Research Article
Volume 1 Issue 1 - 2018
Antibody Response in Broiler Chickens Infected with Different Developmental Stages of Eimeria tenella
1Department of Veterinary Parasitology and Entomology, Faculty of Veterinary Medicine, University of Jos, Nigeria
2Department of Veterinary Parasitology and Entomology, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria, Nigeria
3Department of Veterinary Medicine, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria, Nigeria
2Department of Veterinary Parasitology and Entomology, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria, Nigeria
3Department of Veterinary Medicine, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria, Nigeria
*Corresponding Author: Kaze Paul Davou, Department of Veterinary Parasitology and Entomology, Faculty of Veterinary Medicine, University of Jos, Nigeria.
Received: February 17, 2018; Published: March 06, 2018
Abstract
Antibodies (IgG or IgY) titre values were higher in broilers sera infected with sporulated oocyst and merozoites reaching a peak on day 10 of post primary and secondary infections and day 5 post tertiary infection in sera of broilers (treated and non- treated). At tertiary infection, antibodies increases at day 5, 7, 11 and 14 indicating that antibodies increases in broilers infected with the invasive or zoite stages, (sporozoite and merozoite) of the parasite. There was a significant difference in the antibody output between the sera of the broiler groups (p < 0.05).
Keywords: Antibodies; Sera; Sporulated oocyst; Merozoites; Primary-secondary-tertiary infections; Broilers; invasive or zoite stages; Parasite
Abbreviations: IgG: Immunoglobulin G; IgY: Immunoglobulin Y
Introduction
Coccidiosis caused by protozoans of genus Eimeria tenella is a chicken parasitic disease of great economical importance globally characterized by haemorrhage leading to mortality. Conventional disease control strategies depend on vaccination and prophylactic use of anticoccidial drugs. Research has been carried out worldwide to try to elucidate the mechanism of protective immunity against coccidiosis. It was concluded from early studies that cellular immunity is the key to protection against Eimeria, whereas humoral immunity plays a very minor role in resistance against infection. By contrast, other studies have pointed towards the ability of antibody to block parasite invasion, development and transmission and to provide passive and maternal immunity against challenge infection.
Herein, recent results demonstrate the ability of antibodies (raised by live immunization or against purified stage-specific Eimeria antigens) to inhibit parasite development in vitro and in vivo and readdress the question of the role of antibody in protection against coccidiosis (Wallach, M., 2010). Enzyme-linked immunosorbent assay has already been used extensively to measure anti-Eimeria antibodies in chickens (Smith., et al. 1994). The aim of the study was to determine the antibody response in Broilers infected with different developmental stages of Eimeria tenella.
Materials and Methods
Study Area
The experimental settings was at the PETCA building, Anguldi, 5 kilometers from the National Veterinary Research Institute, Vom, Jos Plateau State, Nigeria, where the laboratory work was carried out. The Jos Plateau lies on the pre-cambian from the cambian to jurasic northern Nigeria crystalline complex in central Nigeria. Its average elevation is about 1,250m above mean sea level. The state is bounded on the north and west by Kaduna plains (on the average of 600 m above mean sea level) and on the south by Benue plains (on the average of 700m above mean sea level), (PADP, 2002). Geographically, the Jos Plateau is located between latitude 08°24'N and longitude 008°32' and 010°38' east.
The experimental settings was at the PETCA building, Anguldi, 5 kilometers from the National Veterinary Research Institute, Vom, Jos Plateau State, Nigeria, where the laboratory work was carried out. The Jos Plateau lies on the pre-cambian from the cambian to jurasic northern Nigeria crystalline complex in central Nigeria. Its average elevation is about 1,250m above mean sea level. The state is bounded on the north and west by Kaduna plains (on the average of 600 m above mean sea level) and on the south by Benue plains (on the average of 700m above mean sea level), (PADP, 2002). Geographically, the Jos Plateau is located between latitude 08°24'N and longitude 008°32' and 010°38' east.
The land surface of Jos Plateau consists of plains, hills, depressions and todes of various forms, shapes and sizes. It is a major tourist centre in Nigeria with agriculture as the main occupation of the people. The high altitude confers on the Plateau lower temperature than those encountered elsewhere in Nigeria except the Obudu and Mambilla Plateau. The dry season is determined by the north easterly tropical continental air masses known as harmattan (from October-April) and the wet season is the most tropical maritime air masses from May-September. The average annual rainfall is about 1,100 mm and is evenly distributed. Another element of climate is temperature December and January experience temperatures of below 150C. During February and March, the temperature rises again about 25°C. Most of the human activities are mining and agriculture involving rearing of chickens in both the rural and urban areas for subsistency and income (PADP, 2002).
Experimental Birds
Four hundred (400) day-old broilers (marshal breed) were purchased from ECWA farms, Jos, brooded and used for the study. The birds were randomly distributed into six different groups of 40 each, in a clean wire cage (n = 40). At two weeks old, each group was again subdivided into two, treated and none treated, of twenty broilers (n = 20) each. The birds were kept in a clean building, and the legs banded or labelled under strict biosecurity measures. Feed (Broiler starter, Grand cereals and oil mills, PLC, Zawan, and Jos-Plateau, Nigeria) and water were provided adlibitum the birds were vaccinated with Newcastle disease vaccine (La-Sota) at day 21 and Gomboro disease vaccines at days 14 and 28.
Four hundred (400) day-old broilers (marshal breed) were purchased from ECWA farms, Jos, brooded and used for the study. The birds were randomly distributed into six different groups of 40 each, in a clean wire cage (n = 40). At two weeks old, each group was again subdivided into two, treated and none treated, of twenty broilers (n = 20) each. The birds were kept in a clean building, and the legs banded or labelled under strict biosecurity measures. Feed (Broiler starter, Grand cereals and oil mills, PLC, Zawan, and Jos-Plateau, Nigeria) and water were provided adlibitum the birds were vaccinated with Newcastle disease vaccine (La-Sota) at day 21 and Gomboro disease vaccines at days 14 and 28.
Experimental infection of broilers with infective materials and monitoring
The experimental birds, except the control were orally given primary and secondary challenge infections with the various developmental stages of Eimeria tenella, respectively at week 2 and 3 1/2 while at week 5 of age, all birds were infected the sporulated oocyst of the parasite (Table 1). Each group was subdivided into Treated (n = 20) and Non- Treated (n = 20). In each infected group, birds in one of the subdivisions were treated with amprolium 250 WSPR Holland was administered in drinking water at a concentration of 250 mg/1 (0.025%) for a period of 5 days as prescribed by the Manufacturer at the appearance of visible clinical signs.
The experimental birds, except the control were orally given primary and secondary challenge infections with the various developmental stages of Eimeria tenella, respectively at week 2 and 3 1/2 while at week 5 of age, all birds were infected the sporulated oocyst of the parasite (Table 1). Each group was subdivided into Treated (n = 20) and Non- Treated (n = 20). In each infected group, birds in one of the subdivisions were treated with amprolium 250 WSPR Holland was administered in drinking water at a concentration of 250 mg/1 (0.025%) for a period of 5 days as prescribed by the Manufacturer at the appearance of visible clinical signs.
To obtain serum, blood samples were collected from the experimental birds using the method described by Talebi and Mulcahy (1995). Briefly, 1 ml of blood sample was obtained from the wing vein of each bird using 20 gauge needle (Becton Dickson co., Plymouth, UK) into a 2 ml vacutainer. Samples were obtained on days 2, 4, 6, 8, and 10 after primary and secondary infections, and on days 5, 7, 11, 14, 17, 20 and 24 after tertiary infection (Rose and Hasketh, 1982). The blood which had been allowed to clot for 1 hour at room temperature, was left over night at 4°C and then centrifuge at 800g for 5 minutes. The serum samples were thereafter heated at 56°C for 30 minutes to inactivate the compliment before storage at -20°C. All sera were analyzed with the developed ELISA Triplicate
| Group Treatment and No. of birds |
Infection type/ Age of bird | |||
| I°/wk 2 | 2°/wk 31/2 | 3°/wk 2 challenge with virulent E. tenella | ||
| I | T (n = 20) | 105 USO | 105 USO | 105 SO |
| NT (n = 20) | 105 USO | 105 USO | 105 SO | |
| II | T (n = 20) | 105 SO | 105 SO | 105 SO |
| N T(n = 20) | 105 SO | 105 SO | 105 SO | |
| III | T (n = 20) | 105 SCZ | 105 SCZ | 105 SO |
| NT (n = 20) | 105 SCZ | 105 SCZ | 105 SO | |
| Iv | T (n = 20) | 105 MRZ | 105 MRZ | 105 SO |
| NT (n = 20) | 105 MRZ | 105 MRZ | 105 SO | |
| V | T (n = 20) | 105 GMT | 105 GMT | 105 SO |
| NT (n = 20) | 105 GMT | 105 GMT | 105 SO | |
| VI | 0 | 0 | 0 | |
| KEY; USO-Unsporulated oocyst | 1°- primary infection | |||
| SO- Sporulated oocyst | 2°- Secondary infection | |||
| SCZ- Schizoites | 3°- Tertiary infection WK-Week T –Treated NT – Non treated |
|||
| MRZ- Merozoites | ||||
| GMT- Gametocytes | ||||
Table 1: Experimental infection of broilers with developmental stages of Eimeria tenella.
Enzyme-linked immunosorbent assay
Hay Dottom Nune certified microtiter plates (Roskilde Denmark) were coated with 50µ1 of soluble E. tenella antigen (sporozoites from characterized sporulated Eimeria tenella oocysts)/web at a concentration of 5µ g/ml carbonate buffer (pH 9.6) for 1 hour at 390C. The plates were rinsed five times with saline/tween (S/T), and treated with 75 µl of PBS containing 3% BSA, 1% rabbit serum and 0.05% sodium azide for 1 hr at room temperature to block non-specific adsorption. The plates were washed five times with saline/tween (S/T). A 50µl test serum sample, diluted 1: 1000 in PBS-T (including 1% rabbit serum and 0.05% sodium azide) was added to each well and incubated for 2 hours at room temperature.
Hay Dottom Nune certified microtiter plates (Roskilde Denmark) were coated with 50µ1 of soluble E. tenella antigen (sporozoites from characterized sporulated Eimeria tenella oocysts)/web at a concentration of 5µ g/ml carbonate buffer (pH 9.6) for 1 hour at 390C. The plates were rinsed five times with saline/tween (S/T), and treated with 75 µl of PBS containing 3% BSA, 1% rabbit serum and 0.05% sodium azide for 1 hr at room temperature to block non-specific adsorption. The plates were washed five times with saline/tween (S/T). A 50µl test serum sample, diluted 1: 1000 in PBS-T (including 1% rabbit serum and 0.05% sodium azide) was added to each well and incubated for 2 hours at room temperature.
The plates were washed 5 times with S/T and 50µl of 1:1000 dilution of rabbit anti chicken immunoglobulin peroxidase (Pelfreeze Rogers, Arkansas) in PBS-T was added. After 2 hours incubation, plates were washed five times with S/T and freshly prepared substrate solution (2 mM OPD 6.15 Mm H2O2 in 0.1M citrate buffer pH 6.0) was added per well. The enzyme-substrate reaction was stopped after 30 minutes by addition of 100µl to each well of 2N H2SO4. Absorbance were measured at 492 nM (A492) in a Biotele ELISA Reader (Ref S1118170, Multiskan Ex, USA).All serum samples from the experime nt were analysed on a single day.
Enzyme linked immunospot assay
The spleen was crushed by pressing on fine mesh Petri dishes containing RPMI-1640 (Sigma, Aldrich Cheme, and GmbH, Germany). The suspension was then passed through nylon cell strainer (70 µm; Becton, Dickson, Lincoln Park, NJ). The filtrate was centrifuged at 250g for 10 minutes at 4oC and the sediment collected. Lysis buffer (1 ml/spleen) was added for erythrocyte lysis and placed on ice for 2 minutes. The suspension was passed through the cell strainer again and was centrifuged again 250g for 10 minutes at 4°C to collect the sediment. The cell suspension (10 µl) was mixed with same amount of trypan blue (Sigma, Aldrich Cheme GmbH, and Germany) and the number of cells was counted in a haemocytometer. The centrifuged ion was adjusted to 106 cells/100 µl with RPMI-1640. Nitro cellulose – microlitre plates (96 wells, Millipode multiscreen MAHA) were used in the ELISPOT assay. Individual wells of the plate were filled with 100 µl of goat anti-chick 1g (H+L) – UNLB (primary antibody) at a final concentration of 2 µl/ml and were allowed to stand overnight at 4°C in a humid chamber. Unadsorbed antibodies were removed by three successive washings with PBS. Wells were immediately filled with 100 µl RPMI – 1640 to saturate the remaining finding sites and incubated at 37°C for 2 hrs.
The spleen was crushed by pressing on fine mesh Petri dishes containing RPMI-1640 (Sigma, Aldrich Cheme, and GmbH, Germany). The suspension was then passed through nylon cell strainer (70 µm; Becton, Dickson, Lincoln Park, NJ). The filtrate was centrifuged at 250g for 10 minutes at 4oC and the sediment collected. Lysis buffer (1 ml/spleen) was added for erythrocyte lysis and placed on ice for 2 minutes. The suspension was passed through the cell strainer again and was centrifuged again 250g for 10 minutes at 4°C to collect the sediment. The cell suspension (10 µl) was mixed with same amount of trypan blue (Sigma, Aldrich Cheme GmbH, and Germany) and the number of cells was counted in a haemocytometer. The centrifuged ion was adjusted to 106 cells/100 µl with RPMI-1640. Nitro cellulose – microlitre plates (96 wells, Millipode multiscreen MAHA) were used in the ELISPOT assay. Individual wells of the plate were filled with 100 µl of goat anti-chick 1g (H+L) – UNLB (primary antibody) at a final concentration of 2 µl/ml and were allowed to stand overnight at 4°C in a humid chamber. Unadsorbed antibodies were removed by three successive washings with PBS. Wells were immediately filled with 100 µl RPMI – 1640 to saturate the remaining finding sites and incubated at 37°C for 2 hrs.
The medium was discarded and the plates were dried with absorbent paper incubation of Ig-secreting cells. A 100 µl cell suspension containing 106 cells was dispensed into each well in duplicate and they were incubated undisturbed at 37°C for 4 hours. The plates were rinsed twice by immersion in PBS containing 0.05% Tween 20 (PBST) for 2-3 minutes. The wash buffer was removed from the plates and the outer surfaces of the plates were dried carefully. A 100 µl of PBST containing Goat anti-chick IgG-AP (1000-fold dilution) were added to each well and the plates incubated at 4°C over night. The plates were then rinsed three times by immersion in PBST and dried. Each well was then filled with 100 µl BCIP/NBT solution; prepared by adding 66µl of NBT (containing 50 µl/ml nitroblue tetrazolium in 70% N, N-dimethylformamide) in alkaline phosphate buffer (containing 5.8 g Nacl, 0.1 g Mgcl2 12.1g Tris). The plates were thoroughly washed with running tap water and air-dried for 24 hours. Blue spots showing fuzzy borders were considered positive for immunoglobulin G (IgG).
Results
Antibodies ( IgG or IgY) titre values were higher in sera from broilers infected with sporulated oocyst and merozoites reaching a peak on day 10 of post primary and secondary infections and day 5 post tertiary infection in both broilers treated and non-treated, (Figure 1, 2 and 3). The antibodies values were relatively low in broilers infected with unsporulated oocysts, schizonts and gametocytes at primary and secondary infection in both treated and non treated broilers at day 10 (Figure 1 and 2). At tertiary infection, antibodies increases at day 5, 7, 11 and 14 (Figure 3) respectively. Generally, antibodies levels of sera from the infected broilers with the different developmental stages of the parasite, treated and non treated increased post inoculation and after reaching peak levels, they began to decline (Figure 3). The control birds show no antibodies in the sera. The study demonstrates a non significant difference in the antibody titre values of the treated and non-treated sera of the infected broilers groups (II and IV), p < 0.05.
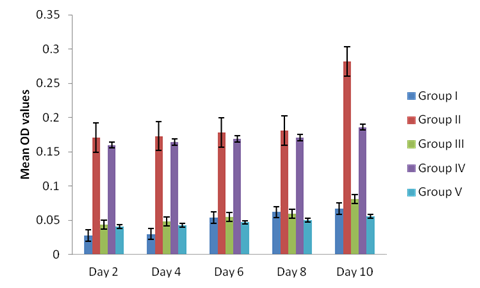
Figure 1: Antibodies level in sera of the experimentally infected broilers with the different stages (unsporulated oocyst, sporulated oocyst, schizonts, merozoites and gametocytes) of Eimeria tenella at optical density (O.D) or absorbance of 492 nm) at primary infection.
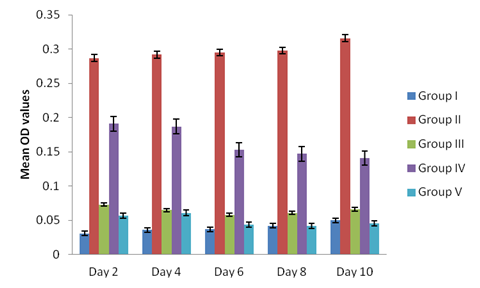
Figure 2: Antibodies level in sera of the experimentally infected broilers with the different stages (unsporulated oocyst, sporulated oocyst, schizonts, merozoites and gametocytes) of Eimeria tenella at optical density (O.D) or absorbance of 492 nm) at secondary infection.
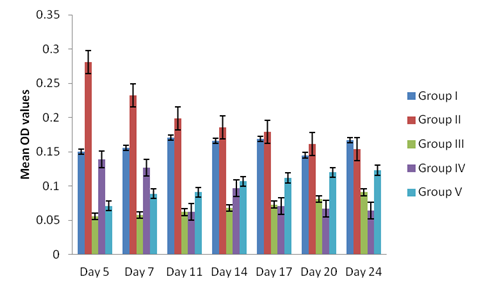
Figure 3: Antibodies level in sera of the experimentally infected broilers with the different stages (unsporulated oocyst, sporulated oocyst, schizonts, merozoites and gametocytes) of Eimeria tenella at optical density (O.D) or absorbance of 492 nm) at tertiary infection.
Discussion
Serum antibody levels increased rapidly on day 10 in the broilers at primary and secondary infections and day 5 at tertiary infection. This varied from the reports Bumstead., et al. (1995) who recorded a peak of humoral immune response between day 14 and 21 post coccidial infection in birds. This may be due to differences in the immunogenic potential of the isolate, age, environment and genetic background of the birds. Antibodies remain significantly high in broilers infected with sporulated oocyst (sporozoite) and merozoite at the end of each infection period, suggesting that the level of antibodies appears to be related to the severity of the developmental stage of the parasite.
This is in concordance with the reports of Constantinoiu., et al. (2007) who reported high antibodies levels persistence in commercial flock after natural exposure to Eimeria or following infection with live vaccine. The present study revealed that there was no significant difference in the antibody titre values in the treated and non treated broilers. This is consistent with the results of Kiani and Farhang (2008), but is inconsistent with the reports of Kurkure., et al. (2006) who stated that chicks treated with coxynil showed higher antibody titre values than those maintained on feed without coxynil. There are still debates on antibodies inducing protective immunity. Dalloul and Lillehoj, (2005) stated that antibodies play a minor role as cell mediated immunity (CMI). Gilbert., et al. (1998), Talebi and Mulcahy (1995), reported that the levels of serum antibodies following infection do not correlate with protection or oocyst output and antibody levels in chickens. This variation may be due to the age, dose and strain of the parasite as well as the genetic background of the broilers. However, the study agrees with the findings of Rose (1987) who showed that antibody could have deleterious effects, including agglutination, lysis, neutralization of infectivity and morphological changes on various developmental stages of Eimeria if they come in close contact with the parasite. The first subunit vaccine (CoxAbicR) is based on transfer of protective antibodies from immunized hens to embryo (Belli., et al. 2004), indicating that antibodies do play an important role in immunity.
Conclusion
The following can be concluded from the results obtained:
- The sporozoites and merozoites showed strong infectivity and elicited stronger antibody titre values in infected broilers with sporulated oocysts and merozoites in infected birds at primary-secondary-tertiary infections, indicating that they might be potential vaccines candidates against avian coccidiosis.
- The immunoglobulins were IgG or IgY
References
- Belli SI., et al. “Characterization of the antigenic and immunogenic properties of bacterially expressed, sexual stage antigens of the coccidian parasite, Eimeria maxima”. Vaccine 22.31.32 (2004): 4316-4325.
- Bumstead JM., et al. “comparision of immune responses in bred lines of chickens to Eimeria maxima and Eimeria tenella”. Parasitology(1995): 143-151.
- Constantinoiu, C., et al. “Development and validation of an ELISA for detecting antibodies to Eimeria tenella in chickens”. Veterinary Parasitology 150.4 (2007): 306-313.
- Conway D and McKenzie M. “Poultry Coccidiosis Diagnostic and Testing Procedures. 3rd Edn, 2121 state Avneu, Ames, lowa, USA”. Veterinary Parasitology 150 (2007): 306-313.
- Dalloul RA and Lillehoj HS. “Recent advances in immunomodulation and vaccination strategies against coccidiosis”. Avian Diseases 49.1 (2005): 1-8.
- Gilbert., et al. “An Enzyme Linked Immunosotbent Assay (ELISA) for coccidiosis in chickens: Correlation of Antibody levels with Prior Exposure to Coccidia in the laboratory and in the field”. Parasite Immunology32.4 (1998): 668-694.
- Kiani R and Farhang HH. “Development of an ELISA test for serological diagnosis of coccidial infections and studying of resistance against coccidiostats based on flock history”. Asian Journal of Biological Sciences 1 (2008): 77-83.
- Kurkure NV., et al. “Evaluation of herbal coccidiostat 'Coxynil' in broiler”. Indian Journal of Experimental Biology44.9 (2006): 740-744.
- Plateau Agricultural Development Programme (PADP). (2002). Annual Report 2002.
- Rose M. “Immunity to Eimeria infections”. Veterinary Immunology and Immunopathology Journal17.1.4 (1987): 333-343.
- Rose ME and Hesketh P. “Immunity to Coccidia in Chickens:Adaptive transfer with peripheral blood lymphocytes and spleen cells”. Parasite Immunology 4.3 (1982): 171-185.
- Smith N., et al. “Maternal transmission of immunity to Eimeria maxima”. Infection and Immunity 62.11 (1994). 1348-1352.
- Talebi A and Mulcahy J. “Correlation between immune responses and oocyst production in chickens monospecifically infected with Eimeria maxima”. Avian Pathology 24.3 (1995): 485-495.
- Wallach M. “Role of antibody in immunity and control of chicken coccidiosis”. Trend in Parasitology 26.8 (2010): 382-387.
Citation:
Kaze Paul Davou., et al. “Antibody Response in Broiler Chickens Infected with Different Developmental Stages of Eimeria
tenella”. Bulletin of Clinical Immunology 1.1 (2018): 1-7.
Copyright: © 2018 Kaze Paul Davou., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.