Research Article
Volume 1 Issue 2 - 2017
The Role of Modifiers in the Enhancement of the Systemic Resistance Inducing Efficacy of Phytoproteins, In Crops against Viruses
1Plant Virus Laboratory, Department of Botany, Lucknow University, Lucknow–226007 (India)
2Department of Plant Pathology, N.D. University of Agriculture and Technology, Kumarganj, Faizabad-224229 (India)
2Department of Plant Pathology, N.D. University of Agriculture and Technology, Kumarganj, Faizabad-224229 (India)
*Corresponding Author: Ahmad Najam, Plant Virus Laboratory, Department of Botany, Lucknow University, Lucknow–226007 (India).
Received: June 06, 2017; Published: August 18, 2017
Abstract
The crude preparations of phytoprotein, isolated from Clerodendrum aculeatum leaves, exhibited significant reduction in number of local lesions, produced by Sun hemp rosette virus on, the leaves of Cyamopsis tetragonoloba plants, treated 24 hours earlier with CA phytoprotein. It was, most probably because of the induction of strong systemic resistance, in non-treated, upper leaves of test host /synthesis of virus inhibitory agent (VIA), induced in test hosts under the stimulus of CA phytoproteins.
Ex-vivo inactivation of virus using VIAs from different sources indicated that the effectiveness of various VIAs was variable and amongst all those used, LS-VIA gave best response when mixed with SHRV. The VIA at a concentration of 1:1 was found to have optimum effect and further increase in its strength did not have any significant effect on the ex vivo inactivation of Virus.
Keywords: Phytoproteins; Systemic Resistance; Virus Inhibitory Agent; Ex Vivo; Antiviral
Introduction
Viruses infect variety of crop plants, cause many important plant diseases and are responsible for losses in yield and quality in all parts of the world. The worldwide losses caused by viral diseases are estimated at about US $ 220 billions per year (Carr and Loebenstein, 2010). Control strategies, for viral diseases, consist of the removal of infected host, breeding for resistance and interruption of the disease cycle by measures such as vector control and screening of partially infected seed lots (Najam, 2008). Direct control measures including chemicals have been tried by many workers but none of the chemicals could prevent or control the infection and spread of the viruses in fields (Awasthi., et al. 1984).
Although the protection of crop plants, from the ravages of pests and diseases, by the use of synthetic pesticides has been the usual practice for many years. The farmers of India usually use more insecticides than the farmers of neighboring countries and the indiscriminate application of pesticides not only adds to high input cost but also leads to undesirable effects to the environment and to human health (Chaube., et al. 2014, 2017). The constraints to use chemically synthesized pesticides for pest control presently include the health and ecological hazardous, development of pesticide resistance in insects and also their use is not economically feasible (Singh., et al. 2017). Therefore, the plant disease control using toxic chemicals are not preferred now days. So, some alternative methods, in place of chemical control are being preferred for the management of diseases (Awasthi., et al. 2006). In recent years, several plant products have been reported to be useful for the management of viral diseases of crop plants (Awasthi., et al. 1987; Sharma and Awasthi, 2017). Botanical pesticides are gaining status and recognition as a possible method for practical control of diseases of crop plants.
Endogenously occurring substances in a few higher exotic plants have been reported to induce systemic resistance in susceptible hosts against viral infections (Awasthi, 1981; Awasthi and Singh, 2015, Awasthi and Verma 2006; Awasthi., et al. 1984). Such plant extracts have been used for protecting economically important crops against viral diseases (Verma., et. al. 1995 a, b). The leaves of C. aculeatum and roots of B. diffusa have been shown to contain potent endogenous virus inhibitory proteins, called as CA-SRIP and BD-SRIP respectively (Awasthi., et al. 1989; Verma., et al. 1996). These phytoproteins confer strong systemic resistance in several plants against a number of plant viruses (Awasthi., et al. 2006; Gupta., et al. 2004; Singh., et al. 2004; Srivastava., et al. 2004 and Verma., et al. 1998). Phytoproteins isolated from C. Aculeatum and B. Diffusa have molecular masses of 34 kDa and 16-20 kDa respectively (Awasthi., et al. 2016). The phytoproteins are highly stable and have been purified (Verma., et al. 1996). Systemic Resistance Inducers (SRIs) do not inactivate viruses in vitro but activate certain host “defence genes”, prompting the host to produce a new virus inhibitory agent (VIA) in the susceptible hosts (Awasthi., et al. 2013). The VIA is trans located to the upper non-treated of the plant and induced short to long-term resistance/immunity to virus infections. The synthesis of VIA can be inhibited by the application of Actinomycin-D, soon after treatment, indicating thereby that the transcription machinery of the host is involved in its synthesis (Verma and Awasthi, 1980).
The present investigations were carried out to study the effect of antiviral resistance inducing activity of isolated phytoproteins and enhancement in their activities by the addition of various bio enhancers of biological origin.
Materials and Methods
Procedure for raising of test plants, maintenance of virus culture, preparation of virus inoculum and the induction of resistance were the same as described earlier (Najam., et al. 2017a, b).
Preparation of virus inhibitor and the isolation of phytoprotein from Clerodendrum aculeatumleaf extract
The lust green leaves from healthy and vigorously growing C. aculeatum plants were harvested and ground in freshly prepared 0.2M phosphate buffer (PB) of pH 6.6 containing 0.1% β mercaptoethanol in the ratio of 1:2. It was then squeezed through double-layered muslin cloth. The extracted sap was centrifuged at 8,000g for 10 minutes to remove the cell debris. The pellet was discarded and supernatant was collected. A saturated solution of ammonium sulphate was added to the supernatant with continuous stirring and then left overnight at 4°C. The mixture was centrifuged at 8,000g for 15 minutes and the precipitate in the form of thick pellets was collected. It was then suspended in a small amount of buffer [20g fresh weight/ml of 0.2M PB (pH 6.6)] and then dialyzed, in a dialysis bag, against running water for overnight, to obtain total protein fraction. The dialyzed protein fraction was either diluted as per requirement or was concentrated through freeze-drying. Lyophilized protein was stored at –20°C. For VIA work sodium acetate buffer was used, instead of phosphate buffer.
The lust green leaves from healthy and vigorously growing C. aculeatum plants were harvested and ground in freshly prepared 0.2M phosphate buffer (PB) of pH 6.6 containing 0.1% β mercaptoethanol in the ratio of 1:2. It was then squeezed through double-layered muslin cloth. The extracted sap was centrifuged at 8,000g for 10 minutes to remove the cell debris. The pellet was discarded and supernatant was collected. A saturated solution of ammonium sulphate was added to the supernatant with continuous stirring and then left overnight at 4°C. The mixture was centrifuged at 8,000g for 15 minutes and the precipitate in the form of thick pellets was collected. It was then suspended in a small amount of buffer [20g fresh weight/ml of 0.2M PB (pH 6.6)] and then dialyzed, in a dialysis bag, against running water for overnight, to obtain total protein fraction. The dialyzed protein fraction was either diluted as per requirement or was concentrated through freeze-drying. Lyophilized protein was stored at –20°C. For VIA work sodium acetate buffer was used, instead of phosphate buffer.
The partially purified C. aculeatum protein fraction was further purified by elution through sephadex G-25 column, following the procedure as described earlier (Verma., et al.1979).
Induction of Virus Inhibitory Agent (VIA) and its Purification
The systemic resistance inducing partially purified C. aculeatum protein (CAP) was applied, by spraying through a spryer, on to the two basal leaves of Cyamopsis tetragonolobaplants. Two basal leaves of an equal number of identical plants, sprayed with distilled water instead of CAP, served as control. Twenty four hours later two upper leaves of each of treated and control plants were harvested separately, weighed, washed well with sterile water and frozen immediately. An overnight frozen leaves were homogenized with an equal amount (W/V) of 0.2M sodium acetate buffer, pH 5.2 containing 0.1% β mercaptoethanol. The homogenate obtained was squeezed through two fold of muslin cloth and the filtrate was centrifuged at 10,000g for 15 minutes. The pellets were discarded and the proteins from the clear supernatant, thus obtained were precipitated by mixing it with an equal amount of saturated solution (60% w/v) of ammonium sulphate. Following overnight of precipitation, the sample was centrifuged and the pellets obtained were dissolved in a minimum amount (W/V) of 0.02M sodium acetate buffer, pH 5.2 containing 0.01% β mercaptoethanol. The dissolved pellets were centrifuged at 10,000g for 15 min. The clear supernatant thus obtained was assayed for VIA activity in the sample against SHRV. The VIA sample was incubated, for 4 hours, with an equal amount of SHRV inoculum and assayed on hypersensitive hosts.
The systemic resistance inducing partially purified C. aculeatum protein (CAP) was applied, by spraying through a spryer, on to the two basal leaves of Cyamopsis tetragonolobaplants. Two basal leaves of an equal number of identical plants, sprayed with distilled water instead of CAP, served as control. Twenty four hours later two upper leaves of each of treated and control plants were harvested separately, weighed, washed well with sterile water and frozen immediately. An overnight frozen leaves were homogenized with an equal amount (W/V) of 0.2M sodium acetate buffer, pH 5.2 containing 0.1% β mercaptoethanol. The homogenate obtained was squeezed through two fold of muslin cloth and the filtrate was centrifuged at 10,000g for 15 minutes. The pellets were discarded and the proteins from the clear supernatant, thus obtained were precipitated by mixing it with an equal amount of saturated solution (60% w/v) of ammonium sulphate. Following overnight of precipitation, the sample was centrifuged and the pellets obtained were dissolved in a minimum amount (W/V) of 0.02M sodium acetate buffer, pH 5.2 containing 0.01% β mercaptoethanol. The dissolved pellets were centrifuged at 10,000g for 15 min. The clear supernatant thus obtained was assayed for VIA activity in the sample against SHRV. The VIA sample was incubated, for 4 hours, with an equal amount of SHRV inoculum and assayed on hypersensitive hosts.
Role of Virus Inhibitory Agent (VIA), produced in different hosts after treatment with the crude extract of Clerodendrum aculeatum leaves, in immunizing the plants against viral infection
Virus Inhibitory Agent (VIA) is an antiviral state, induced concomitantly with the systemic resistance induction in host plant, following treatment of host with the protein isolated from non-host plants, endogenously produced in them. These phytoproteins mediated induced systemic resistance followed partial resemblance with SAR.VIA, is an inducible gene product likewise AVF, IVR and PR-proteins. VIA induction in host plants following the application of endogenously produced plant proteins, are capable of ex vivo virus inactivation.
Virus Inhibitory Agent (VIA) is an antiviral state, induced concomitantly with the systemic resistance induction in host plant, following treatment of host with the protein isolated from non-host plants, endogenously produced in them. These phytoproteins mediated induced systemic resistance followed partial resemblance with SAR.VIA, is an inducible gene product likewise AVF, IVR and PR-proteins. VIA induction in host plants following the application of endogenously produced plant proteins, are capable of ex vivo virus inactivation.
The present experiment was designed to evaluate the production of VIA in different hosts and to study its role in the management of viral infections in plants. The seedlings/plants of bottle gourd (Lagenaria siceraria L Standle), cucumber (Cucumis sativus L) and sun hemp (Crotolaria juncea L) were used as source plants for the production of VIA.
Two basal leaves of healthy and vigorously growing plants of bottle gourd (Lagenaria siceraria), cucumber (Cucumis sativus) and sun hemp (Crotolaria juncea) were sprayedwith leaf extract from Clerodendrum aculeatum plants (CA phytoproteins). Twenty four later, two upper leaves from treated plants, in each case separately, were harvested, weighed, washed, homogenized within a standard protein purification protocol and centrifuged at 12,000g for 20 min. Supernatants obtained from each plant were screened separately for its antiviral state. It was designated as LS-VIA (Lagenaria siceraria -Virus Inhibitory Agent), CS-VIA (Cucumis sativus -Virus Inhibitory Agent) and CJ-VIA (Crotolaria juncea-Virus Inhibitory Agent).
The antiviral efficacy of VIAs (LS-VIA, CS-VIA and CJ-VIA), isolated from different source plant, was assessed separately on the leaves of hypersensitive hosts (Cyamopsis tetagaonoloba L Taube) against sun hemp rosette virus. The suitably diluted VIS, in each case, separately was sprayed on to the leaves C. tetragonoloba plants. Virus in each case was challenge inoculated following 24 hours of VIA application. Leaves of an equal number of identical plants sprayed with distilled water, instead of VIA, served as control. Local lesions appeared, in the form of small necrotic spots on inoculated leaves, 4-6 days after virus inoculation were counted. The data obtained were analyzed statistically for the significance of results (Nedcor, 1961).
Results and Discussion
Induction of systemic resistance in host plants by CA Protein (Crude Extract)
The CA protein (crude leaf extract of C. Aculeatum), sprayed on to the two basal leaves of Cyamopsis tetagaonoloba plants, 24 hours before challenge inoculation with SHRV, exhibited tremendous decrease or reduction in number of local lesions produced by SHRV(Plate-1). The percent reduction in lesion number was highly significant (92%). The reduction in number of local lesions up to this extent was due to the induction of strong systemic resistance, in non-treated upper leaves of test host (basal leaves of which were treated, 24 hours earlier, with CA protein/crude leaf extract of C.aculeatum) against SHRV, by virus inhibitory agent (VIA).
The CA protein (crude leaf extract of C. Aculeatum), sprayed on to the two basal leaves of Cyamopsis tetagaonoloba plants, 24 hours before challenge inoculation with SHRV, exhibited tremendous decrease or reduction in number of local lesions produced by SHRV(Plate-1). The percent reduction in lesion number was highly significant (92%). The reduction in number of local lesions up to this extent was due to the induction of strong systemic resistance, in non-treated upper leaves of test host (basal leaves of which were treated, 24 hours earlier, with CA protein/crude leaf extract of C.aculeatum) against SHRV, by virus inhibitory agent (VIA).
Ex-vivo Inactivation of Virus using VIA
The leaves of Lagenaria siceraria, Cucumis sativus and Crotolaria juncea were harvested separately, 24 hours after treatment with CAP, weighed, washed and homogenized using standard protein purification protocol (Awasthi., et al. 2013) and centrifuged at 12,000g for 15 minutes. Supernatants obtained in each case when screened for their antiviral state revealed that LS- VIA, CS-VIA and CJ-VIA possess a strong ability for ex vivoinactivation of viruses. Thesamples of LS- VIA, CS-VIA and CJ-VIA incubated with SHRV in equal volume for 4 hours, when inoculated separately on to the leaves of Cyamopsis tetagaonoloba plants, exhibited significant decrease in local lesion production as compared with number of local lesions on control plants, inoculated with a mixture of DW-SHRV. Results presented in Table 1,2,3,4,5 & 6 have clearly indicated a significant reduction in virus titer/local lesion production (Plate1, Graph 1,2).
The leaves of Lagenaria siceraria, Cucumis sativus and Crotolaria juncea were harvested separately, 24 hours after treatment with CAP, weighed, washed and homogenized using standard protein purification protocol (Awasthi., et al. 2013) and centrifuged at 12,000g for 15 minutes. Supernatants obtained in each case when screened for their antiviral state revealed that LS- VIA, CS-VIA and CJ-VIA possess a strong ability for ex vivoinactivation of viruses. Thesamples of LS- VIA, CS-VIA and CJ-VIA incubated with SHRV in equal volume for 4 hours, when inoculated separately on to the leaves of Cyamopsis tetagaonoloba plants, exhibited significant decrease in local lesion production as compared with number of local lesions on control plants, inoculated with a mixture of DW-SHRV. Results presented in Table 1,2,3,4,5 & 6 have clearly indicated a significant reduction in virus titer/local lesion production (Plate1, Graph 1,2).
After 48 hours duration treated plants, on comparison with DW-SHRV control plants, showed 88%, 82% and 74% reduction in local lesions by LS-VIA, CS-VIA and CJ-VIA respectively. VIA is an inducible gene product likewise AVF, IVR and PR-proteins. VIA induction in host plant following application of endogenously produced plant protein extracts, are capable of ex vivo virus inactivation. (Verma., et al. 1998). These antiviral proteins may induce the host for the production of virus inhibitory agents. Probably some protein (VIA) diffuses to surrounding tissues and other plant parts (Verma., et al. 1996). The VIA have been isolated from leaves of plants treated with antiviral agents and they have been shown conclusively to inactivate the viruses in vitro (Verma and Awasthi, 1980; Verma., et al. 1996). The release of resistance seems to be an activation of a pre-existing system and hence is easily stimulated. It is probably able to move from one leaf to another through the vascular system of the plant.
Highly potent and broad-spectrum antiviral agents have been isolated and characterized from Boerhaavia diffusa, Cuscuta reflexa, Euphorbia hirta, Clerodendrum aculeatum, Datura metal, Solanum melongena, Bougainvelia spectabilis and many other plants (Verma and Awasthi,1979 a,b;Verma., et al. 1995 a,b 1998; Awasthi., et al. 2014, 2016). The phytoproteins stimulate natural viral defense mechanism existing in susceptible plants and provide systemic protection of a very high degree. The systemic resistance inducers (SRIs) induced strong systemic resistance in several susceptible hosts against viruses, reacting hyper sensitively or systemically, when applied/sprayed 24 hours before virus inoculation. The SRIs were purified using modern protein purification techniques and were identified as basic proteins (Sharma and Awasthi, 2017).
Verma and Awasthi (1980) reported that the synthesis of VIA is inhibited, if Actinomycin D (AMD) is applied soon after extract treatment. The VIA synthesis is neither virus specific nor host specific. Extracts containing VIA when incubated with the viruses reduced their infectivity. VIAs from a few hosts have been characterized. It has been reported that VIA synthesized in the leaves of N.glutinosa treated with B.diffusa root extract reduced infectivity of TMV on N.glutinosa, Datura stramonium and D. metel (Verma and Awasthi, 1980). These experiments resulted in the conclusion that VIA so produced in different host plants viz. bottle gourd (Lagenaria siceraria), cucumber (Cucumis sativus), sun hemp (Crotolaria juncea) and Cyamopsis tetragonoloba can serve as an effective control measures in the treatment of viral diseases of economically important plants.
Following treatment with phytoproteins, the treated hosts accumulate a new virus inhibitory agent (VIA) in treated and non-treated parts of plants. The induced virus inhibitory agent (VIA) shows characteristics of protein and reduces infectivity of viruses both in vitro and in vivo (Verma and Awasthi, 1980). The SRIPs and VIAs are immunologically two distinct proteins. The VIA is neither host nor virus specific and is not accumulated in the presence of actinomycin-D (Awasthi., et al. 2013).
The research work conducted by our group, on the induction of systemic resistance against infection of viruses by non-host plants has demonstrated for the first time that the inducible plant defense system against viruses can be switched on after treatment with certain highly specific basic phytoproteins and has opened a new field of ‘Plant Immunology’. Upon treatment, these systemic resistance inducing proteins (SRIPs) provoke the plant to produce a new defensive protein in the treated plants, which is the actual virus-inhibitory protein (VIA). These SRIPs, like interferon, are the only natural substances with the proven ability to inhibit in vivo virus infection and replication and will be very useful for immunization of susceptible plants against commonly occurring viruses. Although specific immunoglobulins have not been found in plants, but many new defensive proteins are formed in plants following treatment with a large number of different agents. These defensive proteins, effective against cellular pathogens, are more common and induced in greater quantities and number, whereas, induced proteins effective against viruses are synthesized in smaller quantities and hence their detection and purification was difficult. Cellular pathogens during attachment have the ability to elicit defense responses in plants, whereas, viruses since are directly delivered into the cell, the attachment process is by passed and hence they are not able to elicit strong defense response to produce detectable amounts of defensive proteins (Awasthi., et al. 2013).
The possible role of modifiers/bio enhancers in the development of systemic induced resistance, by the application of virus inhibitory agents (VIAs), in the host plants against natural infection of viruses has been established by Awasthi., et al. (2016). It has been demonstrated that in short duration crops, normally weekly or in a few crops/host plants fortnightly treatments are required for the development of defense, against natural infection of viruses, in plants. So repeated doses are required at a regular time interval (Verma and Awasthi, 1979). It has been observed that the application of modifiers/bio enhancers alone, before the treatment with antiviral/phytoproteins, or in combination (phytoproteins mixed with bienhancer) gave very encouraging results. It has been evinced from the observations that plants, treated with phytoproteins, isolated from C.aculeatum, mixed with bioenhancer exhibited strong systemic resistance which persisted/prolonged for a longer duration. The antiviral state in such plants could be maintained for a longer period of time; hence the numbers of treatments required in maintaining host resistant, against a further infection, were reduced. Such plants could defend themselves for a longer duration, against viral infections in fields. Same findings were reported by Singh., et al. --. They could manage papaya leaf curl and papaya ring spot virus infection in papaya orchards by monthly sprayings orchard with a phytoproteins isolated from B. diffusa along with milk protein as a bioenhancer (Singh and Awasthi, 2012).
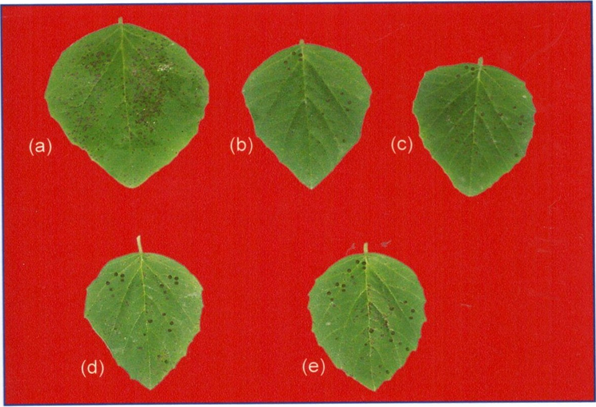
Plate 1: Effect of crude phytoproteins, isolated from the leaves of Clerodendrum
aculeatum (CA phytoproteins), on the production of virus inhibitory agents in
Lagenaria siceraria (LS-VIA), assayed on the leaves of Cyamopsis tetragonoloba.
(a) Control distilled water (DW)
(b) LS-VIA (1:1)
(c) LS-VIA (1:5)
(d) LS-VIA (1:10)
(e) LS-VIA (1:1)
(a) Control distilled water (DW)
(b) LS-VIA (1:1)
(c) LS-VIA (1:5)
(d) LS-VIA (1:10)
(e) LS-VIA (1:1)
| Treatment | Local Lesion at the Site of Application | Average Number of Local Lesions ± SEM | Percent Reduction in Local Lesion Number | |||
| L1 ± SeM | L2 ± SeM | L3 ± SeM | L4 ± SeM | |||
| Control (DW) | 279 ± 2.16 | 288 ± 2.88 | 290 ± 2.92 | 283 ± 1.16 | 285 ± 1.68 | 0 ± 0 |
| LS-VIA (1:1) | 31 ± 2.00 | 37 ± 1.68 | 29 ± 1.68 | 35 ± 0.88 | 33 ± 1.60 | 88 ± 2.88 |
| LS-VIA (1:5) | 64 ± 1.64 | 57 ± 1.44 | 71 ± 1.64 | 65 ± 1.68 | 64 ± 1.72 | 78 ± 1.66 |
| LS-VIA (1:10) | 78 ± 1.68 | 86 ± 1.78 | 79 ± 1.32 | 94 ± 2.14 | 84 ± 2.44 | 71 ± 2.16 |
| LS-VIA (1:20) | 118 ± 2.88 | 123 ± 2.16 | 143 ± 2.22 | 138 ± 1.60 | 131 ± 3.16 | 54 ± 2.14 |
Table 1: Ex-vivo inactivation of SHRV using LS-VIA on Cyamopsis tetragonoloba.
+ SEM = Standard Error of Mean
LS – VIA = Lagenaria siceraria -Virus Inhibitory Agent
L1, L2, L3 and L4 represent the number of leaves treated
1.0g of SHRV infected leaf tissue in 50 ml distilled water [virus inoculum (1:50)].
+ SEM = Standard Error of Mean
LS – VIA = Lagenaria siceraria -Virus Inhibitory Agent
L1, L2, L3 and L4 represent the number of leaves treated
1.0g of SHRV infected leaf tissue in 50 ml distilled water [virus inoculum (1:50)].
| Treatment | Local Lesion at the Site of Application | Average Number of Local Lesions ± SEM | Percent Reduction in Local Lesion Number | |||
| L1 ± SeM | L2 ± SeM | L3 ± SeM | L4 ± SeM | |||
| Control (DW) | 279 ± 2.16 | 288 ± 2.34 | 290 ± 2.18 | 283 ± 3.22 | 285 ± 4.06 | 0 ± 0 |
| LS-VIA (1:1) | 76 ± 1.72 | 79 ± 1.28 | 81 ± 2.00 | 96 ± 1.06 | 83 ± 1.44 | 71 ± 1.72 |
| LS-VIA (1:5) | 102 ± 1.68 | 123 ± 1.12 | 111 ± 1.72 | 125 ± 1.26 | 115 ± 1.32 | 60 ± 1.68 |
| LS-VIA (1:10) | 134 ± 1.78 | 142 ± 1.68 | 132 ± 1.28 | 149 ± 2.16 | 139 ± 2.80 | 51 ± 1.24 |
| LS-VIA (1:20) | 188 ± 1.64 | 194 ± 1.94 | 176 ± 1.24 | 189 ± 2.16 | 187 ± 3.16 | 34 ± 0.98 |
Table 2: Ex-vivo inactivation of SHRV using LS-VIA on Cyamopsis tetragonoloba.
+ SEM = Standard Error of Mean
LS – VIA = Lagenaria siceraria -Virus Inhibitory Agent
L1, L2, L3 and L4 represent the number of leaves treated
1.0g of SHRV infected leaf tissue in 50 ml distilled water [virus inoculum (1:100)]
+ SEM = Standard Error of Mean
LS – VIA = Lagenaria siceraria -Virus Inhibitory Agent
L1, L2, L3 and L4 represent the number of leaves treated
1.0g of SHRV infected leaf tissue in 50 ml distilled water [virus inoculum (1:100)]
| Treatment | Local Lesion at the Site of Application | Average Number of Local Lesions ± SEM |
Percent Reduction in Local Lesion Number | |||
| L1 ± SeM | L2 ± SeM | L3 ± SeM | L4 ± SeM | |||
| Control (DW) | 288 ± 1.26 | 295 ± 2.16 | 271 ± 3.12 | 274 ± 1.88 | 282 ± 1.96 | 0 ± 0 |
| CS-VIA (1:1) | 49 ± 0.88 | 46 ± 0.98 | 56 ± 1.16 | 44 ± 1.22 | 49 ± 1.54 | 82 ± 1.72 |
| CS-VIA (1:5) | 71 ± 0.68 | 76 ± 0.66 | 82 ± 0.76 | 77 ± 0.88 | 77 ± 1.66 | 73 ± 1.72 |
| CS-VIA (1:10) | 85 ± 1.66 | 98 ± 1.54 | 87 ± 1.44 | 90 ± 1.22 | 90 ± 1.16 | 68 ± 1.80 |
| CS-VIA (1:20) | 115 ± 1.64 | 129 ± 1.29 | 156 ± 1.24 | 136 ± 1.36 | 134 ± 1.64 | 52 ± 1.66 |
Table 3: Ex-vivo inactivation of SHRV using CS-VIA on Cyamopsis tetragonoloba.
+ SEM = Standard Error of Mean
CS-VIA = Cucumis sativus -Virus Inhibitory Agent
L1, L2, L3 and L4 represent the number of leaves treated
1.0g of SHRV infected leaf tissue in 50 ml distilled water [virus inoculum (1:50)].
+ SEM = Standard Error of Mean
CS-VIA = Cucumis sativus -Virus Inhibitory Agent
L1, L2, L3 and L4 represent the number of leaves treated
1.0g of SHRV infected leaf tissue in 50 ml distilled water [virus inoculum (1:50)].
| Treatment | Local Lesion at the Site of Application | Average Number of Local Lesions ± SEM |
Percent Reduction in Local Lesion Number | |||
| L1 ± SeM | L2 ± SeM | L3 ± SeM | L4 ± SeM | |||
| Control (DW) | 288 ± 3.16 | 295 ± 2.22 | 271 ± 2.16 | 274 ± 2.34 | 282 ± 2.68 | 0 ± 0 |
| CS-VIA (1:1) | 96 ± 1.16 | 102 ± 1.20 | 109 ± 1.90 | 89 ± 1.38 | 99 ± 2.16 | 64 ± 1.06 |
| CS-VIA (1:5) | 123 ± 1.16 | 136 ± 1.26 | 129 ± 3.36 | 139 ± 1.08 | 132 ± 1.24 | 53 ± 1.38 |
| CS-VIA (1:10) | 149 ± 1.16 | 165 ± 2.16 | 163 ± 1.39 | 144 ± 1.24 | 155 ± 2.64 | 45 ± 1.16 |
| CS-VIA (1:20) | 191 ± 2.16 | 187 ± 1.64 | 198 ± 1.38 | 178 ± 1.74 | 189 ± 1.36 | 33 ± 1.26 |
Table 4: Ex-vivo inactivation of SHRV using CS -VIA on Cyamopsis tetragonoloba.
+ SEM = Standard Error of Mean
CS-VIA = Cucumis sativus -Virus Inhibitory Agent
L1, L2, L3 and L4 represent the number of leaves treated
1.0g of SHRV infected leaf tissue in 100 ml distilled water [virus inoculum (1:100)].
+ SEM = Standard Error of Mean
CS-VIA = Cucumis sativus -Virus Inhibitory Agent
L1, L2, L3 and L4 represent the number of leaves treated
1.0g of SHRV infected leaf tissue in 100 ml distilled water [virus inoculum (1:100)].
| Treatment | Local Lesion at the Site of Application | Average Number of Local Lesions ± SEM |
Percent Reduction in Local Lesion Number | |||
| L1 ± SeM | L2 ± SeM | L3 ± SeM | L4 ± SeM | |||
| Control (DW) | 292 ± 2.06 | 299 ± 2.16 | 289 ± 1.18 | 305 ± 4.18 | 296 ± 2.19 | 0 ± 0 |
| CJ-VIA (1:1) | 71 ± 1.78 | 83 ± 2.36 | 75 ± 2.18 | 79 ± 2.06 | 77 ± 2.16 | 74 ± 0.76 |
| CJ-VIA (1:5) | 143 ± 1.68 | 156 ± 2.18 | 159 ± 3.60 | 163 ± 1.32 | 155 ± 3.15 | 48 ± 0.72 |
| CJ-VIA (1:10) | 189 ± 1.72 | 175 ± 2.16 | 173 ± 1.72 | 187 ± 1.68 | 181 ± 2.16 | 39 ± 0.68 |
| CJ-VIA (1:20) | 191 ± 1.68 | 189 ± 1.29 | 193 ± 3.60 | 199 ± 1.80 | 193 ± 3.15 | 35 ± 0.68 |
Table 5: Ex-vivo inactivation of SHRV using CJ-VIA on Cyamopsis tetragonoloba.
+ SEM = Standard Error of Mean
CJ-VIA = Crotolaria juncea -Virus Inhibitory Agent
L1, L2, L3 and L4 represent the number of leaves treated
1.0g of SHRV infected leaf tissue in 50 ml distilled water [virus inoculum (1:50)].
+ SEM = Standard Error of Mean
CJ-VIA = Crotolaria juncea -Virus Inhibitory Agent
L1, L2, L3 and L4 represent the number of leaves treated
1.0g of SHRV infected leaf tissue in 50 ml distilled water [virus inoculum (1:50)].
| Treatment | Local Lesion at the Site of Application | Average Number of Local Lesions ± SEM |
Percent Reduction in Local Lesion Number | |||
| L1 ± SeM | L2 ± SeM | L3 ± SeM | L4 ± SeM | |||
| Control (DW) | 292 ± 1.96 | 299 ± 2.22 | 289 ± 1.76 | 305 ± 3.60 | 296 ± 3.60 | 0 ± 0 |
| CJ-VIA (1:1) | 121 ± 2.28 | 117 ± 2.14 | 124 ± 1.26 | 111 ± 2.00 | 118 ± 1.78 | 60 ± 0.68 |
| CJ-VIA (1:5) | 173 ± 1.16 | 186 ± 2.16 | 189 ± 1.78 | 175 ± 1.78 | 181 ± 1.26 | 39 ± 2.60 |
| CJ-VIA (1:10) | 191 ± 2.06 | 189 ± 2.32 | 193 ± 2.26 | 196 ± 1.28 | 192 ± 2.26 | 35 ± 1.60 |
| CJ-VIA (1:20) | 199 ± 2.44 | 205 ± 2.64 | 218 ± 1.16 | 213 ± 1.16 | 209 ± 1.26 | 29 ± 2.16 |
Table 6: Ex-vivo inactivation of SHRV using CJ-VIA on Cyamopsis tetragonoloba.
+ SEM = Standard Error of Mean
CJ-VIA = Crotolaria juncea -Virus Inhibitory Agent
L1, L2, L3 and L4 represent the number of leaves treated
1.0g of SHRV infected leaf tissue in 100ml distilled water [virus inoculum (1:100)].
+ SEM = Standard Error of Mean
CJ-VIA = Crotolaria juncea -Virus Inhibitory Agent
L1, L2, L3 and L4 represent the number of leaves treated
1.0g of SHRV infected leaf tissue in 100ml distilled water [virus inoculum (1:100)].
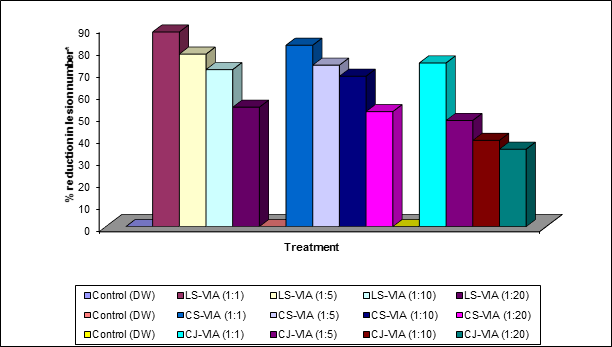
*Challenge inoculation was made using SHRV 1:50 (V/V) dilution.
Graph 1: Ex-vivo inactivation of SHRV using LS-VIA, CS-VIA and CJ-VIA on Cyamopsis tetragonoloba.
Graph 1: Ex-vivo inactivation of SHRV using LS-VIA, CS-VIA and CJ-VIA on Cyamopsis tetragonoloba.
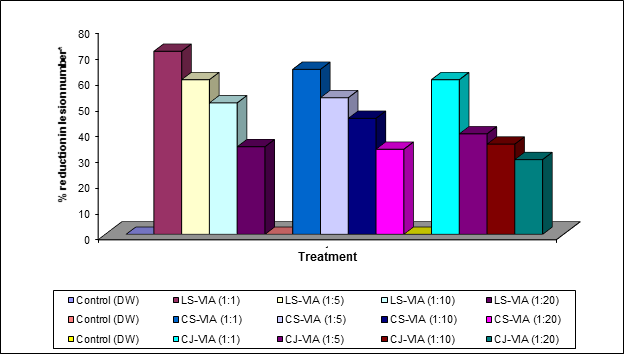
*Challenge inoculation was made using SHRV 1:100 (V/V) dilution
(LS-VIA = Lagenaria siceraria -Virus Inhibitory Agent, CS-VIA = Cucumis sativus -Virus Inhibitory Agent, CJ-VIA = Crotolaria juncea -Virus Inhibitory Agent)
Graph 2: Ex-vivo inactivation of SHRV using LS-VIA, CS-VIA and CJ-VIA on Cyamopsis tetragonoloba.
(LS-VIA = Lagenaria siceraria -Virus Inhibitory Agent, CS-VIA = Cucumis sativus -Virus Inhibitory Agent, CJ-VIA = Crotolaria juncea -Virus Inhibitory Agent)
Graph 2: Ex-vivo inactivation of SHRV using LS-VIA, CS-VIA and CJ-VIA on Cyamopsis tetragonoloba.
References
- Awasthi LP. “The purification and nature of an antiviral protein from Cuscuta Reflexa plants”. Archives of Virology 70.3 (1981): 215-223.
- Awasthi LP., et al. “Clerodendrum– A Novel Herb having Broad Spectrum Antimicrobial Properties”. Asian Agri History 19.1 (2015): 33-44.
- Awasthi LP and Verma HN. “Boerhaavia diffusa – a wild herb with potent biological and antimicrobial properties”. Asian-Agri History 10 (2006): 55-68.
- Awasthi LP., et al. “Prevention of plant virus disease by Boerhaavia diffusa inhibitor”. International Journal of Tropical Plant Diseases 2.1 (1984): 41- 44.
- Awasthi LP., et al. “Characteristics of antiviral agents induced by B. Diffusa glycoprotein in host plants”. Indian Journal of Virology 3 (1989): 156-169.
- Awasthi LP, Kumar P, Singh S Incidence and symptomatology of cucumber mosaic virus in cucumber at farmers field of Faizabad district, U. P. Indian Phytopathology 59.3 (2006): 380.
- Awasthi LP., et al. “Further studies on the antiviral agent(s) isolated from host plants, pre-treated with Boerhaavia diffusa glycoprotein”. Virology and Mycology 3.1(2013): 124.
- Awasthi L. P., et al. “Eco-friendly Management of the Viral Diseases of Chilli (Capsicum annum L.)”. Research & Review: Journal of Agriculture Science and Technology, 3.1 (2014): 11-16.
- Awasthi LP., et al. “A possible mechanism of action for the Inhibition of plant viruses by an antiviral glycoprotein isolated from Boerhaavia Diffusa roots”. Journal of Virology & Antiviral Research 5.3 (2016): 1-8.
- Carr JP and Loebenstein GD. “Natural and Engineered Resistance to Plant Viruses. Part B”. Advancesin Virus Research 76 (2010): 269-282.
- Chaubey AN., et al. “Eco-friendly management of viral diseases of potato”. International Research Journal of Life Sciences 2.1 (2014): 8.
- Awasthi LP., et al. “Ecofriendly management of leaf curl disease of Chilli through botanical bio pesticides”. SciFed Virology Research Journal 1.1 (2017):1-7.
- Gupta RK., et al. “Callus culture and organogenesis in Boerhaavia diffusa: A potent antiviral protein containing plant”. Physiology and Molecular Biology of Plant 10(2004): 263.
- Najam, A Ph.D. Thesis, Lucknow University, Lucknow (2010).
- Najam, A., et al. “Effect of bioenhancer on the antiviral resistance inducing activity of phytoproteins, isolated from roots of Boerhaavia diffusa and leaves of Clerodendrum aculeatum plants”. Sci Fed Journal of Virology (2017).
- Najam. et al. “Management of viral diseases of crops through phytoproteins ,isolated from Boerhaavia diffusa and Clerodendrum aculeatum plants, along with bioenhancer”. Virology: Current Research (2017).
- Sharma NK and Awasthi LP. “Molecular characterization of antiviral Proteins, isolated from host Plants, pretreated with antiviral glycoprotein, isolated from roots of Boerhaavia diffusa plants”. Journal of Human Virology & Retro virology 5.3(2017): 1-5.
- Singh S and Awasthi LP. “Characterization and Management of Viral Diseases of papaya”. Lap Lambert Academic Publishing GmbH KG (2012): 240.
- Awasthi LP., et al. “Studies on the molecular variability in Indian isolates of Papaya ring spot virus”. Virology Research Journal 1.1(2017): 10-16.
- Snedecor G.W. “The statistical methods, Allied Pacific Private Limited, Bombay”.
- Srivastava A., et al. “Micro propagation of Clerodendrum Aculeatum through adventitious shoot induction and production of consistent amount of virus resistance inducing protein". Indian Journal of Experimental Biology 42.12(2004): 1200-1207.
- Verma HN and Awasthi LP. “Prevention of virus infection and multiplication by leaf extract of Euphorbia hirta and the properties of the virus inhibitor”. New Botanist 6(1979): 49-59.
- Verma HN and Awasthi LP. “Antiviral activity of Boerhaavia diffusa root extract and the physical properties of the virus inhibitor”. Canadian Journal of Botany 57.8(1979): 926-932.
- Verma HN and Awasthi LP. “Occurrence of a highly antiviral agent in plants treated with Boerhaavia diffusa inhibitor”. Canadian Journal of Botany 58.20 (1980): 2141-2144.
- Awasthi LP., et al. “Prevention and control of yellow mosaic disease of mungbean through aqueous root extract of Boerhaavia diffusa”. Indian phytopathology 57.3(2004): 303-307.
- Verma HN., et al. “Isolation of the virus inhibitor from root extract of Boerhavvia diffusa inducing systemic resistance in plants”. Canadian Journal of Botany 57.11 (1979): 1214-1217.
- Verma HN., et al. “Antiviral activity of different Clerodendrum L. species. Zeitschrift fur Pflanzenkrantheiten und Flanzcnchvtz”. 91 (1984): 34-41.
- Verma HN., et al. “Endogenous virus inhibitors from plants: Their physical and biological properties. In: Antiviral Proteins in Higher Plants, M. Chessin., et al”. CRC Press (1994): 1-21.
- Verma HN., et al. “Agricultural role in endogenous antiviral substance of plant origin. In: Antiviral Proteins in Higher Plants, M. Chessin., et al”. CRC Press (1994): 23-37.
- Verma HN., et al. “Induction of systemic resistance in plants against viruses by a basic protein from Clerodendrum aculeatum leaves”. Phytopathology 86 (1996): 485-492.
- Verma HN., et al. “Seasonal variation in systemic resistance inducing basic protein isolated from leaves of Clerodendrum aculeatum”. Indian Journal of Experimental Biology 16 (1998): 9-13.
- Verma HN and Baranwal VK. “Antiviral phytoproteins as biocontrol agents for efficient management of plant viruse diseases. In: Biocontrol potential and their exploitation in Crop Pest and Disease Management (Eds. RL Rajak and Rajeev K Upathyay), Aditya Book Pvt. Ltd., New Delhi”. (1998):
Citation:
Ahmad Najam., et al. “The Role of Modifiers in the Enhancement of the Systemic Resistance Inducing Efficacy of Phytoproteins,
In Crops against Viruses”. Clinical Biotechnology and Microbiology 1.2 (2017): 50-59.
Copyright: © 2017 Ahmad Najam., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.






























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.