Research Article
Volume 3 Issue 2 - 2019
Market Assessment Study for Sulbactomax: An Integrated Medicine
Amity University, India
*Corresponding Author: Bhawna Sharma, Amity University, India.
Received: July 04, 2019; Published: July 15, 2019
Abstract
Sulbactomax, an antibiotic combination for the treatment of lower respiratory tract infections, septicemia, meningitis, skin and skin structure infections, urinary tract infections, bone and joint infections, intra-abdominal infections, sexually transmitted infections, and pre and post operative infections, has been marketed in India in the last two decades. Among all the medical practitioners, the pediatrics has the highest utility for this medicine. This research is undertaken to study the pharmaceutical antibiotic market for public health perspective. The study is carried out using data of 200 doctors in major cities in the country ranging from General Surgeon to Urologist. The outcome of this research will be a significant tool in analyzing the current status for integrated medicines (Antibiotics). The basic aim of this research is to provide a basis for marketing strategies for Sulbactomax. Results indicate that the overall satisfaction with the utility of the integrated medicine is high except certain parameters and the property of showing fewer side effects than the traditional antibiotic has a dominant influencing power on the practitioner’s choice for prescription. Further this study also envisages certain points of improvement in the marketing strategy and product promotional policies.
Key Words: Upper respiratory Track Infection; Medicine; Indian Pharma; Antibiotic; Marketing strategies
Introduction
The Indian Pharmaceutical sector had seen tremendous growth over the past three decades. It is currently ranked third in the world and the demand for pharmaceutical drugs is rising. Factors such as: growing population, rising income levels, demand for quality healthcare and the changing lifestyles are responsible for change in disease patterns and hence the increase in demand for new medicines to combat lifestyle related diseases.
Keeping in mind the huge potential of the Indian market, Venus Remedies Ltd, a leading Pharmaceutical company having a huge international presence, also wants to establish itself as a major player in this market too. Venus’s main product range comprises manufacturing of injectables like Onco-Liquid, Onco-lyophilizied, Carbapenem, Prefilled Syringes, Cardiovascular, Small Volume Parentral (SVP), Large Volume Parentral (LVP) and Novel Formulations. Development of world first 14 novel formulations and a grant of 70 patents are some of the major achievements of this company.
In India ,Venus have launched a novel antibiotic combination known as Sulbactomax-an integrated medicine(Levy G. and Nelson, E., pharmaceutical formulation and therapeutic efficacy, Jama, 177,689,1961) for the treatment of Lower Respiratory Tract Infections, Septicemia, Meningitis, Skin & Skin Structure Infections, Urinary Tract Infections, Bone & Joint Infections, Intra Abdominal Infections, Sexually Transmitted Infections and Pre and Post Operative Infections. It is a novel combination of β- Lactam (Ceftriaxone) and β- Lactamase Inhibitor (Sulbactam) along with a chemical vector. Sulbactomax is the breakthrough in antibiotic therapy (Kaplan, S A., Biopharmaceutical consideration in drug formulation design and evaluation, Drug Metlab. Rev., 1, 15, 1972) with triple mechanism of action. It, along with a chemical vector as third ingredient, is used for a wide range of indications due to its unique capability of fighting against resistance (Nigel Shankley Johnson and Johnson pharmaceutical Research and Development, LLC, Merryfield Row, San Diego, California, US)
Rationale of Study
One of the main reasons for the development of Sulbactomax is the emergence of Extended Spectrum ß-Lctamase (ESBLs) as a major threat worldwide with limited treatment options. Carbapenems are the treatment of choice for serious infections due to ESBL-producing organisms, yet Carbapenem-resistant isolates have recently been reported. The available therapeutic options for the treatment of ESBL-associated infections are limited by drug resistance conferred by the ESBLs, along with frequently observed co-resistance to various antibiotic classes, including Cephamycins, Fluoroquinolones, Aminoglycosides, Tetracyclines, and trimethoprim/sulfamethoxazole. All these evidences point towards the development of Sulbactomax which has been developed in such a way that (i) it can kill resistant bacterial strains by multiple mechanisms, (ii) it can break bacterial bio- films and (iii) has efficacy against penem resistant strains.
One of the main reasons for the development of Sulbactomax is the emergence of Extended Spectrum ß-Lctamase (ESBLs) as a major threat worldwide with limited treatment options. Carbapenems are the treatment of choice for serious infections due to ESBL-producing organisms, yet Carbapenem-resistant isolates have recently been reported. The available therapeutic options for the treatment of ESBL-associated infections are limited by drug resistance conferred by the ESBLs, along with frequently observed co-resistance to various antibiotic classes, including Cephamycins, Fluoroquinolones, Aminoglycosides, Tetracyclines, and trimethoprim/sulfamethoxazole. All these evidences point towards the development of Sulbactomax which has been developed in such a way that (i) it can kill resistant bacterial strains by multiple mechanisms, (ii) it can break bacterial bio- films and (iii) has efficacy against penem resistant strains.
Venus had received patent for this product in entire Europe, India, Ukraine, South Africa and Mexico. Sulbactomax with IP protection is an effective means to tackle the growing resistance towards Cephalosporin which has a market worth $9.7 billion globally (http://www.venusmedicineresearchcentre.com).
Research Objectives
The main objective of this research is to study and analyze the major factors in the current therapeutic use of Integrated Medicines (Antibiotics) and to find the Marketing loopholes for Sulbactomax which will help the company to increase its presence in the Indian market.
The main objective of this research is to study and analyze the major factors in the current therapeutic use of Integrated Medicines (Antibiotics) and to find the Marketing loopholes for Sulbactomax which will help the company to increase its presence in the Indian market.
A survey (Fowler, F.J. (2002), Survey Research methods (3rd ed). Newbury Park, C A: sage), which takes into account the opinion of more than 200 doctors around the country has been conducted. An effort has been to elucidate the market gap analysis for Sulbactomax with primary, quantitative and qualitative data and modeling them to fit the marketing gaps.
Research Design
The feedback about the effectiveness of Sulbactomax was collected from more than 200 doctors in major cities in the country (Fink, A., & Kosekoff, J. (1998). How to conduct surveys: A step-by-step guide (Baverly Hills, CA: Sage). A brief distribution of the sample is shown in Table 1.
The feedback about the effectiveness of Sulbactomax was collected from more than 200 doctors in major cities in the country (Fink, A., & Kosekoff, J. (1998). How to conduct surveys: A step-by-step guide (Baverly Hills, CA: Sage). A brief distribution of the sample is shown in Table 1.
| Region | City | General Surgeon | General Physician | General Surgeon | Gynecologist | Pediatrician | Pediatrician | Urologist | Grand Total |
| East | Jorhat (Assam) | 1 | 1 | ||||||
| Kolkata | 1 | 1 | 2 | 3 | 7 | ||||
| Shillong | 1 | 1 | |||||||
| Total | 0 | 1 | 0 | 2 | 0 | 3 | 3 | 9 | |
| North | Agra | 3 | 1 | 4 | |||||
| Aligarh | 5 | 1 | 6 | ||||||
| Alwar | 2 | 2 | 1 | 5 | |||||
| Bikaner | 1 | 1 | 2 | ||||||
| Chandigarh | 1 | 1 | 1 | 2 | 2 | 7 | |||
| Delhi | 2 | 2 | 1 | 1 | 2 | 3 | 11 | ||
| Firajpur Jhirka | 1 | 1 | |||||||
| Jaipur | 3 | 2 | 3 | 4 | 1 | 3 | 16 | ||
| Lucknow | 3 | 3 | 3 | 1 | 1 | 3 | 14 | ||
| Ludhiana | 3 | 3 | |||||||
| Mohali | 1 | 1 | 2 | ||||||
| Panchkula | 1 | 1 | |||||||
| Pratapgarh | 1 | 1 | |||||||
| Varanasi | 2 | 2 | 1 | 1 | 6 | ||||
| Total | 8 | 24 | 9 | 13 | 5 | 7 | 13 | 79 | |
| South | Bangalore | 1 | 3 | 1 | 7 | 2 | 2 | 5 | 21 |
| Chennai | 1 | 2 | 1 | 4 | |||||
| Hyderabad | 2 | 2 | 2 | 2 | 1 | 2 | 11 | ||
| Loni | 1 | 1 | |||||||
| Malakhera | 1 | 1 | |||||||
| Manipal | 1 | 1 | |||||||
| Mysore | 1 | 1 | |||||||
| Pondicherry | 1 | 1 | |||||||
| Secundrabad | 1 | 1 | |||||||
| vijaywada | 2 | 2 | |||||||
| Total | 2 | 6 | 5 | 10 | 6 | 5 | 10 | 44 | |
Table 1: Respondent Demography.
| Region | City | General Surgeon | General Physician | General Surgeon | Gynecologist | Pediatrician | Pediatrician | Urologist | Grand Total |
| West | Ahmedabad | 1 | 3 | 1 | 5 | 3 | 2 | 4 | 19 |
| Amreli, Gujrat | 1 | 1 | |||||||
| Baroda | 1 | 1 | 2 | ||||||
| Basti | 1 | 1 | 2 | ||||||
| Bhopal | 1 | 3 | 4 | ||||||
| Goa | 1 | 1 | |||||||
| Indore | 2 | 1 | 1 | 4 | |||||
| Kanpur | 1 | 1 | |||||||
| Mumbai | 1 | 4 | 3 | 1 | 1 | 4 | 14 | ||
| Pune | 2 | 2 | 4 | 1 | 3 | 2 | 14 | ||
| Solapur (Maharashtra) | 1 | 1 | |||||||
| Surat | 1 | 1 | 2 | 1 | 2 | 7 | |||
| Total | 1 | 9 | 14 | 18 | 7 | 7 | 14 | 70 | |
| Grand Total | 11 | 40 | 28 | 43 | 18 | 22 | 40 | 202 | |
Table 1: Respondent Demography.
The target market segment for the product was framed by pinpointing the utility of the drug to various groups of specialized medical practitioners. Respondents (Doctors) were requested to rate the product (Gaito, J). Measurement Scales and statistics) as per its medical importance: Critically Important, Very Important and Important, depending on their respective experiences and perception (Weights were assigned to each of the ratings based on the rational that ‘critically Important’ is having the highest value and ‘Important’ is having the lowest.
Weighted Average for responses (Cochran, W.G), pertaining to each of the specialization were listed down and their respective standard deviations and coefficient of variations (Mosteller, F., Feinberg, S. E., & Rourke, R. E). The results are mentioned in table 2:
| Specialization | Critically important (3) | C.I- wt. (3) | Important (1) | I.- wt. (1) | Very important (1) | V.I.- wt. (2) | Total | Wt.avg mean | Stdv | CV | Priority Index |
| General Surgeon | 6 | 18 | 9 | 9 | 24 | 48 | 63 | 10.50 | 20.42 | 32% | 2 |
| General Physician | 7 | 21 | 12 | 12 | 21 | 42 | 61 | 10.17 | 15.39 | 25% | 3 |
| Gynaecologist | 5 | 15 | 12 | 12 | 26 | 52 | 69 | 11.50 | 22.28 | 32% | 2 |
| Paediatrician | 7 | 21 | 7 | 7 | 26 | 52 | 66 | 11.00 | 23.03 | 35% | 1 |
| Urologist | 7 | 21 | 12 | 12 | 21 | 42 | 61 | 10.17 | 15.39 | 25% | 3 |
| Grand Total | 32 | 52 | 118 | 236 | 320 |
Table 2: Determining the target segment priority in marketing sulbactomax.
The higher CV value is ranked as ‘1’, showing the maximum utility of the concerned product (Sulbactomax) and the lowest percentage value is ranked lowest, 3 representing the least importance. A relationship between different factors influencing the decision of the medical practitioner in prescribing the drug and the satisfaction level derived out has been developed by using Chi-Square test of independence at 5 per cent level significance.
Prescription Likeliness Vs Side Effect Removal of Cefriaxone/Cefriaxone + Sulbactum
This analysis describes the level of interdependence between importance of the concerned drug in removing the side effect of cefriaxone/cefriaxone + Sulbactum and the likeliness of practitioners prescribing the drug for usage.
This analysis describes the level of interdependence between importance of the concerned drug in removing the side effect of cefriaxone/cefriaxone + Sulbactum and the likeliness of practitioners prescribing the drug for usage.
Satisfied with Efficacy of Current Cefriaxone/cefriaxone + Sulbactum Vs Disease Relapse
This analysis describes the level of interdependence between importance of the concerned drug in removing the chances of disease relapse and the satisfaction level of the medical practitioners derived from the usage of the integrated medicine.
This analysis describes the level of interdependence between importance of the concerned drug in removing the chances of disease relapse and the satisfaction level of the medical practitioners derived from the usage of the integrated medicine.
Satisfied with efficacy of current Cefriaxone/Cefriaxone Vs Duration of Usage
This analysis describes the level of interdependence between importance of the concerned drug in reducing the duration of usage and the satisfaction level of the medical practitioners derived from the usage of the integrated medicine.
Satisfied with Efficacy of Current Cefriaxone/Cefriaxone + Sulbactum Vs Disease Relapse Vs Drug Resistance removal
This analysis describes the level of interdependence between importance of the concerned drug in reducing chances of disease relapse and the satisfaction level of the medical practitioners derived from the usage of the integrated medicine.
This analysis describes the level of interdependence between importance of the concerned drug in reducing chances of disease relapse and the satisfaction level of the medical practitioners derived from the usage of the integrated medicine.
Prescription Likeliness Vs Disease Relapse
This analysis describes the level of interdependence between importance of the concerned drug in reducing the cases of disease relapse and the likeliness of practitioners prescribing the drug for usage.
This analysis describes the level of interdependence between importance of the concerned drug in reducing the cases of disease relapse and the likeliness of practitioners prescribing the drug for usage.
Prescription Likeliness Vs Bacterial Biofilm Rupturing Property
This analysis describes the level of interdependence between importance of the concerned drug in reducing the cases of disease relapse and the likeliness of practitioners prescribing the drug for usage. The Chi- contingency table for above listed factors is shown in Table 3:
This analysis describes the level of interdependence between importance of the concerned drug in reducing the cases of disease relapse and the likeliness of practitioners prescribing the drug for usage. The Chi- contingency table for above listed factors is shown in Table 3:
| Particulars | Approx. Significance |
| Prescription Likeliness Vs Side effect Removal of cefriaxone/cefriaxone + Sulbactum | 0.001 |
| Satisfied with efficacy of current cefriaxone/cefriaxone + Sulbactum Vs Disease Relapse | 0.13 |
| Satisfied with efficacy of current cefriaxone/cefriaxone Vs Duration of Usage | 0.537 |
| Satisfied with efficacy of current cefriaxone/cefriaxone + Sulbactum Vs Drug resistance removal | 0.002 |
| Prescription Likeliness Vs Disease Relapse | 0.566 |
| Prescription Likeliness Vs Bacterial Biofilm Rupturing Property | 0.064 |
Table 3: Chi-Square Contingency Coefficient chart (α= .05).
Table 3 shows that
- The side effect removal property of Sulbactomax significantly influences medical practitioners to consider the drug in their choice while prescribing to patients for usage.
- The satisfaction derived out of usage of integrated medicine (cefriaxone/cefriaxone + Sulbactum) on the factor of its drug resistance inhibiting property is considerable.
The related cases were analyzed using contingency table (Conover, W. J., Practical Nonparametric Statistics, 3rd ed. (New York: Wiley, 2000)), and customer satisfaction level was tested against the importance in choosing the drug for prescription and satisfaction derived out of the use of the integrated medicine respectively using SERVQUAL platform.
SERVQUAL is the most widely used scale for measuring service quality based on the GAP analysis. It is operationalized as Q = P - E, where Q = Service Quality and P = customers perception of service and E = Customer’s service expectation.
The satisfaction level derived out of the usage of integrated medicine (cefriaxone/cefriaxone + Sulbactum) is shown in Table 4:
| Looking for a new alternative | Partially satisfied | Total | |||
| Whether there are problems of bacterial resistance/ESBL (Extended Spectrum ?-Lactamases) with Ceftriaxone | No | Count | 0 | 61 | 61 |
| Expected Count | 7.1 | 53.9 | 61 | ||
| within Whether there are problems of bacterial resistance/ESBL (Extended Spectrum ?-Lactamases) with Ceftriaxone | 0.00% | 100.00% | 100.00% | ||
| within Satisfied with the efficacy of current Ceftriaxone/Ceftriaxone + Sulbactam | 0.00% | 36.70% | 32.40% | ||
| % Total | 0.00% | 32.40% | 32.40% | ||
| Seldom | Count | 4 | 28 | 32 | |
| Expected Count | 3.7 | 28.3 | 32 | ||
| % within Whether there are problems of bacterial resistance/ESBL (Extended Spectrum ?-Lactamases) with Ceftriaxone | 12.50% | 87.50% | 100.00% | ||
| % within Satisfied with the efficacy of current Ceftriaxone/Ceftriaxone + Sulbactam | 18.20% | 16.90% | 17.00% | ||
| % of Total | 2.10% | 14.90% | 17.00% | ||
| Yes | Count | 18 | 77 | 95 | |
| Expected Count | 11.1 | 83.9 | 95 | ||
| % within Whether there are problems of bacterial resistance/ESBL (Extended Spectrum ?-Lactamases) with Ceftriaxone | 18.90% | 81.10% | 100.00% | ||
| % within Satisfied with the efficacy of current Ceftriaxone/Ceftriaxone + Sulbactam | 81.80% | 46.40% | 50.50% | ||
| % of Total | 9.60% | 41.00% | 50.50% | ||
Table 4: Satisfied with the efficacy of current Ceftriaxone/Ceftriaxone + Sulbactam.
In the cross-tabulated data the responses for the questions about the problem of drug resistance were taken against the responses about the satisfaction level derived from the utility of integrated medicine. The observed values and the expected values as calculated were arranged in the form of SERVQUAL Table 5 to perform the Gap analysis.
| Satisfaction/Perception | (O) | (E) | (O – E )/ E | %Satisfaction |
| Looking for a new alternative/No | 0 | 7.1 | 1 | 0% |
| Partially satisfied/No | 61 | 53.9 | -0.13173 | 113% |
| Looking for a new alternative/Seldom | 4 | 3.7 | -0.08108 | 108% |
| Partially satisfied/Seldom | 28 | 28.3 | 0.010601 | 99% |
| Looking for a new alternative/Yes | 18 | 11.1 | -0.62162 | 162% |
| Partially satisfied/Yes | 77 | 83.9 | 0.082241 | 92% |
Table 5: SERVQUAL table to perform the Gap analysis.
In Table 5, the measurement of error deviation and the deviation less than 100% satisfaction level gives the current satisfaction level for different groups of respondents.
Property of Sulbactomax Influencing the Decision of Medical Practitioners
To understand the level of influence of the side effect reduction property of the antibiotic in influencing the practitioner’s prescription decision, the answers given by the respondent doctors against their satisfaction from the side effect reduction property of Cefriaxone/Cefriaxone + Sulbactum were recorded in Table 6 along with the respective doctor’s willingness to prescribe the medicine.
To understand the level of influence of the side effect reduction property of the antibiotic in influencing the practitioner’s prescription decision, the answers given by the respondent doctors against their satisfaction from the side effect reduction property of Cefriaxone/Cefriaxone + Sulbactum were recorded in Table 6 along with the respective doctor’s willingness to prescribe the medicine.
| After reviewing scientific data | Not without published evidence | Without hesitation | Total | |||
| Expectation from Sulbactomax in removing side effects of Ceftriaxone/Ceftriaxone + Sulbactam | Can't Say | Count | 4 | 0 | 1 | 5 |
| Expected Count | 2.3 | 0.5 | 2.2 | 5 | ||
| % within Should be a solution that virtually removes side effects of Ceftriaxone/ Ceftriaxone + Sulbactam | 80.00% | 0.00% | 20.00% | 100.00% | ||
| % within Would you like to prescribe an antibiotic (Sulbactomax) | 4.70% | 0.00% | 1.20% | 2.70% | ||
| % of Total | 2.10% | 0.00% | 0.50% | 2.70% | ||
| No | Count | 19 | 5 | 3 | 27 | |
| Expected Count | 12.4 | 2.6 | 12.1 | 27 | ||
| % within Should be a solution that virtually removes side effects of Ceftriaxone/Ceftriaxone + Sulbactam | 70.40% | 18.50% | 11.10% | 100.00% | ||
| % within Would you like to prescribe an antibiotic (Sulbactomax) | 22.10% | 27.80% | 3.60% | 14.40% | ||
| % of Total | 10.10% | 2.70% | 1.60% | 14.40% | ||
| Yes | Count | 63 | 13 | 80 | 156 | |
| Expected Count | 71.4 | 14.9 | 69.7 | 156 | ||
| % within Should be a solution that virtually removes side effects of Ceftriaxone/Ceftriaxone + Sulbactam | 40.40% | 8.30% | 51.30% | 100.00% | ||
| % within Would you like to prescribe an antibiotic (Sulbactomax) | 73.30% | 72.20% | 95.20% | 83.00% | ||
| % of Total | 33.50% | 6.90% | 42.60% | 83.00% | ||
Table 6: Likely Ness in Prescribing Sulbactomax.
| Likelyness To Prescribe/Perception | (O) | (E) | ( E- O)/(E) | %Satisfaction Level |
| After reviewing scientific data/Can't Say | 4 | 2.3 | -0.74 | 174% |
| Not without published evidence/Can't Say | 0 | 0.5 | - | - |
| Without hesitation/Can't Say | 1 | 2.2 | 0.55 | 45% |
| After reviewing scientific data/No | 19 | 12.4 | -0.53 | 153% |
| Not without published evidence/No | 5 | 2.6 | -0.92 | 192% |
| Without hesitation/No | 3 | 12.1 | 0.75 | 25% |
| After reviewing scientific data/Yes | 63 | 71.4 | 0.12 | 88% |
| Not without published evidence/Yes | 13 | 14.9 | 0.13 | 87% |
| Without hesitation/Yes | 80 | 69.7 | -0.15 | 115% |
Table 7: Chi –Square Contingency Table.
In Table 7, measurement of error deviations and the deviation less than 100% satisfaction level gives the current satisfaction level for different groups of respondents. The table distributes the respondent group into the following subgroups-
1. Those who are not sure about the side effect removal property of Sulbactomax, but
• Rely on scientific data to prescribe it.
• Are unwilling to prescribe them without published evidence.
• Can prescribe them without hesitation.
2. Those who think that Sulbactomax cannot be a solution for side effect reduction of the antibiotic, but
• Can prescribe them after reviewing scientific data.
• Can prescribe them after reviewing published evidence.
• Can prescribe them without hesitation.
3. Those who think that Sulbactomax can be a solution for side effect reduction of the antibiotic ,but
• Can prescribe them without hesitation.
• Can prescribe them after reviewing published evidence.
• Can prescribe them after reviewing scientific data.
• Rely on scientific data to prescribe it.
• Are unwilling to prescribe them without published evidence.
• Can prescribe them without hesitation.
2. Those who think that Sulbactomax cannot be a solution for side effect reduction of the antibiotic, but
• Can prescribe them after reviewing scientific data.
• Can prescribe them after reviewing published evidence.
• Can prescribe them without hesitation.
3. Those who think that Sulbactomax can be a solution for side effect reduction of the antibiotic ,but
• Can prescribe them without hesitation.
• Can prescribe them after reviewing published evidence.
• Can prescribe them after reviewing scientific data.
The percentage satisfaction level shows the likeliness of each of the sub-groups for prescribing the medicine for usage. To achieve a high demand pool it is required to have 100% or above satisfaction level for each of the groups and the others is required to be converted.
Results with Details of Discussions
Determining the target segment priority in marketing Sulbactomax
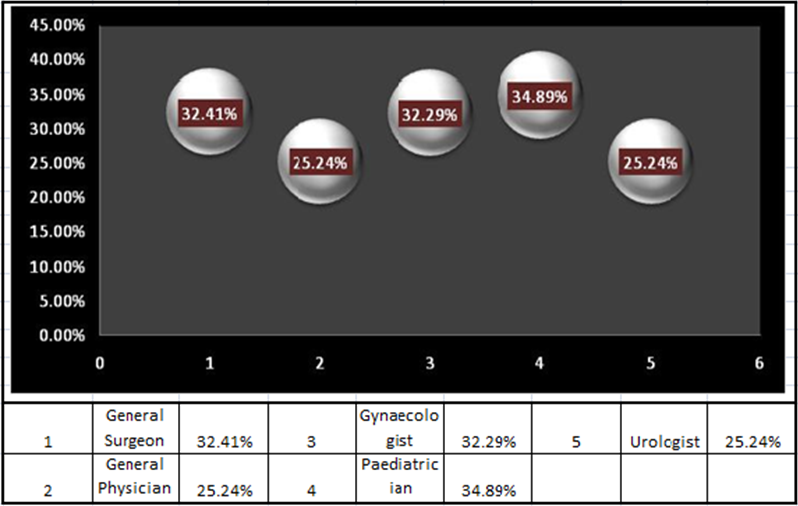
The following graphical representation of the priority matrix shows that the Pediatricians have a very high utility of the product (Sulbactomax) when compared with others.
The following graphical representation of the priority matrix shows that the Pediatricians have a very high utility of the product (Sulbactomax) when compared with others.
The coefficient of variance of percentage of the Pediatricians is 34.89% followed by Gynecologists and General surgeon at 32.41%, and the General Physicians and Urologists at 25.24%. Hence Pediatricians are supposed to have the highest utility of the antibiotic than the others and must be using them to treat diseases like Pneumonia. So, to effectively market the product and to have a high return on the marketing initiative a campaign may be launched involving the pediatricians to highlight them about the better usage of the drug and to share the reports of clinical trials to show them the high efficacy of the drug for pediatric purpose.
General Surgeons and the Gynecologists can be taken into priority and can be approached to inform them about the probable usage of the drug in their respective fields by marketing executives/ Medical Representatives and an idea about their expectations from the medicine can be taken as a feedback, which can be further analyzed to favorably position the drug.
The cause of the low importance of the drug for the General Physicians and Urologists can also be analyzed in the aforesaid manner and the data can be analyzed for probable resolution.
The satisfaction level derived out of the usage of integrated medicine as a solution against drug resistance.
Responses were analyzed about drug resistance experienced against Cefriaxone. This data was used to carry out the Gap analysis to understand the level of satisfaction out of integrated medicine for drug resistance inhibition. The analysis divides the respondents into following sub-groups-
1. Those who are not sure about drug resistance of pathogens against Cefriaxone and are
• Still looking for new alternative.
• Partially satisfied with the efficacy of Ceftriaxone/Ceftriaxone + Sulbactam.
2. Those who find seldom cases of drug resistance against cefriaxone and are
• Partially satisfied with the efficacy of Ceftriaxone/Ceftriaxone+ Ulbactam.
• Looking for new product alternatives.
3. Those who accept that there are cases of drug resistance against Cefriaxone and are
• Looking for new alternatives.
• Partially satisfied with the efficacy of Ceftriaxone/Ceftriaxone + Sulbactam.
Responses were analyzed about drug resistance experienced against Cefriaxone. This data was used to carry out the Gap analysis to understand the level of satisfaction out of integrated medicine for drug resistance inhibition. The analysis divides the respondents into following sub-groups-
1. Those who are not sure about drug resistance of pathogens against Cefriaxone and are
• Still looking for new alternative.
• Partially satisfied with the efficacy of Ceftriaxone/Ceftriaxone + Sulbactam.
2. Those who find seldom cases of drug resistance against cefriaxone and are
• Partially satisfied with the efficacy of Ceftriaxone/Ceftriaxone+ Ulbactam.
• Looking for new product alternatives.
3. Those who accept that there are cases of drug resistance against Cefriaxone and are
• Looking for new alternatives.
• Partially satisfied with the efficacy of Ceftriaxone/Ceftriaxone + Sulbactam.
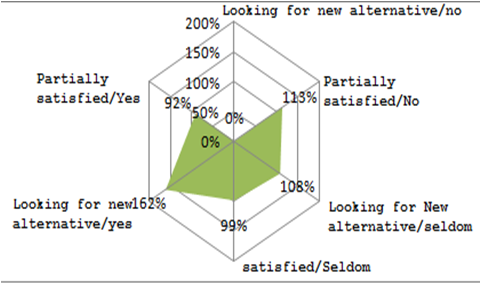
Graphical representation shows that the satisfaction level of ‘those who find seldom cases of drug resistance against cefriaxone and are partially satisfied with the efficacy of Ceftriaxone/Ceftriaxone + Sulbactam’ is at 99%. All other sub-groups have a high satisfaction level. Only the satisfaction level of those who do not feel that there is a drug resistanceproblem with Cefriaxone but still are looking for new alternatives have their satisfaction level at ‘0%’.
The high level of satisfaction across different sub-groups reflects that there is not much pull in the market for a new product on account of β-lactamase inhibition. However, the loophole identified is in the segment where practitioners are not sure about β-lactamase inhibition of Cefraxione.
Marketing initiatives may be undertaken by distributing journals and compilations of cases about drug resistance against cefriaxone and the scientific reports of the product’s capability of reducing β-lactamese inhibition and other USPs.
Medical conferences can also be held at regional/central level involving practitioners to discuss about ‘Drug Resistance’ cases amongst them and presentations about the efficacy of the product may be delivered.
Side effect reduction property of Sulbactomax in influencing the decision of medical practitioners in considering the medicine for Prescription
The analysis was carried out by creating a cross tabulation between the answers given by the respondents about their perception on the product’s efficiency in removing the side effects of Cefriaxone and their level of willingness to prescribe the drug to their patients for usage.
The analysis was carried out by creating a cross tabulation between the answers given by the respondents about their perception on the product’s efficiency in removing the side effects of Cefriaxone and their level of willingness to prescribe the drug to their patients for usage.
The below mentioned figure represents the willingness of the practitioners to prescribe Sulbactomax to their patients on account of their side effect reduction property of the core antibiotic Cefriaxone in form of percentage satisfaction.
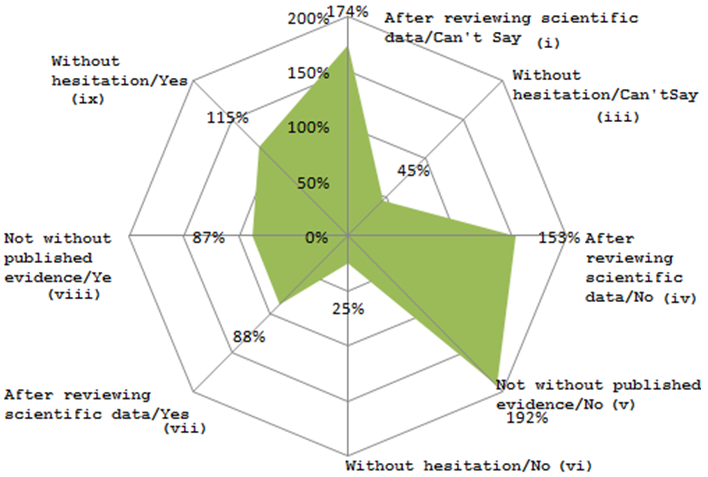
All other groups except sub-group (iii), (vi), (vii), (ix) shows high level of satisfaction. However, for group (iii), the satisfaction level is at only 45%. The low value may be due to their low perception about the product since they do not go with the idea that the new drug can have fewer side effects than the conventional one.
For group (vi), the satisfaction level is 25%. The low value may have resulted as the respondents concerned do not accept that the new product can have fewer side effects than the traditional one though they are willing to prescribe it without hesitation.
For group (vii), the satisfaction level is at 88%. Here the respondents are willing to review scientific data as they lack confidence about the efficacy of the new drug though they do also perceive that Sulbactomax can have fewer side effects than the traditional alternatives.
For group (viii), the satisfaction level is at 87%. Here the respondents are willing to review published evidence as they lack confidence about the efficacy of the new drug though they do also perceive that Sulbactomax can have fewer side effects than the traditional alternatives.
Conclusion and Recommendation
It can be concluded from the result that few initiatives may be undertaken in developing the marketing strategy for Sulbactomax-
- The competitive advantage of the new product over the traditional cefriaxone and Cefriaxone+sulbactum complex should be highlighted.
- There is a requirement to highlight the drug resistance pattern of pathogens against Cefriaxone through different scientific research evidences and published figures.
- Salient features of the product viz. fewer side effects than the traditional antibiotics should be discussed with key practitioners for better positioning of the product.
References
- FOWLER FJ. Survey research methods, Newbury Park, CA: Sage (2002)
- Johnston, L.D., O’ MALLEY, P.M., BACHMAN, J.G. (1991). Drug use among American high school Seniors, college student and young adults, 1975-1990 (2 Volumes), Rockville, MD: National Institute of Drug Abuse.
- YIN RK. “Case study research: Design and methods”. (2nd Edition). Newbury Park, CA: Sage (1994)
- Daniel ww. “Applied Nanparametric statistics”. 2nd edition (Boston: PWS kent, 1990)
- Hollander M and DA Wolfe. “Nonparametric Statistical Methods”. Newyork: Willey, (1999).
- Fisher, R. A., and F. Yates, Statistical Tables for Biological, Agricultural and Medical Research, 5th ed. (Edinburgh: Oliver and Boyd, 1957)
- Levy G and Nelson E. “Pharmaceutical formulation and therapeutic efficacy”. JAMA 177 (1961): 689.
- Nigel Shankley Johnson & Johnson Pharmaceutical Research & Development, LLC, Merryfield Row, San Diego, California, U.S.
- http://www.venusremedies.com
- http://www.venusmedicineresearchcentre.com
- http://www.venuspharmagmbh.de
Citation:
Bhawna Sharma and J.K Sharma. “Market Assessment Study for Sulbactomax: An Integrated Medicine”. Medical Research and Clinical Case Reports 3.2 (2019): 50-60.
Copyright: © 2019 Bhawna Sharma. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.