Research Article
Volume 1 Issue 2 - 2018
Bigunidine Metformin Pharmacologically Efficacious Than Herbal Therapies and Its Preparation, The Safest Biguanide Derivates in Use by T2dm Patients
IPL Research Centre, Editoral Board Member- Asscoiate editor OAJTMS –(Open acc.J.of Toxicol. Hospital and Clinical Pharmacy)
*Corresponding Author: Krishnasarma Pathy, IPL Research Centre, Editoral Board Member- Asscoiate editor OAJTMS –(Open acc.J.of
Toxicol. Hospital and Clinical Pharmacy), India.
Received: March 09, 2018; Published: March 22, 2018
Abstract
Metformin usage may be considered for people with type 2 diabetes to help them control their blood sugar levels a secondary side effect may be weight loss, which leads some to believe it may be a viable medication to use for weight loss. However, researchers are still not clear on whether or not metformin has an actual impact on weight, or if weight loss is due to lifestyle changes in people with type 2 diabetes. Metformin (Glucophage, Glumetza, Fortamet) is typically prescribed to counteract the effects of insulin resistance -- the body's sluggish response to the blood-sugar-lowering hormone insulin. Insulin resistance can lead to high blood sugars and may eventually progress to prediabetes or type 2 diabetes (T2DM). Metformin improves insulin sensitivity of the body tissues and reduces liver glucose production, both of which help lower blood sugar levels. The American Diabetes Association recommends metformin as a first-choice medicine to treat T2DM. It is also sometimes used in combination with exercise and weight loss in people with prediabetes. Some evidence suggests that a few herbs might mimic some of the effects of metformin. However, no herb is a proven alternative to metformin
Introduction
N-dimethyl imidodicarbonimidic diamide is a biguanide drug, the generic name of which is metformin. When this drug is administered to type 2 diabetic patients or glucose intolerant patients, it can exhibit blood glucose lowering action by controlling glucose formation in the liver and increasing glucose utilization in muscles and improve lipid metabolism, thus preventing the development and deterioration of diabetes complications and treating diabetes complications.
It can be seen in several papers that only metformin among oral anti-diabetic drugs is a first-choice drug. Particularly, it was proved that metformin has the effect of activating AMPK, and thus the propriety of clinical effects thereof was demonstrated.
It was reported that AMPK is a key enzyme physiologically controlling metabolism of carbohydrate and lipid, and metformin is effective in normalizing high glucose level, improving the condition of lipid, normalizing amenorrhea, ovulation and pregnancy, treating fatty liver, and preventing and treating p53 gene-deficient cancers by activating said enzyme. According to a report by the Abramson Cancer Center of the University of pennsylvania, metformin, an AMPK activator, is effective for the prevention and treatment of p53 gene-deficient cancers [Monica Buzzai., et al. Systemic Treatment with the Antidiabetic Drug Metformin Selectively Impairs p53 gene-Deficient Tumor Cellgrowth, Cancer Res 2007; 67:(14); July 15, 2007].
Discussion
People who do not have type 2 diabetes should likely seek out more traditional methods of losing weight through diet and exercise, or possibly other medications or procedures to control their weight.
Two guanide molecules joined together are known as biguanides, which is a name that was given by Rathke in 1879 when he obtained a new compound after a condensation reaction of thiourea and phosphorus trichloride with guanidine. The synthesis was henceforth improved by using a condensation reaction at 110ºC of cyanoguanidine with an ammoniac solution of cupric sulfate in a sealed tube.
In 1892 it was discovered that biguanides can be obtained via direct fusion of ammonium chloride with cyanoguanidine at 195ºC for a couple of minutes. This procedure is still employed in the synthesis of substituted bigunaides to detect the presence of biguanide.
To achieve ecofriendly synthesis of the target molecule, the starting materials are made to react by adjusting the reaction conditions in such a way that the by-products and wastes are eliminated, with minimal use of organic solvents. Thin layer chromatography has been described as a tool for reaction optimization in microwave assisted synthesis.
Metformin is a white, hygroscopic crystalline powder with a bitter taste. Chemically it is 1,1 dimethyl-biguanide hydrochloride with a mode of action and uses similar to other biguanides. This small molecule is soluble in water and 95% alcohol; on the other hand, it is practically insoluble in ether or chloroform. Its structure was generally represented in a wrong tautomeric form for several years, but that was corrected in 2005.
When heated to decomposition, metformin emits toxic fumes of nitric oxides. It undergoes negligible hepatic metabolism and is excreted by the kidney with a half-life of approximately two hours. Gas-liquid chromatography, mass fragmentography and high-pressure liquid chromatography are highly specific and most often employed as analytic laboratory methods.
Several studies reported on crystallographic structure of metformin and its derivates – namely metformin hydrochloride, N, N-dimethylbiguanidium nitrate and metal complexes with metformin. These studies accentuate the importance of π-conjugation (multiple bond systems) and inter-molecular hydrogen bonding.
Four primary end products of oxidation that result from the direct attack of hydroxyl radicals on metformin are a covalent dimer of metformin, hyproperoxide of metformin, methyl-biguanide and 2-amino-4-methylamino-1,3,5-triazine. Under similar conditions, the superoxide radicals are poor initiators of metformin oxidations, suggesting that metformin is not a powerful antioxidant.
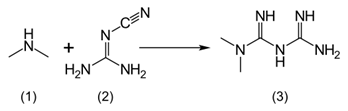
Stage I: Diemthylamine Hydrochloride is reacted with cyano guanidine in presence of Isopropyl alcohol to give Metformin Hydrochloride crude.
Stage II: Metformin Hydrochloride crude is purified in methanol and carbon gives pure Metformin Hydrochloride. Schematic diagram of Metformin Hydrochloride.
Preparation of Metformin Hydrochloride (I)
Isoproylalcohol (500 mL) and dicyanodiamide or cyanoguanidine (100g) were taken in a round-bottom flask at 25°C. to 35°C. The reaction mixture was heated at 78-80°C. Dimethylamine hydrochloride (117g) was added portion-wise within 2 hours. The reaction mass was stirred for 3 hours. The reaction mixture was further heated to 80° to 82°C. followed by heating to reflux temperature. The reaction mass was stirred for 4 hours and cooled to 80°C. treated with charcoal (3g) and stirred for 20 minutes. The reaction mass was filtered through hyflowbed and washed with isopropyl alcohol (50 mL). The filtrate was taken in a round-bottom flask at 50°C. and N2 gas was purged for 30 minutes.
Isoproylalcohol (500 mL) and dicyanodiamide or cyanoguanidine (100g) were taken in a round-bottom flask at 25°C. to 35°C. The reaction mixture was heated at 78-80°C. Dimethylamine hydrochloride (117g) was added portion-wise within 2 hours. The reaction mass was stirred for 3 hours. The reaction mixture was further heated to 80° to 82°C. followed by heating to reflux temperature. The reaction mass was stirred for 4 hours and cooled to 80°C. treated with charcoal (3g) and stirred for 20 minutes. The reaction mass was filtered through hyflowbed and washed with isopropyl alcohol (50 mL). The filtrate was taken in a round-bottom flask at 50°C. and N2 gas was purged for 30 minutes.
The filtrate was distilled to remove isopropyl alcohol completely under vacuum at 45°C. The residue thus obtained was treated with methanol (110 mL) at 40°C. to 45°C. and cooled to 20°C. to 25°C. The product was filtered and washed with chilled methanol (50 mL). The wet-cake thus obtained was treated with water at 50°C. along with N2 gas purging for 30 minutes. The solution was distilled to remove water completely under vacuum at 65°C. The residue thus obtained was treated with methanol (80 mL) at 40°C. to 45°C. to prepare the slurry. The slurry was pulverized under high-speed grinder for wet grinding for 25 minutes. The reaction mass was filtered and dried. The wet-cake was washed with chilled methanol (30 mL). The product was dried at 65°C. to 70°C. to obtain 155g metformin hydrochloride having dimethylamine content less than 5 ppm.
Goat’s rue, or Galega officinalis, is an age-old remedy. In times past, it was used for assorted ailments, including diabetes. Metformin is a man-made chemical that's closely related to a substance found in goat’s rue. Animal studies from the 1970s and 1980s established that substances in goat's rue have blood-sugar-lowering effects. Some of these chemicals can be toxic, however, so human studies are lacking. A recent animal study was published in April 2008 in the "British Journal of Pharmacology." Researchers found that mice fed galegine -- a chemical found in goat's rue -- ate less, lost weight and had reduced blood sugar levels, compared to mice that weren't fed the chemical.
Goat's rue is not approved for diabetes treatment by the U.S. Food and Drug Administration or Germany's Commission E, a scientific advisory board that reviews and approves herbal medicines. Commission E noted significant health risks with goat's rue and the availability of more effective diabetes treatments. Importantly, animal studies show that goat's rue may be harmful during pregnancy
Bitter Melon
Bitter melon has long been used as a folk remedy for diabetes. Several substances found in the fruit and seeds of bitter melon reportedly have blood-sugar-lowering effects. However, research on the effectiveness of bitter melon as a diabetes remedy has been mixed. A February 2002 "Alternative Medicine Review" article reported that 2 small studies conducted in people with diabetes suggested blood-sugar-lowering effects. However, a December 2014 "Nutrition and Diabetes" article that analyzed results from 4 studies reported that people with T2DM experienced no significant reduction in blood sugar, compared to people taking an inactive substance. Bitter melon is not approved for diabetes treatment by the FDA. Animal studies indicate bitter melon may reduce fertility and increase the risk for miscarriage.
Bitter melon has long been used as a folk remedy for diabetes. Several substances found in the fruit and seeds of bitter melon reportedly have blood-sugar-lowering effects. However, research on the effectiveness of bitter melon as a diabetes remedy has been mixed. A February 2002 "Alternative Medicine Review" article reported that 2 small studies conducted in people with diabetes suggested blood-sugar-lowering effects. However, a December 2014 "Nutrition and Diabetes" article that analyzed results from 4 studies reported that people with T2DM experienced no significant reduction in blood sugar, compared to people taking an inactive substance. Bitter melon is not approved for diabetes treatment by the FDA. Animal studies indicate bitter melon may reduce fertility and increase the risk for miscarriage.
Berberine
Berberine is a potentially powerful chemical derived from different plants, including goldenseal, goldenthread and tree turmeric. This herbal remedy has been used in traditional Chinese medicine to treat various conditions, including diabetes. An October 2012 "Evidence-Based Complementary and Alternative Medicine" article reported on the pooled results from 14 studies examining berberine for T2DM treatment. The researchers found that berberine plus diet and exercise led to lower blood sugar levels than diet and exercise alone. They also noted evidence that adding berberine to oral diabetes medicines leads to lower blood sugars than oral diabetes medicines alone. The researchers cautioned, however, that the quality of the studies was generally poor, which could lead to inaccurate conclusions.
Berberine is a potentially powerful chemical derived from different plants, including goldenseal, goldenthread and tree turmeric. This herbal remedy has been used in traditional Chinese medicine to treat various conditions, including diabetes. An October 2012 "Evidence-Based Complementary and Alternative Medicine" article reported on the pooled results from 14 studies examining berberine for T2DM treatment. The researchers found that berberine plus diet and exercise led to lower blood sugar levels than diet and exercise alone. They also noted evidence that adding berberine to oral diabetes medicines leads to lower blood sugars than oral diabetes medicines alone. The researchers cautioned, however, that the quality of the studies was generally poor, which could lead to inaccurate conclusions.
Large, well-designed studies are needed to determine whether berberine may be useful for diabetes treatment. It is not FDA-approved for diabetes treatment as of 2016. Large doses of berberine may cause digestive system upset, flulike symptoms, shortness of breath and low blood pressure. It may also increase the risk for miscarriage by stimulating uterine contractions.
Cinnamon
Cinnamon has also been suggested as a possible herbal treatment for diabetes. A September 2013 "Annals of Family Medicine" article examined the pooled results from 10 studies wherein 543 people with T2DM took daily cinnamon or an inactive placebo for 4 to 18 weeks. Many study participants also took oral diabetes medicines. The researchers found that participants taking cinnamon had lower fasting blood sugar levels than those taking a placebo. However, there was no effect on A1C, a test to measure long-term blood sugar control. The researchers cautioned that insufficient research has been performed to apply these findings to routine diabetes treatment. More research is also needed to determine dosing amounts, the best form of cinnamon to use and to clarify for whom this herbal remedy might be useful. As of 2016, cinnamon is not FDA-approved for diabetes treatment. While cinnamon is generally safe, animal studies indicate that large doses may trigger liver inflammation and reduced clotting ability. Safety studies for pregnant and breastfeeding women are lacking.
Cautionary Note
Cinnamon has also been suggested as a possible herbal treatment for diabetes. A September 2013 "Annals of Family Medicine" article examined the pooled results from 10 studies wherein 543 people with T2DM took daily cinnamon or an inactive placebo for 4 to 18 weeks. Many study participants also took oral diabetes medicines. The researchers found that participants taking cinnamon had lower fasting blood sugar levels than those taking a placebo. However, there was no effect on A1C, a test to measure long-term blood sugar control. The researchers cautioned that insufficient research has been performed to apply these findings to routine diabetes treatment. More research is also needed to determine dosing amounts, the best form of cinnamon to use and to clarify for whom this herbal remedy might be useful. As of 2016, cinnamon is not FDA-approved for diabetes treatment. While cinnamon is generally safe, animal studies indicate that large doses may trigger liver inflammation and reduced clotting ability. Safety studies for pregnant and breastfeeding women are lacking.
Cautionary Note
Conclusion
No herb has been proved a safe and effective alternative to metformin. You should never change the dosage or stop taking metformin without first consulting your doctor. Stopping or reducing diabetes medications could lead to high blood sugar levels that could be life-threatening. It's also important not to start taking herbs in combination with your diabetes medicines without discussing it with your doctor, as dangerously low blood sugar levels could occur. Herbal supplements could also interact with other medications you're taking, making them less effective or more toxic. Provided that the dose is adjusted for renal function, metformin treatment appears to be safe and still pharmacologically efficacious in moderate-to-severe CKD.
I am thankful to suggestions from Dr. Sunil nadkarni -torrent pharma researchcentre-ahmedabad. Dr. Vijaykumarhonda, Aurbindopharma hyderabad, Dr. Mc sriramn, Deepest gratitude to Dr. P Atchutha ramie, Ex professor-Andhra university.
References
- www.nhs.uk/news/2010/09September/Pages/rosiglitazone-avandia-drug-suspended-heart- risk.aspx
- www.medicines.org.uk/emc/medicine/1043
- The New England Journal of Medicine 346 (2002): 393–403.
- Agalloco J and Carleton J. “Validation of Pharmaceutical process. Why Validation?” Informa Healthcare:NY, 3rd edition 3.
- Ahmed S U., et al. Scale up process validation & Technology Transfer, In:Shargel, L., Kanfer, I.(Eds.). Generics Drug Product Development: Solid Oral Dosage form, Marcel Dekker, New York 143 (2005): 95-136.
- J Clin Endocrinol Metab 93 (2008): 2559–2565.
- Bankar GS., et al. “The Theory and Practice of Industrial Pharmacy”. Varghese Publishing House. Bombay (1986): 293-342, 343-348.
- Chao AY., Forebes, FJ., Johnson, RF. Prospective Process Validation, In: Nash, R. A., Watcher A. H. (Eds.), Pharmaceutical Process Validation, Marcel Dekker, New York/Basel 129 (2003): 7-31.
- Metabolism 57 (2008): 712–717
- Manthan DJ., et al. “Facets of Technology Transfer: A Perspective of Pharmaceutical Industry”. Journal of Intellectual property Right 13 (2008): 28-34.
- Mehta RM., et al. Processing of Tablets, pharmaceutics-1, Vallabh Prakashan, Delhi (2002): 238-267.
- Patel JK., et al. “Aqueous-Based Film Coating of Tablets: study the effect of critical process parameters”. International Journal of PharmTech Research 1.2 (2009): 235-240.
- Pfeffer., et al. Direct Compression Formulation and Process: US Patent 20060210627 (2006) 21.
- Rane M and Parmar J. “Tablet Formulation design and Manufacturing: Oral Immediate Release Application”. Pharma Times 41 (2009): 21-24.
- Sweetman SC. “Metformin Hydrochloride, Sweetman, S. C. (Ed.) The complete drug references”. Pharmaceutical Press, London/Chicago 1. (2007): 411-412.
- Med Sci Monit 17 (2011): RA164–7
- Evid Based Complement Alternat Med 2012 (2012): 591654.
Citation:
Krishnasarma Pathy. “Bigunidine Metformin Pharmacologically Efficacious Than Herbal Therapies and Its Preparation, The Safest
Biguanide Derivates in Use by T2dm Patients”. Medical Research and Clinical Case Reports 1.2 (2018): 61-65.
Copyright: © 2018 Krishnasarma Pathy. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.