Research Article
Volume 2 Issue 2 - 2019
Tumor Melts like Ice – An Earliest Response to Gefitinib
1Head of Department of Pulmonary Medicine, Ruby Hall Clinic, Pune, India
2Senior Resident, Department of Pulmonary Medicine, Ruby Hall Clinic, Pune, India
2Senior Resident, Department of Pulmonary Medicine, Ruby Hall Clinic, Pune, India
*Corresponding Author: R K Chopra, H.O.D Department of Chest Medicine, Cancer Building 1st Floor, Ruby Hall Clinic, Pune, Maharashtra, India.
Receive: January 03, 2019; Published: January 31, 2019
Abstract
Lung cancer is one of the most common cancer in the world. Non-small cell lung cancer (NSCLC) comprises 80% of all lung cancer. Incidence of adenocarcinoma has drastically increased since last decade. A 64-year-old non smoking Indian women was diagnosed to have right upper lobe mass lesion whose bronchoscopy revealed right sided endobronchial obstruction. Endobronchial biopsy confirmed adenocarcinoma with epidermal growth factor receptor (EGFR) gene mutation in the primary tumor specimen that uncovered an exon 19 mutation. PET scan showed metastatic deposits in the liver, vertebra, sacrum. She was treated by Gefitinib therapy, which showed a dramatic response by both primary tumor and peripheral metastases in one month with complete resolution of endobronchial obstruction and opening up of collapsed lung. It was described as the tumor melted like ice after seeing followup PET scan. So through this paper we want to show the earliest response seen in stage IV adenocarcinoma treated with Gefitinib.
Keywords: Endobronchial biopsy; Gefitinib; Epidermal growth factor receptor; Non-small cell lung cancer
Abbreviations: Tyrosine kinase inhibitors (TKI); Response Evaluation Criteria in Solid Tumors Group (RECIST); Time to response (TTR)
Introduction
Protein kinase activation by somatic mutation or chromosomal alteration is a common mechanism of tumorigenesis [1]. Exon 19 and 21 mutations were the first mutations discovered. These mutations predict the response associated with tyrosine kinase inhibitors (TKIs) [2]. They have a higher frequency of occurrence and are associated with improved outcomes on treatment with TKI. They are called classic activating mutations. Patients with uncommon EGFR mutations are less responsive to Gefitinib compared with patients with L858R and exon 19 deletions [3].Tumor markers and mutation studies has caused paradigm shift in treatment of stage IV disease with improved survival rate and quality of life. Here we now report a case of NSCLC harboring EGFR exon 19 deletion treated with Gefitinib with remarkable response in 1 month.
Case History
A 64-year-old, Indian, non-smoker, female patient presented with history of right sided lung collapse with pleural effusion. CT- scan revealed irregular mass lesion in right perihilar region encasing right main bronchus (Figure 3E) with high SUV uptake of 7.8 on PET- scan (Figure 3G). It also showed peripheral metastatic uptakes in liver, vertebra and sacrum. Bronchoscopy was done showing complete occlusion of right main bronchus with endobronchial mass lesion (Figure 2C). Endobronchial biopsy was taken that confirmed adenocarcinoma. Further on immunohistochemistry TTF-1 was positive. EGFR mutation study showed deletion of exon 19. Stage IV lung adenocarcinoma originating from the right upper lobe was confirmed. She was started with TKI- Gefitinib 250mg o.d and asked for follow up after 10 days, patient improved symptomatically with complains of few episodes of diarrhea which subsided with loperamide. Repeat chest x ray and bronchoscopy was done after 1month of treatment, which showed good expansion of right upper and middle lobe lung fields (Figure 1b) and complete resolution of endobronchial mass lesion leading to opening up of upper and lower lobe airways (Figure 2b).
CHEST X RAY – P.A VIEW
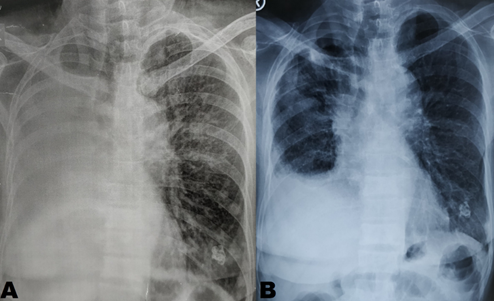
Figure 1: (A) shows homogenous opacity with bronchial cut off sign in right side. (B) shows expansion of right upper and middle lobe after 1month of treatment with gefitinib.
CT and PET Images
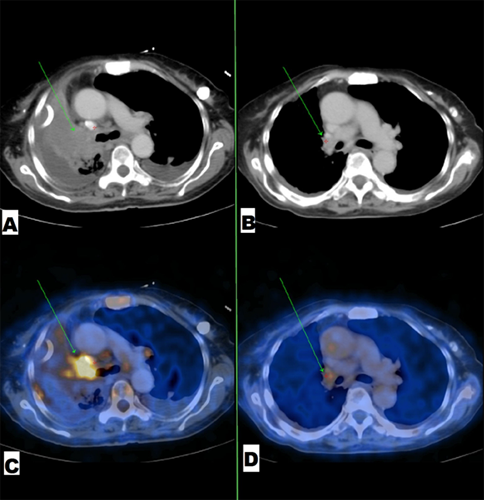
Figure 2: (A) and (C) Mass lesion in the right hilar region with significant FDG uptake. (B) and (D) significant resolution of same mass lesion with decreased FDG uptake after 1month of treatment by Gefitinib.
BRONCHOSCOPY
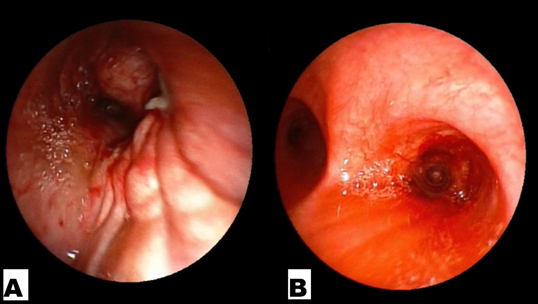
Figure 3A and B: Endobronchial images showing mass lesion completely obstructing the right main bronchus and complete resolution of mass opening up upper and middle lobe airways after 1 month of treatment with gefitinib respectively.
Discussion
After the discovery of EGFR-TKIs, the management of lung cancer has completely changed. Initially platinum based chemotherapy was the standard first line treatment for advanced NSCLC. The introduction of EGFR-TKIs has improved the prognosis of EGFR-mutated NSCLC patients with a median overall survival of 20–30 months and a median progression-free survival of 10–14 months [4]. A retrospective Japanese study reported that 28 (8.4%) of 335 NSCLC patients treated with Gefitinib survived for more than 5 years and the longest survival time was more than 10 years (3,867 days) [5]. Gefitinib belongs to group of synthetic anilinoquinazolines that selectively and reversibly prevent ATP binding and autophosphorylation of the EGFR tyrosine kinase. RECIST criteria have been used in phase II trials of molecular targeted agents. In a study done by Masayuki Takeda et al, of the 68 selected patients, 6 achieved a Complete Response (CR), 42 achieved Partial Response (PR), and 14 Stable disease (SD). Among the CR/PR group, the median maximal tumor shrinkage relative to baseline was 56%, and the median time to response (TTR) was 4.2 weeks [6]. In our case after treatment with gefitinib, response was seen earliest which was witnessed by chest x ray findings as described in Figure 1B, and also endobronchial resolution was seen in bronchoscopy as described in (Figure 2D). The tumor responded in a span of 4 weeks which melted like ice. The overall survival on treatment with Gefitinib is 8 to13 months which may prolong up to 36 months seen in south East Asian, nonsmoking females [7]. In various trials of gefitinib as shown in table no1, RR varied from 8% to 32% and PFS was seen from 2.1 months to 9 months. Afatinib is the 2nd generation TKI which is also used against adenocarcinoma which is pan ErbB inhibitor. Patients who has developed resistance to first and second generation TKI are subjected to liquid biopsy or re biopsy among which 60% of cases show T790M mutation on exon 20, who are treated with Osimertinib with improved survival rate.
| Trial | No Of Patients | RR (%) | SDR (%) | PFS (mo) | MST (mo) | Treatment Regimen |
| Cappuzzo., et al. [8] | 63 | 15.9 | 42.8 | ----- | 4.1 | Gefitinib 250mg |
| Fukuako., et al. [9] | 103 | 17.5 | 35.9 | 2.7 | 7.6 | Gefitinib 250mg |
| Thatcher., et al. [10] | 1129 | 8 | 32 | 3 | 5.6 | Gefitinib 250mg |
| Wu., et al. [11] | 40 | 32 | 45 | 9 | 15 | Gefitinib 250mg |
| Maruyama., et al. [12] | 245 | 22.5 | 34 | 2.1 | 5 | Gefitinib 250mg |
RR- Response rate, SDR- Stable disease rate, PFS- Progression free survival, MST-Mean survival time.
Table 1: Response to gefitinib in various trials.
Table 1: Response to gefitinib in various trials.
Conclusion
By presenting this case we want to conclude that patients with activating EGFR mutations especially exon 19 deletion, South East Asian nonsmoking women of age >40years show earliest response and are best benefited by these drugs.
Conflict of interest
Nil
Nil
References
- Sawyers, Charles L. "Opportunities and challenges in the development of kinase inhibitor therapy for cancer." Genes & development 17.24 (2003): 2998-3010.
- Paez, J. Guillermo., et al. "EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy." Science 304.5676 (2004): 1497-1500.
- Watanabe, Satoshi., et al. "Effectiveness of gefitinib against non–small-cell lung cancer with the uncommon EGFR mutations G719X and L861Q." Journal of thoracic oncology 9.2 (2014): 189-194.
- Riely, Gregory J., et al. "Clinical course of patients with non–small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib." Clinical cancer research 12.3 (2006): 839-844.
- Nishino, K., et al. "A retrospective analysis of 335 Japanese lung cancer patients who responded to initial gefitinib treatment." Lung cancer 82.2 (2013): 299-304.
- Takeda, Masayuki ., et al. "Survival Outcome Assessed According to Tumor Response and Shrinkage Pattern in Patients with EGFR Mutation–Positive Non–Small-Cell Lung Cancer Treated with Gefitinib or Erlotinib." Journal of Thoracic Oncology 9.2 (2014): 200-204.
- Park, Keunchil., et al. "Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial." the Lancet oncology 17.5 (2016): 577-589.
- Morgillo, Floriana., et al. "Mechanisms of resistance to EGFR-targeted drugs: lung cancer." ESMO open 1.3 (2016): e000060.
- Ricciuti, Biagio, and Rita Chiari. "First line osimertinib for the treatment of patients with advanced EGFR-mutant NSCLC." Translational lung cancer research 7.2 (2018): S127-S130.
- Tamura, K., et al. "Multicentre prospective phase II trial of gefitinib for advanced non-small cell lung cancer with epidermal growth factor receptor mutations: results of the West Japan Thoracic Oncology Group trial (WJTOG0403)." British journal of cancer 98.5 (2008): 907-914.
- Fukuoka, Masahiro., et al. "Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non–small-cell lung cancer." Journal of clinical oncology 21.12 (2003): 2237-2246.
- Thatcher, Nick., et al. "Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer)." The Lancet 366.9496 (2005): 1527-1537.
- Wu, Chi., et al. "Gefitinib as palliative therapy for lung adenocarcinoma metastatic to the brain." Lung Cancer 57.3 (2007): 359-364.
- Maruyama, Riichiroh., et al. "Phase III study, V-15-32, of gefitinib versus docetaxel in previously treated Japanese patients with non–small-cell lung cancer." Journal of Clinical Oncology 26.26 (2008): 4244-4252.
Citation:
R K Chopra., et al. “Tumor Melts like Ice – An Earliest Response to Gefitinib”. Pulmonary Research and Respiratory Care 2.2 (2019): 174-178.
Copyright: © 2019 RK Chopra., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.





























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.