroinvertebrates and periphyton of River Gora, a tributary of Blue Nile during the dry season (December2015 to February 2017) was studied at Jeldu district in Ethiopia. The water flow rate fluctuated from 0.32 to 0.6 m3/sec. The water temperature did not vary considerably and pH was slightly alakaline. The mean dissolved oxygen varied from 5.5 mg/l and 11.12 mg/l and water hardeness ranged from 608 to 640 mg/l. The mean values of total nitrate and phosphorus contents were 0.38 and 0.52 mg/l and 1.35 and 6.58 mg/l respectively. The organic carbon and organic matter of the bottom soil varied considerably in February. The diatoms dominated in terms of species as well as population density among the periphyton while the membersof the order Diptera was the most abundant benthic invertebrate group. The variations in the biotic components with respect to water quality were also discussed.
Keywords: Periphyton; Macroinvertebrates; Soil organic carbon; Organic matter
Introduction
Rivers play significant roles in the provision of water for domestic and industrial purposes. Nevertheless, land use dynamics continue to impact on river catchments which have negative repercussions for river health. Aquatic organisms are often considered as engineers’ of aquatic ecosystems, play a vital role in the nutrient cycle [1] and also serve as useful protein materials for fish and shellfish [2]. The periphyton community is the slimy coating that adheres to rocks and other stable substrates that comprise of variable proportions of algae, fungi and bacteria as well as organic matter entrained from stream flow. In fast-flowing, oligotrophic lotic habitats, attached algal communities (periphyton) are often the only primary producers, play an important role in the food web through the transformation of energy [3] Periphyton communities have been used as biotic indicators of ecological condition and change in response to human and natural disturbances [4]. Similarly the benthic invertebrates are important in moving energy through food webs which usually inhabit bottom substrates for at least part their life cycle [5] These organismas are diferentially sensitive to many biotic and abiotic factors in their environment [6]. Among these insects [7] are more diverse in lotic systems It is stated that less than 3% of all species of insects begin their life cycle as aquatic larvae before emerging as adults [8]. Several studies have been undertaken to understand the streams and rivers with respect to peripyton and benthos in Ethiopia [9,10]. The Gora River is one of the tributaries of Blue Nile, supports the livelihood and water requirements of a large number of people and hence it is crucial to assess the water quality using biotic indicator communities which is comparatively rapid and and reliable method. The present study mainly emphasizes the composition and distribution of periphyton and benthic invertebrates with respect to water quality parameters and nature of bottom substratum in the River Gora.
Materials and Methods
Three stations were selected along the course of Gora River at Jeldu district, Ethiopia for the present study. Station 1 is located between 03”90’619” N and 10”32’571”E, Station 2 between 03”91’192” N and 10”33’030 E and Station 3 between 03”89’958”N and 10”33’283’'E. Water temperature and pH were measured in the field by using digital probes after making standard calibrations. The dissolved oxygen and total hardness of the water samples were estimated by Winkler’s Azide and EDTA methods respectively [11]. Nitrate and phosphate were estimated as described by EPA (12) and Olsen., et al. [13]. The river flow velocity was measured by float method [14]. The soil organic carbon and organic matter were estimated by and Walkley and Black method [15]. Periphyton and benthic fauna samples were collected during December 2015-April 2016 by using quadrate method. All benthic samples were sieved through 200-500 μm seive and fixed in 5% formaldehyde for further identification. The qualitative and quantitative analyses were done as described by Arbačiauskas., et al. [16]. For the periphyton, the organisms attached to submerged stones, rocks and wood logs were collected by scrapping from a unit area and preserved for qualitative and quantitative analyses.
Results
The water flow rate at all the stations showed decreasing trend from December to February. The maximum flow was recorded in December (0.6 m3/sec) at station 1 and minimum (0.32 m3/sec) in February at station III [Table1]. The water temperature remained between 20.3 and 20.5°C at all the three stations. The pH of water in Gora River was minimum 7.63, 6.4 and 7.03 and maximum 8.06, 8.23 and 8.15 in station I, II and III respectively.
| Parameters | Station I | Station II | Station III | ||||||
| Dec | Jan | Feb | Dec | Jan | Feb | Dec | Jan | Feb | |
| Water flow m3/sec | 0.6 | 0.54 | 0.35 | 0.4 | 0.35 | 0.35 | 0.34 | 0.32 | 0.32 |
| Water temperature (°C) | 20.5 | 20.4 | 20.5 | 20.4 | 20.3 | 20.3 | 20.5 | 20.5 | 20.4 |
| pH of water | 8.06 | 7.63 | 7.84 | 8.23 | 6.44 | 7.60 | 8.15 | 7.04 | 7.83 |
| DO (mg/l) | 5.61 | 7.07 | 11.12 | 6.55 | 8.08 | 11.12 | 5.5 | 9.09 | 11.01 |
| NO3-N (mg/l) | 0.39 | 0.52 | 0.44 | 0.49 | 0.45 | 0.38 | 0.44 | 0.43 | 0.44 |
| Phosphate (mg/l) | 1.35 | 1.79 | 3.37 | 6.58 | 4.62 | 1.54 | 3.44 | 2.42 | 1.69 |
| Hardness (mg/l CaCO3) | 608 | 640 | 629 | 628 | 612 | 625 | 628 | 624 | 612 |
| Soil Organic carbon (%) | 0.49 | 0.49 | 0.91 | 1.08 | 1.09 | 0.36 | 0.64 | 1.27 | 0.33 |
| Soil Organic matter (%) | 0.85 | 0.83 | 1.57 | 1.86 | 1.96 | 0.62 | 1.11 | 2.20 | 0.57 |
Table 1: Physico-chemical parameters of water and soil.
The dissolved oxygen increased from December (5.61 mg/l, 6.55 mg/l and 5.5 mg/l) to February (11.12mg/l, 11.12 mg/l and 11.01mg/l) at stations I, station II and station III respectively. Total hardness varied between the stations (608 mg/L to 640 mg/L in station I, 612 mg/L to 628 mg/L in station II and III). The nitrate (NO3-N) noticeable variations between the sampling months the lowest and highest values (0.38 mg/l and 0.52 mg/l respectively) were observed during different months.and 0.44 mg/l in December, January and February respectively. At station II the values showed decreasing trend from 0.49 mg/l (December) to 0.38 mg/l (February). However, the values did not vary at station III and it was 0.44 mg/l, 0.43 mg/l and 0.44 mg/l in December, January and February respectively. The total phosphorus (TP) concentration in water samples showed increasing trend at station I, the values were 1.35 mg/l, 1.793 mg/l and 3.376 mg/l in December, January and February respectively. However, the values decreased from 6.582 mg/l to 1.54 mg/l (Station II) and 3.439 mg/l to1.688 mg/l at stations II and III respectively. The amount of organic carbon level in the bottom soil at station I varied from 0.495 % (December) to 0.915 % (February). At station II the values were higher than station I (1.08% 1.09% December and January respectively) and decreased to 0.36% in February. Station III showed much fluctuation and the values were 0.645 % in December, 1.275 in January and 0.33 in February. The organic matter (OM) in the soil was maximum (2.20%) and minimum (0.57 %) in station III. The OM values in station I was 0.83 % in December and January and increased to 1.57 % in February. At station II the values were found to be 1.86 %, 1.96 % and 0.62 % in December, January and February respectively.
Periphyton
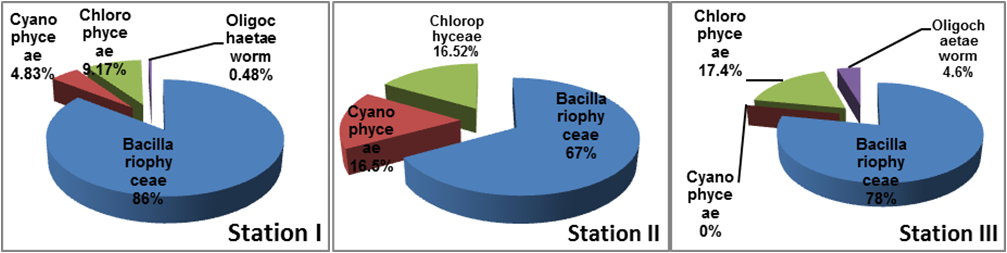
Percentage composition of periphyton population observed in the River Gora is presented in Figure 1. A total of 21 genera belonging to Bacillariophyceae, Cyanophyceae, Chlorophyceae were found along with oligochate worms. Bacillariaophyceae comprised 86% in station I, 67% in Station II and 78% in station III. There were 12 species of diatoms viz., Pinnularia sp., Gomphoneis sp., Navicula spp., Nitzchia sp., Cyclotella sp., Asterionella, sp., Cymbella sp., Diatoma sp., Fragillaria sp., Gyrosigma spp., Gomphonema sp., and Tabellaria sp. The second most abundant group Chlorophyceae comprised 9.17%, 16.52% and 17.38 % in station I, station II and station III respectively represented by Actinastrum sp., Ankistrodesmus sp., Cryptophyta sp., Cosmarium sp., and Monorophidium sp. The Cyanophyceae comprised 4.83% and 16.48% respectively in station I and station II represented by Anabaena sp., Oscillatoria sp., and Lyngbya sp. The oligochaetes were found at station I (0.48%) and station III (4.6%).
Benthic fauna
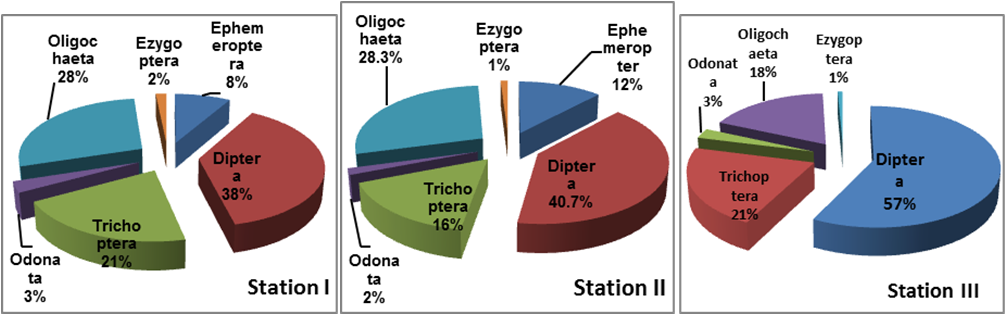
The benthic invertebrates identified were categorized into Ephemeroptera (May flies), Diptera, Trichoptera (Caddis flies), Odonata (Dragon Flies), Ezygoptera (Damsel flies) and Oligochaeta (worms). Diptera was found to be the most abundant forming 38%, 40.7% and 57% at stations I, II and III respectively. The Trichopteran species were comprised 21% in station I, 16% in station II and 21% in station III. Ephemeroptera constituted 8%, and 12% at station I and station II respectively, whereas in station III they are absent. Odonata and Ezygoptera were poorly represented. Oligochaeta was the second important group dominant group respectively 28% (station I), 28.3% (station II) and 18% (station III).
The benthic invertebrates identified were categorized into Ephemeroptera (May flies), Diptera, Trichoptera (Caddis flies), Odonata (Dragon Flies), Ezygoptera (Damsel flies) and Oligochaeta (worms). Diptera was found to be the most abundant forming 38%, 40.7% and 57% at stations I, II and III respectively. The Trichopteran species were comprised 21% in station I, 16% in station II and 21% in station III. Ephemeroptera constituted 8%, and 12% at station I and station II respectively, whereas in station III they are absent. Odonata and Ezygoptera were poorly represented. Oligochaeta was the second important group dominant group respectively 28% (station I), 28.3% (station II) and 18% (station III).
Discussion
Physico-chemical parameters of water
The rate of river flow during the study period decreased from station I to station III which might be due to the increase in the width of the channel and the presence of more number of of deeper pools at station II and III [17]. The flow variability depends on the structural pattern of rivers [18]. Temperature exerts a major influence on biological activity and growth of organisms that can live in rivers and lakes. The temperature in the study site didn’t vary considerably and to affect the organisms. Water pH levels play an important role on the health of bodies of water and their ecosystems. pH value in the present study also in the limits [19]. Rapidly moving water, such as mountain streams or a large river, tends to contain a lot of dissolved oxygen. The relatively high level of dissolved oxygen during February is due to the absence of suspended soil particles in the water and exposure to air [20] and the presence of large quantity of filamentous alagae and periphyton contribute oxygen through photosynthesis. Bottom feeders need minimal amounts of oxygen (1-6 mg/L) [21]. Based on hardness, water classified into three different categories; soft water (0 to 75 mg/L), moderately hard water (76 to 150 mg/L) and hard water (151 to 300 mg/L) [22]. In the present study the total hardness of the water remains higher. This might be due to the presence of limestone rocks all along the river bed in the study sites and the natural weathering of Ca+ and Mg+ rich rocks bordering the river bank [23,24]. The nitrate content in the Gora river water ranged from 0.38 mg/l to 0.52 mg/l. The values are slightly higher in station III where small tributaries join together. This is also related to the disposal of domestic and agricultural wastes containing fertilizers around the river banks [25]. The increase in phosphate valu at site1 could be also due to the mineralization of organic matter at the bottom and the whethering of the medium to large size stones at the bottom.The observed values of nitrate and total phosphorous values were within the WHO standards [26]. The stones at site 1 holds large amounts of sediment admixed with particulate materials and alage during the februarywhen the flow rate was reduced.The high rate of decomposition of the settled particulate organic materials could have contributed towards the high rate of organic matter than the other sites.
The rate of river flow during the study period decreased from station I to station III which might be due to the increase in the width of the channel and the presence of more number of of deeper pools at station II and III [17]. The flow variability depends on the structural pattern of rivers [18]. Temperature exerts a major influence on biological activity and growth of organisms that can live in rivers and lakes. The temperature in the study site didn’t vary considerably and to affect the organisms. Water pH levels play an important role on the health of bodies of water and their ecosystems. pH value in the present study also in the limits [19]. Rapidly moving water, such as mountain streams or a large river, tends to contain a lot of dissolved oxygen. The relatively high level of dissolved oxygen during February is due to the absence of suspended soil particles in the water and exposure to air [20] and the presence of large quantity of filamentous alagae and periphyton contribute oxygen through photosynthesis. Bottom feeders need minimal amounts of oxygen (1-6 mg/L) [21]. Based on hardness, water classified into three different categories; soft water (0 to 75 mg/L), moderately hard water (76 to 150 mg/L) and hard water (151 to 300 mg/L) [22]. In the present study the total hardness of the water remains higher. This might be due to the presence of limestone rocks all along the river bed in the study sites and the natural weathering of Ca+ and Mg+ rich rocks bordering the river bank [23,24]. The nitrate content in the Gora river water ranged from 0.38 mg/l to 0.52 mg/l. The values are slightly higher in station III where small tributaries join together. This is also related to the disposal of domestic and agricultural wastes containing fertilizers around the river banks [25]. The increase in phosphate valu at site1 could be also due to the mineralization of organic matter at the bottom and the whethering of the medium to large size stones at the bottom.The observed values of nitrate and total phosphorous values were within the WHO standards [26]. The stones at site 1 holds large amounts of sediment admixed with particulate materials and alage during the februarywhen the flow rate was reduced.The high rate of decomposition of the settled particulate organic materials could have contributed towards the high rate of organic matter than the other sites.
Periphyton composition
Periphyton is a complex matrix of algae and heterotrophic microbes attached to submerged substrata in all aquatic ecosystems [3]. Excessive periphyton growth can occur in rivers as a result of high water temperature from reduced and managed flows [27]. In the present study relatively more number of genera were represented by Bacillariophyceae and their abundance was also high than those belonging to green and bluegreen algae. This could be due to the presence of high mineral content, temperature and light intensity favouring their rapid multiplication. The higher number of diatom in the periphyton indicates the alteration of pH, liter falls underlying the river bed impacts on the water through the process of decay thereby introducing humic substances [28,29]. Some rivers manifest community structure in patches as well as in continuous patterns, usually one more than the other and the levels of periphyton were highly variable in different zones along streams [30]. Similar results were observed in the distribution of diatoms in the present study. The other groups like Chlorophyceae, Cyanophyceae, and cryptophyceae were found lesser in number due the less adherent character and flow rate of water [30,31].
Periphyton is a complex matrix of algae and heterotrophic microbes attached to submerged substrata in all aquatic ecosystems [3]. Excessive periphyton growth can occur in rivers as a result of high water temperature from reduced and managed flows [27]. In the present study relatively more number of genera were represented by Bacillariophyceae and their abundance was also high than those belonging to green and bluegreen algae. This could be due to the presence of high mineral content, temperature and light intensity favouring their rapid multiplication. The higher number of diatom in the periphyton indicates the alteration of pH, liter falls underlying the river bed impacts on the water through the process of decay thereby introducing humic substances [28,29]. Some rivers manifest community structure in patches as well as in continuous patterns, usually one more than the other and the levels of periphyton were highly variable in different zones along streams [30]. Similar results were observed in the distribution of diatoms in the present study. The other groups like Chlorophyceae, Cyanophyceae, and cryptophyceae were found lesser in number due the less adherent character and flow rate of water [30,31].
Benthic macro invertebrates
In the study sites Dipterans were more abundant followed by oligochaete worms, Trichoptera, Ephemeroptera, Odonata and Ezygoptera.The benthic macroinvertebrates respond to fluctuations in the level of dissolved oxygen, pH, and alkalinity [31]. Oligochaeta are aerobic worms which live in soil where more amount of oxygen is available. In the present study the high oxygen content in the water would have influenced the growth of oligochaetes. Generally the chironomids are influenced by water flow, temperature, amount of food and nutrients in the water [32] while the Ephemeropterans are sensitive to environmental perturbations and usually live in clean and well oxygenated waters [33] and their species diversity is influenced by substratum type, riparian vegetation, catchment land use and human activities [34]. Among benthos Caddis fly (Trichoptera) and May fly are less abundant in the river water –soil interface because they tend to prefer specific substratum type in the streams [35]. In the light of the present study, the river Gora at Jeldu district is less polluted and can be recommended for domestic utilization.
In the study sites Dipterans were more abundant followed by oligochaete worms, Trichoptera, Ephemeroptera, Odonata and Ezygoptera.The benthic macroinvertebrates respond to fluctuations in the level of dissolved oxygen, pH, and alkalinity [31]. Oligochaeta are aerobic worms which live in soil where more amount of oxygen is available. In the present study the high oxygen content in the water would have influenced the growth of oligochaetes. Generally the chironomids are influenced by water flow, temperature, amount of food and nutrients in the water [32] while the Ephemeropterans are sensitive to environmental perturbations and usually live in clean and well oxygenated waters [33] and their species diversity is influenced by substratum type, riparian vegetation, catchment land use and human activities [34]. Among benthos Caddis fly (Trichoptera) and May fly are less abundant in the river water –soil interface because they tend to prefer specific substratum type in the streams [35]. In the light of the present study, the river Gora at Jeldu district is less polluted and can be recommended for domestic utilization.
References
- Ostroumov SA. “On the multifunctional role of the biota in the self-purification of aquatic ecosystem”. Russia Journal of Ecology 36.6 (2005): 452-459.
- Sharma S., et al. “Benthic macro invertebrates abundance and its correlations with physico-chemical parameters from Kunda river Khargorie (M.P.) India”. International Journal of Advanced Research 1.2 (2013): 1-8.
- Finlay JC., et al. “Spatial scales of carbon flow in a river food web”. Ecology 83.7 (2002): 1845-1859.
- Denicola DM., et al. “Using epilithic algal communities to assess trophic status in Irish lakes”. Journal of Phycology 40.3 (2004): 481-495.
- Richardson JS. “Organic matter dynamics in small streams of the pacific northwest. Journal of the American Water Resources Association 41.4 (2005): 921-934.
- Rosenberg DM and ReshVH. “Introduction to freshwater biomonitoring and benthic macroinvertebrates. In: D.M. Rosenberg and V.H. Resh, eds. Freshwater Biomonitoring and Benthic Macroinvertebrates”. Chapman and Hall, New York (1993):
- Cushing CE and Allan JD. “Streams: their ecology and life”. Academic Press, San Diego (2001): 366.
- Ward JV and Tocker K. “Biodiversity of plod plain River ecosystems acetones and connectivity regulated”. River research and managements 10 (2001): 159-168.
- Harrison AD and Hynes HBN. “Benthic fauna of Ethiopian mountain streams and rivers”. Hydrobiologia 1 (1988): 1-36.
- Seyoum L., et al. “Characterization of Tannery wastewater and assessment of downstream pollution profiles along Modjo River in Ethiopia”. Ethiopian Journal of Biological Sciences 2.2 (2003): 157-168.
- Strickland JDH and Parsons TR. “A practical handbook of seawater analysis”. Fish. Res. Board Can. Bull 167.2nd ed. (1972): 310.
- EPA. “Determination of Nitrate-Nitrite Nitrogen by Automated Colorimetry”. In. Environmental Protection Agency, Ed. James W.O'Dell, U.S. Cincinnati, Ohio (1993):
- OlsenP. “Estimation of available phosphorus in soils by extraction with sodium bicarbonate”. USDA Circular Nr 939, US Gov. Print. Office, Washington, D.C (1954):
- Graf WH and Altinakar NS. “Fluvial hydraulics.Flow and transport processes in channels of simple geometry”. Wiley and Chester (1998):
- Walkley A and Black A. “An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method”. Soil Science 37.1 (1934): 29-38.
- Arbačiauskas K., et al. “Assessment of bio contamination of benthic macro invertebrate communities in European inland waterways”. Aquatic Invasions 3.2 (2008): 211-230.
- Knights P. “Environmental flows: lessons from an Australian experience”. Proceedings of International Conference: Dialog on Water, Food and Environment. Hanoi, Vietnam (2002): 1-19.
- Ward JV., et al. “Biodiversity of floodplain river ecosystems: ecotones and connectivity”. Rivers: Research and Management 15.1-3 (1999): 125-139.
- Wurts WA and Durborow RM. “Interactions of pH, carbon dioxide, alkalinity and hardness in fish ponds”. Southern Regional Aquaculture Center Publication (1992): 464.
- Russel A and Robert G. “The effects of turbidity on dissolved oxygen levels in various water samples”. California state science fair (2006):
- Osmond DL., et al. “WATERSHEDS: Water, Soil and Hydro-Environmental Decision Support System”. North Carolina State University (1995):
- Soni HB., et al. “Surface water quality assessment and conservation measures of two pond ecosystems of Central Gujarat”. International Research Journal of Chemistry 3.3 (2013): 69-81.
- Murhekar GH. “Determination of Physico-Chemical Parameters of Surface Water Samples in and around Akot City”. International Journal of Research in Chemistry and Environment 1.2 (2011): 183-187.
- Zeitoun MM and Mehana ESE. “Impact of Water Pollution with Heavy Metals on Fish Health: Overview and Updates”. Global Veterinaria 12.2 (2014): 219-231.
- Ewers BE., et al. “Carry-over effects of water and nutrient supply on water use of Pinus taeda. Ecological application 9.2 (1999): 513-525.
- WHO. “Guidelines for drinking-water quality - Volume 1: Recommendations Third edition, incorporating first and second addenda”. (2008): ISBN 978 92.
- Giorgi A and Malacalza L. “Effect of an industrial discharge on water quality and periphyton structure in a Pampeam stream”. Environmental Monitoring and Assessment 75.2 (2002): 107-119.
- Akpan SB., et al. “Analysis of total factor productivity among smallholder vegetable farmers in akwa-ibom state, Nigeria”. Nigerian journal of agriculture, food and environment 7.4 (2011): 68-74.
- Wright KK and Li JL. “From continua to patches: examining stream community structure over large environmental gradients”. Canadian Journal of Fisheries and Aquatic Sciences 59.8 (2002): 1404-1417.
- Kadiri MO. “A spatial profile of net Phytoplankton in the lower River Niger Recorded in the wet season”. Acta hydrobiologica 41.3-4 (1999): 247-258.
- Udoh EJ and Akpan SB. “Measuring Technical Efficiency of Waterleaf (Talinum triangulure) production in Akwa Ibom State, Nigeria”. American Eurasian Journal of Agriculture and Environment Science 2.5 (2007): 518-522.
- Cummins KW., et al. “The use of macroinvertebrate functional groups to characterize ecosystem attributes in selected streams and rivers in south Brazil”. Studies on Neotropical Fauna and Environment 40.1 (2005): 69-89.
- Oliveira ALH and NessimianJL. “Spatial distribution and functional feeding groups of aquatic insect communities in Serra da Bocaina streams, southeastern Brazil”. Acta Limnologica Brasiliensia 22.4 (2010): 424-441.
- Bispo PC., et al. “Ephemeroptera, Plecoptera and Trichoptera assemblages from riffles in mountain streams of Central Brazil: environmental factors influencing the distribution and abundance of immature”. Brazilian Journal of Biology 66.2 (2006): 611-622.
- Selvakumar C., et al. “Impact of riparian land-use patterns on Ephemeroptera community structure in river basins of the southern Western Ghats, India”. Knowledge and Management of Aquatic Ecosystems 412.11 (2014): 1-15.
Citation:
Prabhadevi L., et al. “Studies on the Influence of Water Quality on Periphyton and Benthic Fauna along the River Gora A Tributary
of Blue Nile, Ethiopia”. Innovative Techniques in Agriculture 2.1 (2017): 301-306.
Copyright: © 2017 Prabhadevi L., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.





























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.