Research Article
Volume 1 Issue 1 - 2018
Homocysteine Serum Levels and Methylenetetrahydrofolate (MTHFR) C677T Genotype in Patients with Parkinson's Disease
Neurology Clinic Clinical Center of Serbia, Faculty of Medicine University of Belgarde, Serbia
*Corresponding Author: EleonoraDžoljić, Neurology Clinic Clinical Center of Serbia, Faculty of Medicine University of Belgarde, Serbia.
Received: February 22, 2018; Published: March 09, 2018
Abstract
Both methylenetetrahydrofolate (MTHFR) C677T genotype and levodopa treatment may give rise to elevated serum homocysteine levels in parkinsonian patients. We aimed to clarify the interplay of these factors in pathogenesis of Parkinson's disease (PD)-related hyperhomocysteinemia. Total serum levels of homocysteine (tHcy) and MTHFR C677T genotype were investigated in levodopa-treated and -untreated parkinsonian (“de novo”) patients, as well as in control healthy subjects matched by age and gender (N = 83, 30 and 53, respectively). MTHFR C677T genotypes were equally distributed in PD patients and control subjects, the T allele homozygosity being observed in app. 12-17% cases. tHcy concentrations were significantly higher in both levodopa-treated and -untreated PD patients than in control subjects, and in TT homozygotes than in CT or CC genotype carriers. tHcy levels significantly correlated with the duration of the disease in PD treated patients only, reaching the maximum after 3-6 years. However, there was no correlation between tHcy levels and total daily intake of levodopa in the same group of PD patients. In conclusion, MTHFR C677T genotype is a significant factor for hyperhomocysteinemia in patients with PD, levodopa-untreated and probably even more in levodopa-treated PD patients.
Keywords: Parkinson's disease; Hyperhomocysteinemia; C677T methylenetetrahydrofolate reductase polymorphism; Levodopa
Introduction
Homocysteine is an amino acid arising by methylation of methionine that does not participate in the synthesis of proteins. Recently, it has received particular attention, because of its association with pathogenesis of several neurological, cerebrovascular and cardiovascular disorders (e.g. stroke, Alzheimer's disease, vascular dementia, depression, Parkinson's disease-PD, and coronary heart disease) [1-5] . An increase in total homocysteine (tHcy) concentrations was observed in serum of PD patients [6–8] . The pro-oxidant and pro-apoptotic effects of homocysteine have been confirmed in in vitro models of PD; e.g. homocysteine aggravated neurotoxic effects of pesticide rotenon in human dopaminergic cells, inducing oxidative stress, mitochondrial dysfunction and apoptosis [9] .
Intracellular concentrations of homocysteine are tightly maintained within a narrow range, even though the serum levels vary considerably. The optimal serum tHcy concentrations for healthy individuals are less than 9 μmol/l, while moderately and highly increased atherosclerosis risks are observed in the range of 9.1–15.0 μmol/l, and at values of more than 15.0 μmol/l, respectively [10] . There are many possible causes of hyperhomocyteinemia: genetic factors, nutritional causes, systemic disorders, medication, as well as physiological and lifestyle factors.
Methyl group for Hcy methylation derives from 5-methyl-tetrahydrofolate (5-methyl-THF), which itself provided by the action of 5, 10-methylenetetrahydrofolate reductase (MTHFR). A common C677T polymorphism in MTHFR gene has been identified and shown to influence tHcy levels in general population [11]. In Caucasians, 9-17% of individuals is identified as TT homozygotes that have reduced enzyme activity in comparison with the CT and CC individuals [3,11–13] . The decreased MHTFR activity impairs the remethylation of homocysteine to methionine, the process catalyzed by the enzyme methionine synthase that requires folate and vitamin B12 as cofactors. Folate supplementation in food supply was supposed to reduce serum tHcy levels in middle-aged and older adults [14] . Recent studies pointed out the impact of MTHFR polymorphism on serum tHcy levels in PD patients as well [15,16].
Another possible cause of the rise of serum tHcy concentrations in PD patients could be the biotransformation of levodopa, the drug that remains the mainstay of the therapy of PD patients [15,17] . Both levodopa and its active metabolite dopamine are subjected to methylation, with S-adenosylmethionine (SAM) being a methyl group donor. In turn, the increased conversion rate of levodopa and dopamine depletes the SAM (and methyl group) body reserve, the condition that could be aggravated with folate and vitamin B12 deficiency [18,19] . Consequently, the lack of methyl groups results in the reduced probability of homocysteine being remethylated to methionine. This can be further aggravated by the homozygosity for the MTHFR C677T allele, consequent reduction of MTHFR activity and a decline in 5-methyl-THF levels. In addition, other genetic or acquired factors can play a role. For example, impairment of the metabolic transsulfuration of homocysteine to cystathione could also contribute to the levodopa-induced hyperhomocysteinemia [20] .
Given this hypothesis as well as inconsistencies across the literature regarding relationship between levodopa treatment and the elevation of serum tHcy levels [16,22] our objectives were: to compare the serum tHcy concentrations of levodopa-treated and -untreated PD patients and the corresponding healthy controls; to establish the possible correlation between tHcy levels and daily intake of the drug in levodopa-treated PD patients as well as to investigate the influence of MTHFR C677T genotype on serum tHcy concentrations. Such analysis would provide further insight into the pathogenesis of the disease and the mechanisms of levodopa-induced adverse effects in PD patients.
Materials and Methods
Thirty PD patients without l-DOPA and 83 PD patients with l-DOPA therapy were initially selected and gave their consent to participate in the study and the Institutional ethics committee approved this study. The diagnosis of PD has been established by a neurologist based on the presence of at least two cardinal signs plus unequivocal response to l-DOPA: Unified Parkinson's Disease Rating Scale (UPDRS)-motor score [22] ; Hoehn and Yahr Staging (HY) [23] ; Hamilton Depression Rating Scale (HDRS) [24] ; Beck Depression Rating Scale [25] ; and Mini Mental State Examination (MMSE) [26] . Exclusion criteria were as follows: 1) stages III, IV and V in Hoehn and Yahr's staging; 2) patients with prominent depression or dementia according to Hamilton and Beck Depression Rating Scales, and MMSE (cut-off scores of > 23, > 30 and < 25, respectively) [27] ; 3) history of drug toxicity including hallucinations, delirium, or confusional events; 4) any history of stroke, neurological disease other than PD or any concomitant serious medical illness.
Levodopa was administered as levodopa + benserazide, at a daily dose of levodopa of 531.95 ± 183.00 mg (mean ± S.D., range: 100–1000). The duration of levodopa + benserazide administration was 3.70 ± 2.70 years (mean ± S.D., range: 2–13).
Analyses of total serum levels of homocysteine (tHcy) and MTHFR C677T genotype were performed in all of 113 PD patients (30 levodopa-untreated and 83 levodopa-treated) and in control group (C) of 53 healthy subjects, matched by age and gender.
Standardized measurement of tHcy was only performed in subjects with no metabolic disturbances such as diabetes mellitus, hypertension and without clinical signs of deficiency of vitamines B6, B12 or folic acid, as well as with normal serum creatinine concentrations (men, 53–124 μmol/l; women, 53–106 μmol/l) and MCV values (men, 80–94 fl; women, 81–99 fl). Each participant fasted and was off medication for at least 10 h before blood samples were taken in the morning. Thus we avoided influence of acute levodopa/dopaminergic-drugs intake. Total serum homocysteine (tHcy) levels have been estimated using HPLC with fluorescence detection. Prior sera preparation, derivatization and high-performance liquid chromatography (HPLC) separation have been done using original HPLC reagent kit, produced by “RECIPE”, Cat. No 23000, with original reversed phase column, Cat. No. 23030 produced by the same manufacturer. HPLC separation has been done on HPLC line, which was consisted of isocratic HPLC pump “BIO-RAD 1350T”, column heater “BIO-RAD”, autosampler “AS-100 BIO-RAD”, and fluorescence detector “HEWLETT PACKARD 1046A” (for details see Ref. [28] .
Genomic DNA for MTHFR C677T genotype analysis was extracted from whole blood samples by salting-out procedure. The presence of MTHFR C677T polymorphism was detected as previously described by Hinf I (Fermentas, Lyt.) digestion of PCR products encompassing polymorphic MTHFR C677T site [11]. One-way analysis of variance (ANOVA) with post-hoc Bonferroni's multiple comparison and two-tailed t-test were used in analysis of parametric data, and chi-square and Mann–Whitney tests otherwise (*1). In addition, parametric (Pearson) and nonparametric (Spearman) correlations were calculated when appropriate. Statistical analysis was performed using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego California USA).
Results
The demographic and clinical characteristics of the PD patients and healthy controls, as well as MTHFR across these groups, are shown in Table 1.
| Parameter | Control | PD(−l-DOPA) | PD(+l-DOPA) |
| N | 53 | 30 | 83 |
| Age (years) | |||
| Mean ± S.E.M. | 60.83 ± 13.13 | 59.13 ± 8.65 | 61.86 ± 9.14 |
| Minimum | 37 | 41 | 43 |
| Maximum | 83 | 72 | 83 |
| Gender F/M | 19/34 | 12/18 | 38/45 |
| Duration of the disease | – | 1.69 ± 0.59 | 3.70 ± 2.70 |
| l-DOPA (mg) | – | – | 531.95 ± 183.00 |
| MMSE | – | 28.87 ± 0.86 | 27.92 ± 1.32b |
| HY | – | 1.63 ± 0.43 | 2.37 ± 0.61b |
| UPDRS | – | 33.00 ± 12.22 | 59.59 ± 25.77b |
| Hamilton | – | 11.97 ± 6.01 | 14.80 ± 8.16 |
| Beck | – | 5.93 ± 3.06 | 7.36 ± 3.98 |
| PDQ39 | – | 83.70 ± 25.28 | 106.57 ± 26.09b |
| tHcy (μmol/l) | 13.13 ± 4.25 | 16.93 ± 7.08a | 17.05 ± 6.25a |
| MTHFR gene genotypes | |||
| N (%) | |||
| – CC | 16 (30.19) | 8 (26.67) | 39 (46.99) |
| – CT | 28 (52.83) | 17 (56.67) | 34 (40.96) |
| – TT | 9 (16.98) | 5 (16.67) | 10 (12.05) |
a, b-Significant difference vs. Control and PD(−L-DOPA) groups, respectively (P < 0.05).
Table 1: Characteristics of control subjects and PD patients (without and with levodopa therapy) (mean ± S.D.).
Table 1: Characteristics of control subjects and PD patients (without and with levodopa therapy) (mean ± S.D.).
There was no significant difference of MTHFR C677T genotypes among control, PD (−L-DOPA) and PD (+L-DOPA) groups: (P = 0.2; chi-square); the T allele homozygosity was observed in 9/53 (16.98%), 5/30 (16.67%) and 10/83 (12.05%) subjects, respectively. As would be expected, MMSE, HY, UPDRS, and PDQ39 scores were significantly worse in PD (+L-DOPA) than in PD (−L-DOPA) (“de novo”) patients (P = 0.0004; P < 0.0001; P < 0.0001; and P = 0.0002, respectively; Mann–Whitney test). However, PD groups with and without levodopa therapy did not differ in Hamilton and Back Depression Rating Scales (P > 0.05, both; Mann–Whitney test).
Total serum homocysteine (tHcy) levels were significantly lower in controls than in both PD groups, giving rise to the overall difference observed (all genotypes included) (P = 0.001; one-way ANOVA) (Table 1). However, tHcy did not differ significantly between PD (−L-DOPA) and PD (+L-DOPA) patients (P > 0.05). Similar results, i.e. higher tHcy concentrations in L-DOPA-treated and -untreated PD patients than in controls were observed when tHcy concentrations were compared between subgroups of patients with the specific MHTFR genotypes (Figure 1).
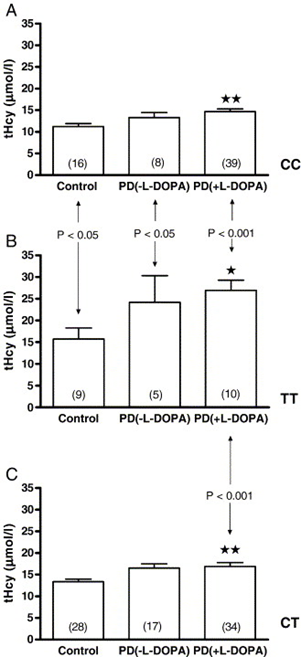
Figure 1: Plasma total homocyteine (tHcy) concentrations in healthy controls (Control), levodopa-untreated (PD (−L-DOPA)), and levodopa-treated patients (PD(+L-DOPA)), with CC, TT, and CT MTHFR genotypes (panels A, B, and C, respectively). Vertical bars represent mean ± standard error of the mean (S.E.M.) of n observations (indicated in the bracket). ⁎⁎⁎ indicate level of significance of P < 0.05 and P< 0.01, respectively for the difference between tHcy level of PD patients (both untreated and treated) vs. controls in each genotype group. Vertical arrow denotes significant difference of tHcy levels between TT allele carriers (panel B) and other genotype groups. The level of significance is indicated on the plots. The results are obtained by one-way ANOVA and post-hoc Bonferroni's multiple comparison test.
On the other hand, tHcy levels were significantly higher in TT allele homozygotes than in CC homozygous individuals in all analyzed groups: controls, PD (−L-DOPA) and PD (+L-DOPA). In addition, tHcy concentrations in TT subjects were significantly higher than in patients with CT genotype in PD (+L-DOPA) group only (Figure 1). Serum total homocysteine concentrations dependent on the duration of the disease in all levodopa-treated and -untreated PD patients are shown in Table 2.
| Duration of the disease (years) | PD(+l-DOPA) | PD(−l-DOPA) | ||||
| N | Mean | S.D. | N | Mean | S.D. | |
| < 3 | 50 | 15.88 | 6.60 | 29 | 16.89 | 7.20 |
| 3–6 | 17 | 19.84a | 5.30 | 1 | 18.16 | |
| > 6 (*2) | 16 | 17.73 | 5.23 | |||
A-Significant difference vs. corresponding < 3 years group (P < 0.05; Student's t-test).
Table 2: tHcy concentration distribution in relation to the duration of the disease in levodopa-treated and -untreated PD patients.
Table 2: tHcy concentration distribution in relation to the duration of the disease in levodopa-treated and -untreated PD patients.
In PD(+L-DOPA) group, significantly higher tHcy levels were seen in patients whose disease lasted between 3 and 6 years than in patients with the duration of the disease of less than 3 years (P = 0.029; Student's t-test). Likewise, significant correlation was observed between tHcy concentrations and the duration of the disease in PD treated patients (Spearman coefficient of correlation, r = 0.3106; P = 0.0043) (Figure 2).
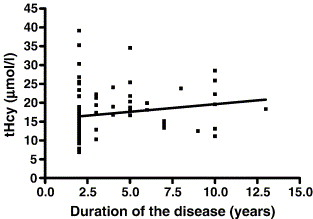
Figure 2: Relation between plasma total homocysteine concentrations and duration of the disease in 83 PD patients treated with levodopa, PD (+L-DOPA) group. Spearman correlation coefficient r = 0.3106, P = 0.004.
On the other hand, there was no significant correlation between the total daily intake of levodopa and tHcy concentrations in the same group of PD (+L-DOPA) patients (all genotypes included) (Pearson correlation coefficient, r = 0.107; P > 0.05). The same lack of correlation was observed in the further analysis of PD (+L-DOPA) patients with the particular (CC, CT or TT) genotype (r = − 0.036; 0.070; and − 0.254, respectively; P > 0.05 in all cases). Also, there was no significant correlation between tHcy concentrations and clinical condition parameters (MMSE, HY, UPDRS, Hamilton, Beck, and PDQ39), regarding all genotypes or a particular one (CC, CT, TT).
In PD (−L-DOPA) group neither duration of the disease nor clinical condition parameters significantly correlated with serum tHcy values (P > 0.05, all cases).
Discussion
We found significant elevation of serum homocysteine levels in PD patients in comparison with the corresponding controls, especially in TT homozygotes, the finding that is in agreement with previous studies [6–8,15,16]. The serum tHcy values observed in our present study were similar in both levodopa-treated and -untreated PD patients. Moreover, no correlation between serum tHcy levels and daily levodopa intake was seen. This is in agreement with previous results, as well as with the observed lack of correlation between plasma homocysteine levels and the concentrations of l-DOPA or its metabolite [21].
It is possible that the average daily levodopa dose of ≈ 530 mg in our study was not high enough to significantly contribute to hyperhomocysteinemia in levodopa-treated PD patients [15,29]. In other words, the “threshold” intake of levodopa that is necessary for the induction of hyperhomocysteinemia in PD patients (app. 1000 mg according to Ref. [29] has not been reached in the present study. The lack of difference in tHcy levels and depression rating scores between levodopa-treated and -untreated PD patients in our study supports such hypothesis. It is possible that higher daily doses of l-DOPA than used in our study could additionally affect tHcy levels in PD patients. Yasui et al. [15] also observed higher tHcy levels in PD (+l-DOPA) patients compared to the control subjects in all genotypes. However, tHcy concentrations in PD(+l-DOPA) patients with TT genotype in the later study were similar to the values obtained in the same group of patients in our study (27.2 ± 23.3 and 26.91 ± 7.43 μmol/l, respectively), despite significant difference in daily doses of l-DOPA used (1497.6 and 531.95 mg, respectively).
Patients from the present study were in early stages of PD (HY I, II), neither depressed (depression rating scores: Hamilton < 17, and Beck < 10) nor demented (MMSE > 27). That could possibly explain the lack of correlation of tHcy and scores of the depression rating scales (Hamilton, Beck) in this study compared to results of O'Suilleabhain [5] . Additionally, our finding of the positive correlation between tHcy values and duration of the disease in levodopa-treated patients only in the group with longer disease duration seems to be in agreement with this notion as well.
Kuhn., et al.[16] suggested MTHFR genotyping before initiation of levodopa treatment and homocysteine monitoring during levodopa long-term treatment. Hyperhomocysteinemia is thought to contribute to side effects of levodopa, especially development and fluctuation of neuropsychiatric symptoms [15,29]. Furthermore, it probably increases mortality from cardiovascular diseases and stroke in treated parkinsonian patients, although this is still a matter of debate [20,30,31]. Conversion of levodopa and dopamine to 3-o-methyldopamine and 3-o-methylthyrosine, respectively, depletes the pool of available methyl groups, necessary for the remethylation of homocysteine to methionine. If this is the case, early combination of levodopa not only with vitamin B12 and folate, but also with catecholamine o-methyltransferase (COMT) inhibitors (tolcapone, entacapone) would prone to be beneficial, reducing homocysteine levels [29]. In other words, COMT inhibitors would diminish the supposed levodopa-induced increase in tHcy.
However, O'Suilleabhain., et al.[32] have recently shown that neither COMT inhibitor entacapone nor levodopa reduction itself significantly reduced tHcy concentrations in PD patients, suggesting a complex etiology of levodopa-induced hyperhomocysteinemia. The Hcy levels in levodopa-treated patients can be further increased in individuals that are homozygous for MTHFR T allele due to the reduction of 5-methyl-THF concentrations. Accordingly, Blandini., et al. [21] hypothesized that levodopa could affect serum tHcy levels only in PD patients that are TT homozygotes.
This is not clearly supported by the present results since our average daily levodopa dose did not reach the threshold level mentioned above [29] . Hcy levels were increased in TT individuals in both levodopa-treated and -untreated patients. Likewise, we found no significant correlation between serum tHcy levels and daily levodopa intake in TT subgroup of PD patients. However, we observed maximal tHcy levels in TT homozygous PD patients treated with levodopa, the values being significantly higher than in CT heterozygotes or CC individuals. And finally, in contrast to Yasui., et al. [15], we have observed that MTHFR genotype significantly affected serum tHcy concentrations in control subjects too, the highest values being detected in TT homozygotes. Such a difference in tHcy levels of control subjects may be the result of some other contributing factors such as nutritional status, genetic causes (cystathione β-synthase and/or methionine synthase deficiency) or lifestyle factors.
In conclusion, C677T mutation of the gene encoding for the enzyme MTHFR significantly affects the serum homocysteine levels in PD patients, the homozygote TT individuals being at the highest risk. The increase in serum tHcy levels significantly correlates with the duration of the disease reaching the plateau after 3–6 years. If levodopa therapy contributed to the increase in serum tHcy concentrations, higher doses would be necessary for such effect. Further investigations are needed to elucidate the interplay between drugs used in Parkinsonism (levodopa, COMT inhibitors, etc.) and different genetic factors in modulation of tHcy levels in PD patients.
References
- KS McCully. “Vascular pathology of homocysteinemia: implications for the pathogenesis of atherosclerosis.” American Journal of Pathology 56.1 (1969): 111-128.
- CJ Boushey SA., et al. “A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes.” JAMA 274.13 (1995): 1049-1057.
- GJ Hankey and JW Eikelboom. “Homocysteine and vascular disease.” Lancet 354.9176 (1999): 407-413.
- R Diaz-Arrastia. “Homocysteine and neurologic disease.” Archives of Neurology 57.10 (2000): 1422-1427.
- PE O'Suilleabhain., et al. “Elevated plasma homocysteine level in patients with Parkinson disease: motor, affective, and cognitive associations.” Archives of Neurology 61 (2004): 865-868.
- P Allain A., et al. “Sulfate and cysteine levels in the plasma of patients with Parkinson's disease”. Neurotoxicology 16.3 (1995): 527-529.
- W Kuhn., et al. “Elevated plasma levels of homocysteine in Parkinson's disease”. European Neurology 40.4 (1998): 225-227.
- T Muller., et al. “Nigral endothelial dysfunction, homocysteine, and Parkinson's disease”. Lancet 354. 9173 (1999): 126-127.
- W Duan., et al. “Dietary folate deficiency and elevated homocysteine levels endanger dopaminergic neurons in models of Parkinson's disease”. Journal of Neurochemistry 80.1 (2002): 101-110.
- O Nygård., et al. “Plasma homocysteine levels and mortality in patients with coronary artery disease”. The New England Journal of Medicine 337.4 (1997): 230-236.
- P Frosst., et al. “A candidate genetic risk factor for vascular disease: a common mutation methylenetetrahydrofolate reductase”. Nature Genetics 10.1 (1995): 111-113.
- JG Ray., et al. “Common C677T polymorphism of the methylenetetrahydrofolate reductase gene and the risk of venous thromboembolism: meta-analysis of 31 studies”. Pathophysiology of Haemostasis and Thrombosis 32.2 (2002): 51-58.
- J Gasparovic., et al. “Effect of C677T methylenetetrahydrofolate reductase gene polymorphism on plasma homocysteine levels in ethnic groups”. Physiological Research 53.2 (2004): 215-218.
- PF Jacques., et al. “The effect of folic acid fortification on plasma folate and total homocysteine concentrations”. The New England Journal of Medicine 340.19 (1999): 1449-1454.
- K Yasui., et al. “Plasma homocysteine and MTHFR C677T genotype in levodopa-treated patients with PD”. Neurology 55.3 (2000): 437-440.
- W Kuhn., et al. “Plasma homocysteine and MTHFR C677T genotype in levodopa-treated patients with PD”. Neurology 56.2 (2001): 281-282.
- JD Rogers., et al. “Elevated plasma homocysteine levels in patients treated with levodopa: association with vascular disease”. Archives of Neurology 60.1 (2003): 59-64.
- B Widner., et al. “Moderate hyperhomocysteinaemia and immune activation in Parkinson's disease”. Journal of Neural Transmission 109.12 (2002): 1445-1452.
- JW Miller., et al. “Effect of l-DOPA on plasma homocysteine in PD patients: relationship to B-vitamin status”. Neurology 60.7 (2003): 1125-1129.
- P O'Suilleabhain and R Diaz-Arrastia. “Levodopa elevates homocysteine: is this a problem?” Archives of Neurology 61.5 (2004): 633-634.
- F Blandini., et al. “Plasma homocysteine and l-DOPA metabolism in patients with Parkinson disease”. Clinical Chemistry 47.6 (2001): 1102-1104.
- S Fahn, and the members of the UPDRS Development Committee Unified Parkinson's Disease Rating Scale S. Fahn, C.D. Marsden, M. Goldstein, D.B. Calne (Eds.), Recent development in Parkinson's disease, vol. II, MacMillan, Florham Park (1987): 153-163.
- M Hoehn and M Yahr “Parkinsonism: onset, progression and mortality”. Neurology 17.5 (1967): 427-442.
- M Hamilton. “A rating scale for depression”. Journal of Neurology, Neurosurgery, and Psychiatry 23 (1960): 56-62.
- AT Beck., et al. “An inventory for measuring depression”. Archives of General Psychiatry 4 (1961): 561-571.
- M Folstein., et al. ““Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician”. Journal of Psychiatric Research 12.3 (1975): 189-198.
- American Psychiatry Association: Committee on Nomenclature and Statistics Diagnostic and statistical manual of mental disorders-revised (3rd ed), American Psychiatric Association, Washington, DC (1980)
- Accinni R., et al. “High-performance liquid chromatographic determination of total plasma homocysteine with or without internal standards”. Journal of Chromatography A 828(1–2) (1998): 397–400.
- L Brattstrom. “Plasma homocysteine and MTHFR C677T genotype in levodopa-treated patients with PD”. Neurology 56.2 (2001): 281-282.
- JM Gorell., et al. “Parkinson's disease and its comorbid disorders: an analysis of Michigan mortality data, 1970 to 1990”. Neurology 44.10 (1994): 1865-1868.
- Y Ben-Shlomo and MG Marmot. “Survival and cause of death in a cohort of patients with parkinsonism: possible clues to aetiology?” Journal of Neurology, Neurosurgery, and Psychiatry 58.3 (1995): 293-299.
- PE O'Suilleabhain., et al. “Modest increase in plasma homocysteine follows levodopa initiation in Parkinson's disease”. Movement Disorders 19 (2004): 1403-1408.
Citation:
EleonoraDžoljić and VladimirKostić. “Homocysteine Serum Levels and Methylenetetrahydrofolate (MTHFR) C677T Genotype
in Patients with Parkinson’s Disease”. Medical Research and Clinical Case Reports 1.1 (2018): 14-22.
Copyright: © 2018 EleonoraDžoljić and VladimirKostić. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.