Research Article
Volume 2 Issue 4 - 2018
Rationale of Ornidazole and Ofloxacin in Management of Diarrhoea
1MBBS (MGIMS); MD (Internal Medicine); DNB (E&M); PhD, Postgraduate in Endocrinology & Metabolism, (AIIMS Delhi), Chairman, National Institute of Health & Research, Warisaliganj (Nawada) Bihar India
2BAMS (BRABU); MHA, Director (Hon), Aarogyam Punarjeevan, Ram Bhawan, Ara Garden Road, Jagdeopath, Baily Road, Patna
3BAMS (BRABU), Ex Director, Centre for Indigenous Medicine & Research, Senior Research Fellow, Regional Research Institute of Ayurveda, Itanagar, Arunachal Pradesh Bahrain
2BAMS (BRABU); MHA, Director (Hon), Aarogyam Punarjeevan, Ram Bhawan, Ara Garden Road, Jagdeopath, Baily Road, Patna
3BAMS (BRABU), Ex Director, Centre for Indigenous Medicine & Research, Senior Research Fellow, Regional Research Institute of Ayurveda, Itanagar, Arunachal Pradesh Bahrain
*Corresponding Author: Avinash Shankar, MBBS (MGIMS); MD (Internal Medicine); DNB (E&M); PhD, Postgraduate in Endocrinology & Metabolism, (AIIMS Delhi), Chairman, National Institute of Health & Research, Warisaliganj (Nawada) Bihar India.
Received: October 04, 2018; Published: October 25, 2018
Abstract
Diarrhoea a commonest food and water born disease affecting equally from neonates to geriatrics due to varied causative pathogens, commonly presents with loose motion may be watery or varied natured stool, principally due to altered water and electrolyte absorption as a result of altered sodium potassium ATPase pump caused by altered mechanism of normally secreted bio molecules Enkephalin.
For diarrheal disease control the common prescription these days is combination of broad spectrum anti protozoal and broad spectrum anti-bacterial, among them are tinidazole–norfloxacin and ornidazole-ofloxacin, and tinidazole and co trimoxazole but unusual presentation observed and reported by patients or parent with ofloxacin and ornidazole consumption, necessitate the present evaluation which affirm superiority of Tinidazole-norfloxacin and tinidazole and co trimoxazole as compared to Ofloxacin and ornidazole in both therapeutic outcome, safety profile and drug adversity as–Ofloxacin -Ornidazole both acts on DNA and alters DNA function, possess high volume distribution thus both remain in GIT for very short duration thus fails to ensure sustained availability to sterilize the GIT while other anti-diarrhoeal composite Norfloxacin never binds with DNA but to DNA substrate, possess very low volume distribution, thus remain in GIT for comparatively longer duration, ensure gut sterility, Co trimoxazole inhibit dihydrofolate enzyme inhibiting synthesis of folinic acid, possess low volume distribution and stay longer in GIT thus check post diarrheal sequel i.e- mucous colitis and Urinary tract infection. All the three anti-diarrheal combination proved safe for haematological, hepatic and renal parameter. Thus Ofloxacin-Ornidazole remain no longer choice for Diarrheal management considering its hazards and outcome.
Key Words: Diarrhoea; Enkephalin; Sodium potassium ATPase pump volume distribution; DNA substrate; Dihydrofolate; Folinic acid; Diarrheal sequel; Mucous colitis
Introduction
Based on the characteristics of characteristic feature of stool, diarrhoea is classified as [3] – Acute Diarrhoea is the 3rd leading cause of childhood morbidity in India and responsible for 13% of all death among children of age < 5 yrs and decrease in diarrheal death in India is very slow. [1,2]
| Watery diarrhoea : lasts for several days Acute diarrhoea with blood : also known as dysentery Persistent diarrhoea : lasts for 14 days or more |
Diarrhoea, a commonest GIT problem causing encumbrance to both patients and parent. Considering a synergistic pathogenesis of diarrhoea due to [4-6] protozoa and bacterial super infection, a combination of antiprotozoal and broad spectrum quinolones are quite in vogue. Commonest among them is Norfloxacin, a quinolone with least volume distribution and plasma binding capacity i.e.- Norfloxacin was quite in use, but these days an isomer of 5 fluoroquinolone having high volume distribution and plasma binding capacity.
i.e Ofloxacin is in rampant use. Considering the pharmacokinetics of Ofloxacin and Norfloxacin, ofloxacin is more toxic than norfloxacin and due to high volume distribution and common toxicity i.e- cartilaginous osteoarthropathy, ofloxacin is never a choice at least in paediatrics. [7-9]
For diarrhoeal disease management, the composite must ensure
- Potent effect against intestinal micro organisms
- Must have low absorption and low volume distribution to have maximum effect to counter intestinal infection
- Must get excreted through kidney as migration of intestinal commensal through systemic circulation may cause post diarrhoeal urinary tract infection which in turn results in suppression of erythropoietin and erythropoiesis leading to chronic anaemia
- Amoebicidal must be potent and active against both local and extra intestinal protozoal infection
- It should not pose any toxic or untoward effect
Objective of the study
Evaluate the rationality of ofloxacin use in diarrhoea management
Evaluate the rationality of ofloxacin use in diarrhoea management
Duration of study
Study was conducted during January 2014 to December 2016 and cases were followed for 6 months post therapy for drug related or disease related untoward effects.
Study was conducted during January 2014 to December 2016 and cases were followed for 6 months post therapy for drug related or disease related untoward effects.
Ethical status
Ethical committee of the National Institute of Health & Research duly permitted the study, based on case data.
Ethical committee of the National Institute of Health & Research duly permitted the study, based on case data.
Material and Method
Material
Patients attending Centre For Research in Diarrhoeal Disease, National Institute of Health & Research, Warisaliganj (Nawada) Bihar and Aarogyam Punarjeevan, Ara Garden Road Jagdeo path, Baily Road, Patna 14 suffering with lose motion were selected for the evaluation and justification for use of Ofloxacin in management of diarrhoea. Patients of diarrhoea with septicaemia or any other complication were excluded from the study.
Patients attending Centre For Research in Diarrhoeal Disease, National Institute of Health & Research, Warisaliganj (Nawada) Bihar and Aarogyam Punarjeevan, Ara Garden Road Jagdeo path, Baily Road, Patna 14 suffering with lose motion were selected for the evaluation and justification for use of Ofloxacin in management of diarrhoea. Patients of diarrhoea with septicaemia or any other complication were excluded from the study.
Method
Data sheet of patient of diarrhoea attended for treatment at RA Hospital & Research Centre are analysed for the clinical presentation, duration of illness, therapeutics taken, their response and were duly investigated for basic bio parameters, stool examination, urine routine and culture to asses clinical effect, disease sequel and drug adversity.
Data sheet of patient of diarrhoea attended for treatment at RA Hospital & Research Centre are analysed for the clinical presentation, duration of illness, therapeutics taken, their response and were duly investigated for basic bio parameters, stool examination, urine routine and culture to asses clinical effect, disease sequel and drug adversity.
Based on clinical presentation as per data sheet selected patients were graded for the disease severity as per following index –
| Degree of severity | Characteristic features |
| Mild | Lose motion with mucous without fowl smell Frequency of motion 5/day Mild dehydration |
| Moderate | lose motion with mucous with fowl smell Frequency up to 10 /day Moderate dehydration |
| Severe | Watery fowl smelling stool Frequency >12/day Severe dehydration |
State of dehydration was adjudged as per following [10,11]
| Dehydration status | Characteristics |
| Mild | irritable, thirsty |
| Moderate | irritable, weak pulse, reduced urine output Anterior fontanelle depressed, eye ball sunken, face dry and parched lips and buccal mucosa dry, skin turgor lost thirsty |
| Severe | moribund, apathetic, pulse weak, thread Marked reduced in the urine volume Fontanelle depressed, eye ball markedly sunken Lips parched, face markedly dried and pinched Buccal mucosa dry, loss of skin turgor and thirsty |
Cases of diarrhoea were classified as per therapeutic regime -
Ornidazol and Ofloxacin: Group A
Tinidazol and Norfloxacin: Group B
Tinidazole and Co trimoxazole: Group C
Dose Schedule:
Children: 2.5-5 ml every 12 hours
Adult: 1 tab every 12 hours
Ornidazol and Ofloxacin: Group A
Tinidazol and Norfloxacin: Group B
Tinidazole and Co trimoxazole: Group C
Dose Schedule:
Children: 2.5-5 ml every 12 hours
Adult: 1 tab every 12 hours
Patients of either group were also taking Racecadotril to monitor Sodium Potassium ATPase pump to bioregulate absorption of fluid and electrolyte in following dose schedule -
Children: 10-15mg sachet every 8 hours
Adult: 100mg Cap every 8 hours
Children: 10-15mg sachet every 8 hours
Adult: 100mg Cap every 8 hours
Patients were analysed for outcome of therapy i.e.- decrease in frequency of stool and water loss, change in faecal matter consistency, total duration in achieving formed stool, post diarrhoeal sequel i.e. urinary tract infection, mucous colitis and nephritis.
Post therapy status of stool, urine, haematological, hepatic and renal bio parameters were analysed to ascertain the clinical outcome and safety profile.
In addition any unusual presentation were duly recorded in either group of patients. Clinical response achieved was graded as per following index of achievement
| Clinical grades | Characteristics |
| Grade I (Excellent) | Decline in frequency and change in consistency of Stool, formed stool in 12 hrs. Recovery with minimal Water and electrolyte supplementation, reduced Duration of illness and ultimately cost of therapy Without any drug or diseases related untoward effect No reversal, No post therapy sequel |
| Grade II (Good) | Decline in frequency and change in consistency of stool in 48 hours, recovery on fluid and electrolyte intravenous Supplementation, persistence of 2-4 lose mition daily with post therapy sequel |
| Grade III (Poor) | No response, worsening of diarrhoea |
Observations >
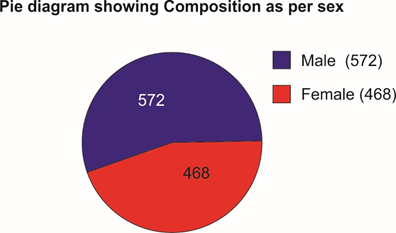
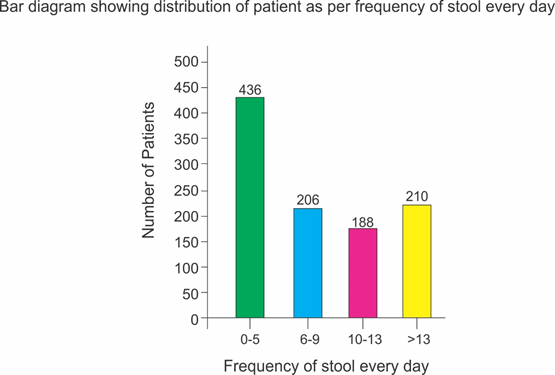
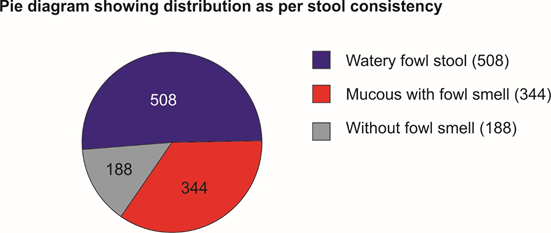
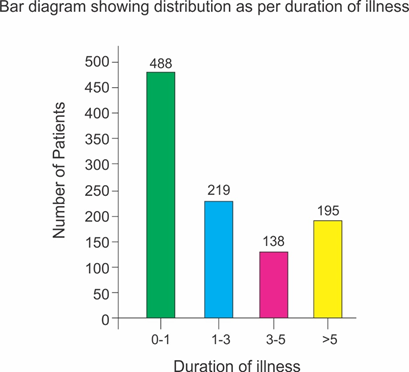
Selected patients were of age group 5-50 years and 572 were male and 468 female (T-1, figure 1). Out of all majority patients 436 (42%) were presenting with 5 motions per day while 210(20.2%) were with >12 motions per day (Figure 2) 508(48%) patients were presenting with watery fowl smelling stool, 344 (33%) with mucous and fowl smell while 188(18.1%) were with non-fowl smelling stool presenting since long duration (figure -3). Out of all 488 patients presented within 24 hours of illness while 198 after 5 days (figure -4). Stool examination reveals 276 (26.5%) viral, 504 (48.5%) bacterial, 140(13.5%) protozoal and 120 (11.5%) parasitic. (T-2). 59.6% patients are on Ofloxacin-Ornidazol, 27% Norfloxacin-Tinidazole and 13.4% on Co trimoxazole-Tinidazole combination (T-3)
Selected patients were of age group 5-50 years and 572 were male and 468 female (T-1, figure 1). Out of all majority patients 436 (42%) were presenting with 5 motions per day while 210(20.2%) were with >12 motions per day (Figure 2) 508(48%) patients were presenting with watery fowl smelling stool, 344 (33%) with mucous and fowl smell while 188(18.1%) were with non-fowl smelling stool presenting since long duration (figure -3). Out of all 488 patients presented within 24 hours of illness while 198 after 5 days (figure -4). Stool examination reveals 276 (26.5%) viral, 504 (48.5%) bacterial, 140(13.5%) protozoal and 120 (11.5%) parasitic. (T-2). 59.6% patients are on Ofloxacin-Ornidazol, 27% Norfloxacin-Tinidazole and 13.4% on Co trimoxazole-Tinidazole combination (T-3)
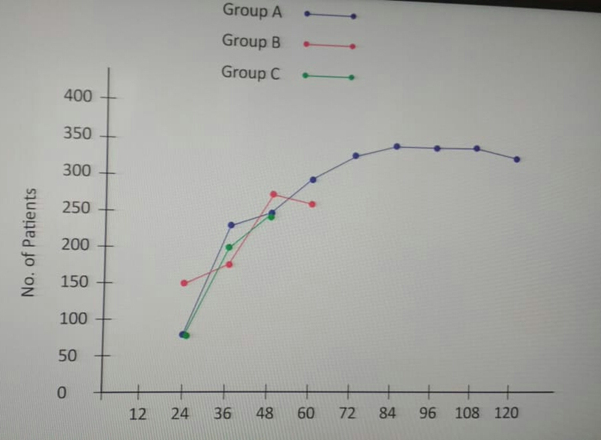
Patients taking Ofloxacin and Ornidazole though achieves changes in faecal matter consistency in 24 hours of therapy but lose motion persist for more than 3-4 days with pain in abdomen. Heaviness in the abdomen recurrent fever, mucous in the stool, lethargy and in some cases (30%) agonizing leg cramps. 46% patients needed fluid and electrolyte intravenous supplementation. (T-4, Figure -5)
| Age group (In years) |
Number of patients | ||
| Male | Female | Total | |
| 5-10 | 28 | 32 | 60 |
| 10-15 | 32 | 24 | 56 |
| 15-20 | 74 | 65 | 139 |
| 20-25 | 64 | 57 | 121 |
| 25-30 | 70 | 64 | 134 |
| 30-35 | 68 | 49 | 117 |
| 35-40 | 105 | 78 | 183 |
| 40-45 | 86 | 62 | 148 |
| 45-50 | 44 | 37 | 81 |
Table 1: Distribution of patients as per age and sex.
| Isolated organism | Number of patients | Percentage |
| Viruses | 276 | 26.5 |
| Bacteria | 504 | 48.5 |
| Protozoa | 140 | 13.5 |
| Helminthes | 120 | 11.5 |
Table 2: Showing distribution of patients as per causative pathogens.
| Therapeutic group | Number of patients | Percentage |
| Ofloxacin -Ornidazole (A) | 620 | 59.6 |
| Norfloxacin-tinidazole (B) | 280 | 27.0 |
| Cotrimexazole-tinidazole (C) | 140 | 13.4 |
Table 3: Showing distribution of patients as per their therapeutic status.
| Clinical presentation | Number of patients | ||
| Group A | Group B | Group C | |
| Nausea | 510 | 12 | 8 |
| Vomiting | 210 | 10 | 2 |
| Abdominal pain | 385 | 6 | - |
| Abdominal distension /heaviness | 402 | 7 | - |
| Uneasiness | 600 | 7 | 02 |
| Dryness of mouth | 578 | 3 | 01 |
| Headache | 108 | 2 | - |
| Dizziness | 103 | - | - |
| Vertigo | 103 | - | - |
| Rash | 84 | 8 | - |
| Pruritis | 84 | 8 | - |
| Insomnia | 128 | - | - |
| Visual disturbances | 34 | - | - |
| Leg cramps | 398 | - | - |
Table 4: Showing presentation during therapy.
Patients taking norfloxacin with tinidazole and co trimoxazole with tinidazole achieved formed stool in 30 hours without any untoward effect like pain in abdomen, mucous colitis, heaviness in abdomen, nausea vomiting and fever. No patients required any fluid and electrolyte replacement.
Post therapy stool examination reveals – 40% patients taking Ornidazole -Ofloxacin, positive for causative pathogen while others taking norfloxacin-tinidazole and Cotrimoxazole-tinidazole shows complete absence of causative pathogen, in addition mucous was predominant in 30% cases on Ornidazole -ofloxacin therapy.
On completion of therapy clinical response grading reveals grade I response in 98% cases of both taking Norfloxacin-Tinidazole and Cotrimoxazole -Tinidazole, 80% cases on ofloxacin- ornidazole shows grade II response in 86% while rest shows grade III response. No patients of either group show any alteration in haematological, hepatic and renal parameters. (Table-5)
| Characteristics | Number of patients | ||
| Group A | Group B | Group C | |
| Duration required for | |||
| Change in faecal matter Consistency | 24 Hrs | 30 Hrs | 36 Hrs |
| Diarrhoea persistence | 3-4 days | - | - |
| Post therapy stool status | |||
| Positive for pathogen | 40% | - | - |
| Sterile | 60% | 98% | 94% |
| Needed fluid & electrolyte | |||
| Replacement | 46% | None | None |
| Post diarrheal mucous colitis | 30% | None | None |
| Post therapy Urine status | |||
| Sterile | All | All | All |
| Status of bio parameter: | |||
| Haemopoietic | Unaltered | Unaltered | Unaltered |
| Hepatic | Unaltered | Unaltered | Unaltered |
| Renal | Unaltered | Unaltered | Unaltered |
| Clinical Grade: | |||
| Grade I | - | 98% | 94% |
| Grade II | 80% | 02% | 06% |
| Grade III | 20% | - | - |
Table 5: Showing outcome of therapy.
Diarrhoea, usually a symptom of intestinal tract infection or intoxication is a second leading cause of death in children i.e.- 1.7-5 millions death per year common in developing country and usually infection is caused by virus, bacteria ,protozoa and parasites. [12,13]
Commonly prescribed antidiarrheals consist of potent antiprotozoal and antibacterial combination irrespective of age considering protozoal infection a common associate but considering diarrhoea etiopathogenesis i.e- [14-16]
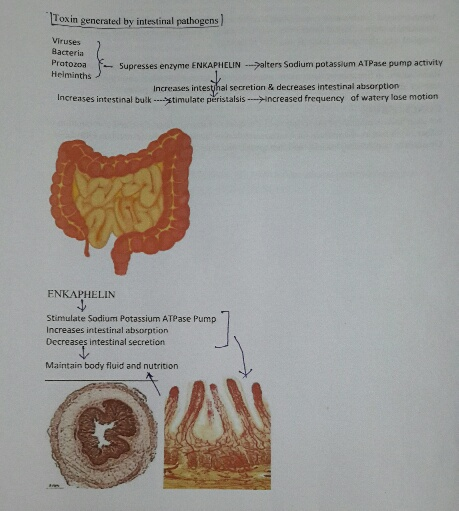
Alteration in Sodium potassium ATPase activity decreases intestinal absorption and increases intestinal mucosal secretion resulting in increased intestinal bulk and irritation of intestinal mucosal nerve ends causing hyperperistalsis presenting as lose motion and electrolyte and water loss.
Thus in present analysis a common composite prescription i.e.- Racecadotril to every patient irrespective of anti-diarrhoeal regime ,which activate the enzyme modulated Sodium Potassium ATPase pump i.e.- Enkephalin ,secreted by the intestinal gland which stimulate Sodium Potassium ATPase pump and promote intestinal absorption and restrict intestinal secretion of intracellular fluid, thus help achieve formed stool. [17-20]
Antiprotozoal and antibacterial are prescribed to combat infection and super infection, as normal commensal become pathogenic due to migration from its normal site. The prescribed composites are Ofloxacin-Ornidazole. Norfloxacin Tinidazole and Co- trimoxazole – Tinidazole.
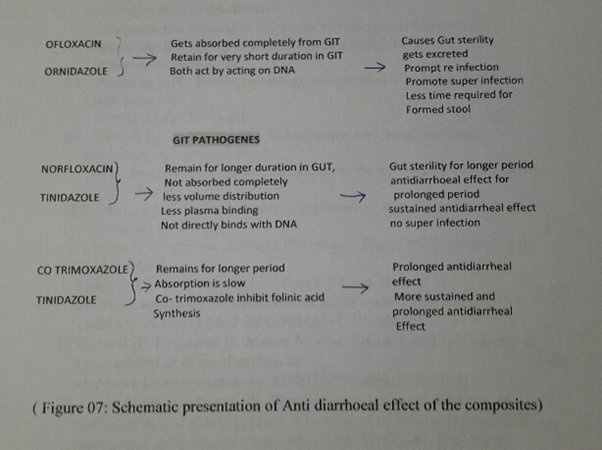
Clinical superiority of two combinations i.e- Tinidazole – Norfloxacin and Tinidazole – cotrimoxazole over commonly prescribed Ofloxacin-Ornidazole, can be explained as [21-29]. Ornidazole, a nitro group of drug reduced by redox protein to reactive nitro radicals, which produces cytocidal action by destabilizing DNA helix and posses high volume distribution. Ofloxacin, a rapidly and completely absorbed after oral ingestion, widely distributed in the body due to its high volume distribution and acts as bactericidal by acting on DNA (DNA gyrase and topoisomerase II & IV), prevent DNA transcription to RNA and subsequent protein synthesis. While in other two combination, antimicrobial absorbs very slowly and posses low volume distribution thus remain longer in the GIT, facilitate longer action on intestinal pathogen ensuring early recovery and cure without any untoward effects.
Thus still Norfloxacin -Tinidazole combination remains best option as antidiarrheal than Ofloxacin-Ornidazole. Hence prescription of Ofloxacin -Ornidazole be restricted for diarrhoea management, considering the therapeutic effect and observed hazards.
Norfloxacin never binds with DNA but binds with substrate DNA, while Ornidazole and Ofloxacin both acts on DNA, poses combined toxicity and hazards commonly observed by patients of either age.
Tinidazole is nitro imidazole which has broad spectrum cidal activity against Protozoa and some anaerobic bacteria. Its selective toxicity to anaerobic microbes enters the cell by diffusion .Nitro group of drug is reduced by redox proteins present only in anaerobic organisms to reactive nitro radical which exerts cytotoxic action by DNA helix destabilization &strand breakage. Co trimoxazole inhibit successive steps in the folate synthesis pathway by its effect on enzyme dehydrofolate reductase (Figure 6, Figure 7)
Result
Antidiarrheal combination constituting Norfloxacin -Tinidazole and Tinidazole -Co trimoxazole shows clinical superiority over Ofloxacin -Ornidazole in outcome, quality of life, hospital stay, therapeutic hazards and cost of therapy.
In addition to bio regulate most cumbersome fluid and electrolyte loss prescription of Racecadotril in therapeutic dose seems mandatory to increase intestinal absorption and restrict intestinal secretion by modulating Sodium potassium ATPase pump.
Conclusion
For diarrhoea management prescription of Racecadotril in prescribed dose and combination of either Norfloxacin -Tinidazole or Cotrimoxazole-tinidazole to be preferred than Ofloxacin -Ornidazole considering toxicity and therapeutic outcome.
References
- Diarrhoeal disease Fact sheet N°330". World Health Organization. April 2013. Archived from the original on 17 July 2014. Retrieved 9 July 2014.
- Gupta P, Murali MV, Seth A. Epidemiology of diarrhea in urban slums. Indian Pediatr. 35 (1998): 147–51. [PubMed]
- Park K. Textbook of Preventive and Social Medicine. 19th ed. Jabalpur, India: Banarsidas Bhanot; 2007. Epidemiology of communicable disease; pp. 142–7.
- "The Treatment of Diarrhea, A manual for physicians and other senior health workers" (PDF). Sometimes needs to be downloaded twice. See "4.2 Treatment Plan A: home therapy to prevent dehydration and malnutrition," "4.3 Treatment Plan B: oral rehydration therapy for children with some dehydration," and "4.4 Treatment Plan C: for patients with severe dehydration" on pages 8 to 16 (12–20 in PDF). See also "8.
- Management of Diarrhoea with Severe Malnutrition" on pages 22–24 (26–30 in PDF) and "Annex 2: Oral and Intravenous Rehydration Solutions" on pages 33–37 (37–41 in PDF). World Health Organization. 2005. Archived (PDF) from the original on 19 October 2011
- .Blacklow NR, Cukor G. Viral gastroenteritis. N Engl J Med. 1981 Feb 12;304(7):397–406.[PubMed]
- DeWitt TG, Humphrey KF, McCarthy P. Clinical predictors of acute bacterial diarrhea in young children. Pediatrics. 1985 Oct;76(4):551–556. [PubMed]
- Shankar ,A etal ; Comparative evaluation of norfloxacin – Tinidazole versus Norfloaxcin _ metronidazole in management of pediatric diarrhea , TheAntisepic Vol 97,No; 10357-359:2000
- Portnoy BL, DuPont HL, Pruitt D, Abdo JA, Rodriguez JT. Antidiarrheal agents in the treatment of acute diarrhea in children. JAMA. 1976 Aug 16;236(7):844–846. [PubMed]
- Shankar A etal ; Norfloxacin in management of Cholera ,presented at International conference of diarrhoeal disease
- Shankar A; Norfloxacin and Tinidazole combination in management of Diarrhea ,presented at IMA national conference 1993 at Calicut ,Published in Current Medical Practice
- Shankar A ,Nalidixic acid and metronidazole combination in management of diarrhoea ,presented at IMA conference Dhanbad 1985 ,published in Current Medical Practice
- Dutta D, Bhattacharya SK, Bhattacharya MK, et al. Efficacy of norfloxacin and doxycycline for treatment of Vibrio cholerae 0139 infection. J Antimicrob Chemother 1996;37:575–81. [PubMed]
- Bassily S, Hyams KG, El-Masry NA, et al. Short-course norfloxacin and trimethoprim-sulfamethoxazole treatment of shigellosis and salmonellosis in Egypt. Am J Trop Med Hyg 1994;51:219–23. [PubMed]
- Gotuzzo E, Oberhelman RA, Maguina C, et al. Comparison of single-dose treatment with norfloxacin and standard 5-day treatment with trimethoprim-sulfamethoxazole for acute shigellosis in adults. Antimicrob Agents Chemother 1989;33:1101–4. [PMC free article] [PubMed]
- Shankar A etal Racecadotril in bioregulation of water and electrolyte flux in diarrhoea
- International Journal of Clinical Chemistry and Laboratory Medicine (IJCCLM) Volume 2, Issue 2, 2016, PP 1-6 www.arcjournals.org
- Farthing MJG, Pathophysiology of infective diarrhoea,Eur.J Gastroenterol Hepatol 1993;5:796-807(Pubmed)
- Turvill JL,Farthing MJG,Enkephalins and enkephalinase inhibitors in intestinal fluid and electrolyte transport , Eur J Gastroenterol Hepatol 1997;9:877-880(Pubmed)
- Farthing MJG,Enkephalinase inhibition ;a rational approach to antisecretory therapy for acute diarrhoea ,Aliment pharmacol Ther 1999;13(suppl -6 ):1-2 (Pubmed)
- Salazar-Lindo E, Santisteban-Ponce J, Chea-Woo E, et al. Racecadotril in the treatment of acute watery diarrhea in children. N Engl J Med 2000; 343:463–7. [PubMed]
- Cezard JP, Duhamel JF, Meyer M, et al. Efficacy and tolerability of
- Racecadotril in acute diarrhoea in children, N. Engl J Med2000:343:463-467 (Pub med)
- "Ofloxacin". The American Society of Health-System Pharmacists. Archived from the original on 28 December 2016. Retrieved 8 December 2016.
- British national formulary: BNF 69 (69 Ed.). British Medical Association. 2015. pp. 409, 757, 782. ISBN 9780857111562.
- WHO Model Formulary 2008 (PDF). World Health Organization. 2009. p. 140. ISBN 9789241547659. Archived (PDF) from the original on 13 December 2016. Retrieved 8 December 2016.
- Pharmacotherapy: Official Journal of the American College of Clinical Pharmacy Print ISSN 0277-0008 Volume: 25 | Issue: 1 Cover date: January 2005 Page(s): 116-118
- Mehlhorn AJ, Brown DA (November 2007). "Safety concerns with fluoroquinolones". Annals of Pharmacotherapy. 41 (11): 1859–66. doi:10.1345/aph.1K347. PMID 17911203.
- Rutgeerts P, Van Assche G, Vermeire S, et al. (April 2005). "Ornidazole for prophylaxis of postoperative Crohn's disease recurrence: a randomized, double-blind, placebo-controlled trial". Gastroenterology. 128 (4): 856–61. doi:10.1053/j.gastro.2005.01.010. PMID 15825069.
- 1020. doi:10.1021/jm00255a026. ISSN 0022-2623.
- Rowen RC1, Michel DJ, Thompson JC. Norfloxacin: clinical pharmacology and clinical use. Pharmacotherapy. 1987;7(4):92-110.
- Hoffer, Max; Grunberg, Emanuel (1974). "Synthesis and antiprotozoal activity of 1-(3-chloro-2-hydroxypropyl)-substituted nitroimidazoles". Journal of Medicinal Chemistry. 17 (9): 1019.
Citation:
Avinash Shankar., et al. “Rationale of Ornidazole and Ofloxacin in Management of Diarrhoea”. Medical Research and Clinical Case
Reports 2.4 (2018): 248-259.
Copyright: © 2018 Avinash Shankar., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.