Research Article
Volume 1 Issue 2 - 2017
Determination of the Presence and Spatial Prediction of Mastomys Rodents in a Lassa fever Endemic Zone of Nigeria
1Department of Community Medicine, Irrua Specialist Teaching Hospital, Irrua, Edo State, Nigeria
2Department of Community Medicine, Irrua Specialist Teaching Hospital, Irrua, Edo State, Nigeria
3Department of Community Medicine, Ahmadu Bello University Teaching Hospital, Zaria, Kaduna, Nigeria
2Department of Community Medicine, Irrua Specialist Teaching Hospital, Irrua, Edo State, Nigeria
3Department of Community Medicine, Ahmadu Bello University Teaching Hospital, Zaria, Kaduna, Nigeria
*Corresponding Author: Ike CG, Department of Community Medicine, Irrua Specialist Teaching Hospital, Irrua, Edo State, Nigeria.
Received: June 13, 2017; Published: August 28, 2017
Abstract
Background: Lassa fever is a severe acute infection caused by Lassa virus, a member of the Arenaviridae family of viruses. The
disease is endemic in West Africa. Countries such as Nigeria, Sierra Leone, Guinea and Liberia are geographical locations where the
disease is endemic. The reservoir of Lassa virus is the multimammate rat, Mastomys natalensis. However, information on the presence
of Mastomys in some active foci where the disease has been reported is sketchy.
Objective: To determine the presence of the rodent vector in the endemic community through geospatial analysis.
Materials and Methods: A prospective study of the presence and location of the relative abundance of Mastomys was carried out between March-May, 2014 in Edo Central Senatorial District of Edo State, Nigeria. A total of 19 trap sites was set at locations of known outbreaks of Lassa fever. Geographical coordinates of sites where rodents were captured were taken. ArcGIS 10.2 Arc Map, was used to visualize the location of all captured rodents, while the Inverse Distance Weighted and Kriging Interpolation techniques were used to predict the relative abundance of Mastomys within the study area.
Results: All the 19 trap sites yielded a total of 96 rodents. Mastomys rats (n = 11) constitute 11.5% of all captured rodents. A higher proportion of Matomys rodents are predicted along the South-Western part of the study area.
Conclusion: Mastomys, the rodent vector for Lassa fever transmission are found in the Edo Central Senatorial District in Edo State, Nigeria, and a higher proportion of the rodents are found towards the South-Western part of the District.
Keywords: GIS; Kriging; IDW; Lassa fever; Mastomys
Background
Lassa fever is a severe acute infection caused by Lassa virus, a member of the Arenaviridae family of viruses [1]. The disease is endemic in West Africa [2]. Countries such as Nigeria, Sierra Leone, Guinea and Liberia are geographical locations where the disease is endemic. [2-6] The disease has also been reported in the United Kingdom and the United States. [7-13] Lassa fever was first discovered in Nigeria in 1969. [14] Since the first report of an outbreak in the Northern part of Nigeria, more than 21 states in Nigeria have reported confirmed cases of the disease. Edo State is one of the States in Nigeria with constant outbreaks of the disease. In endemic regions, Lassa fever infects over 300,000 individuals per year, causing more than 3,000 deaths [14]. The case fatality ranges from 15% to 50%. [15-18] The incubation period is between 5-21 days [19], and it presents initially with symptoms similar to malaria. In late stages, it presents as a multisystem disease and often hemorrhagic. People of all ages are at risk of the disease. [14] About 80% of people infected have mild or no observable symptoms. [14] Presentation within the first few days of illness and high index of suspicion improve the prognosis of treatment. Reverse Transcriptase –PCR is the gold standard for the laboratory diagnosis of Lassa fever. Laboratory diagnosis and research are carried at Biosafety level three (BSL 3) laboratories. The only drug of choice is intravenous Ribavirin. There is yet no available vaccine for Lassa fever. Public health control of the disease emphasises good personal and environmental hygiene for the public and enforcement of standard precautions for health care workers.
The reservoir of Lassa virus is the multimammate rat, Mastomys natalensis. This small rodent has been found in Sierra Lone, Nigeria and Guinea. [20-22] Mastomys rats are ubiquitous in rural areas of Africa [15], and they are usually found in large quantity near human dwellings during the dry season. [23] One possible reason for dry season migration is the increase in human activity such as urbanization and farming, which are commonplace during the dry season. These small mammals are peace-loving animals that will not hesitate to leave their usual niche at the least disturbance. The rodent vector sheds the virus in their excreta, and humans are infected by contact with the animal or its excrements. [13] Clinical studies have established the presence of Lassa virus in Mastomys and locations with a positive seroprevalence of Lassa virus have also reported the presence of the rodent vector. [24-27]
However, there is no available data on the presence of Mastomys in all the areas where the disease has occurred or has been reported. There are several reasons why it may not be feasible to determine all the sites where the rodent vector dwells: It is capital intensive to conduct a census; it is not possible to trap all possible locations of the rodent vector due to its varied migratory habit; some locations are either not accessible, or access is denied. In a few locations, civil conflicts might hamper field studies.
Geographical Information System (GIS) has been extensively utilized in determining the locations of epidemics of infectious diseases [28-31], and in the estimation of the presence of diseases or vectors at unsampled locations. ArcGIS has the capability of using interpolation tools to measure points and estimate related features at unmeasured sites. Two commonly used estimation tools are the Inverse distance weighted (IDW) [32] and Kriging techniques [33]. The first utilizes a deterministic interpolation technique (IDW) based on measured points and the second, a geostatistical technique based on statistics. IDW works on the assumption that objects or events that are close to one another are more alike than those farther away. Technically, interpolation is based on the principle of spatial autocorrelation or spatial dependence. Kriging provides some measure of the accuracy of the prediction.
The aim of this study, therefore, is to determine the presence of Mastomys in locations where human cases of Lassa fever have been mapped in a related study [34] and use the result available to predict the dispersion of the rodent vector at unsampled locations.
Materials and Methods
Site selection for rodent trapping was selected based on earlier spatial information on the hotspot and clusters of Lassa fever cases. [34] Trap locations were close to houses, and where appropriate, inside houses markets and dump sites. Snap-traps were used combined with rodent-bait poisoned with a rodenticide, zinc phosphide, and fish, feed and sometimes, bread. The bait was prepared and applied at each selected site on the evening of the trapping. An average of 10 baits per site were applied. Care was taken not to harm other livestock or humans with the chemical. Equipment used for handling dead or dying rodents were based on BSL3 standards. Proper informed consent to enter sites were obtained.
Geographical Coordinates of trap sites
The geographical coordinates used for locating the sites for rodent trapping were based on WGS 84 while measuring units were in decimal degrees (dd). The accuracy of measuring distance was set at 3meters. The Garmin handheld GPS device was used to pick the coordinate points.
The geographical coordinates used for locating the sites for rodent trapping were based on WGS 84 while measuring units were in decimal degrees (dd). The accuracy of measuring distance was set at 3meters. The Garmin handheld GPS device was used to pick the coordinate points.
Study Site
Edo central senatorial district of Edo State, Nigeria was selected for the study. Anecdotal reports suggest the area has a higher number of hospitalized patients than every other part of the state. Five local government area such as Esan West, Central, North East, Southeast make up the district with Ekpoma, Irrua, Uromi , Ubiaja and Igueben as Area headquarters. Unpublished and anecdotal reports suggest that the study sites have a higher proportion of Lassa fever cases than other locations in Nigeria. Despite this common knowledge, there are studies that need further scientific validation on the ecological niche of the rodent vector, Mastomys rodent within the district.
Edo central senatorial district of Edo State, Nigeria was selected for the study. Anecdotal reports suggest the area has a higher number of hospitalized patients than every other part of the state. Five local government area such as Esan West, Central, North East, Southeast make up the district with Ekpoma, Irrua, Uromi , Ubiaja and Igueben as Area headquarters. Unpublished and anecdotal reports suggest that the study sites have a higher proportion of Lassa fever cases than other locations in Nigeria. Despite this common knowledge, there are studies that need further scientific validation on the ecological niche of the rodent vector, Mastomys rodent within the district.
Farming is the main occupation of the population. Apart from farming, other agricultural activities such as hunting (especially of
small rodents)account for increased man-rodent contact. The rapid change in land use due to urbanization, burning of bushes, and wood
felling for timber and as a major source of fuel (which is in high demand) for the increasing population of the district, disrupts the ecological
niche of small rodents, and that of other animal, causing them to migrate to human settlements. Another agricultural activity which
exposes the community to the risk of Lassa fever transmission is their habit of open- drying of food produce near their settlements. This
food preservation method usually attracts small rodents. Hospital records from the Institute of Lassa Fever Research and Control of Irrua
Specialist Teaching Hospital suggest that a higher proportion of Lassa fever patients come from Ekpoma and Irrua axis of the district.
Although, there may be a bias in the statistics given the relative distance of the different villages and towns within the district from the
tertiary hospital facility. It is of note that the district is made up of urban, sub-urban and rural areas. As should be expected, there are
different housing quality characteristics of these areas. The difference in housing quality and the immediate surrounding environmental
hygiene may also account for the presence of the rodent vector within the community.
Rodent Survey and Identification
A total of 19 trap sites was selected from the four Local Government Area that make up the district. Other locations failed to be used due to security and terrain issues. Part of the planning for harvesting trapped rodents was the use of standard precautions based on the BSL3 requirement for handling dangerous pathogens. Pre and post exposure status of the research team were determined before and after the handling of rodents. Rodents were trapped at dump sites, bush paths, markets and close to residential areas. The trap site selection was guided by the number of reported sites of high rodent activity within the study area. Daily Rodent trapping duration was from March through May 2014 (An average of 66 trapping days).
A total of 19 trap sites was selected from the four Local Government Area that make up the district. Other locations failed to be used due to security and terrain issues. Part of the planning for harvesting trapped rodents was the use of standard precautions based on the BSL3 requirement for handling dangerous pathogens. Pre and post exposure status of the research team were determined before and after the handling of rodents. Rodents were trapped at dump sites, bush paths, markets and close to residential areas. The trap site selection was guided by the number of reported sites of high rodent activity within the study area. Daily Rodent trapping duration was from March through May 2014 (An average of 66 trapping days).
Rodent Identification
All the rodents caught were identified using external morphological characteristics.
All the rodents caught were identified using external morphological characteristics.
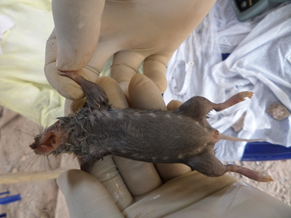
Figure 1: The ventral surface of a multimammate rat caught in one of the trap sites, showing multiple breasts (10 breasts on each side).
Results
Trap yield
A total of 96 rats was trapped at 19 sites. Excluding unsuccessful trap days. The success of traps depended on the presence of rainfall, malfunctioning of traps, activities of humans and livestock. Some traps could not be found, while others did not catch any rodent. Each successful trap site yielded an average of 5 rats per day. All rodents and equipment used were handled based on Biosafety level three (BSL3) standard for handling highly infectious pathogens. The disposable equipment was incinerated; some rodents preserved as museum specimens and the rest buried.
A total of 96 rats was trapped at 19 sites. Excluding unsuccessful trap days. The success of traps depended on the presence of rainfall, malfunctioning of traps, activities of humans and livestock. Some traps could not be found, while others did not catch any rodent. Each successful trap site yielded an average of 5 rats per day. All rodents and equipment used were handled based on Biosafety level three (BSL3) standard for handling highly infectious pathogens. The disposable equipment was incinerated; some rodents preserved as museum specimens and the rest buried.
Only physical features of the rodents were characterized. Rattus (roof house rat) made up about 86.5% (n = 83) of all the rodents caught. Mastomys rats constitute 11.5% (n = 11); while only 1%(n = 1) were made up shrew and Praomys rats were caught respectively.
Spatial Analyses
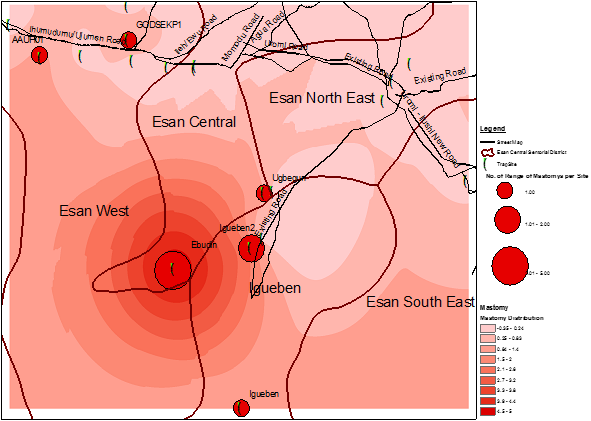
GPS data of trapped Mastomys were transferred to ArcGIS 10.2 software for spatial analysis. The trap sites were viewed in an ArcMap [Figure 2]. The map shows ranges of numbers of Mastomys caught per trap sites. The size of the red dots reflects the number of Mastomys caught per trap sites (green dots). The map also shows that Mastomys are evenly distributed within the study sites.
GPS data of trapped Mastomys were transferred to ArcGIS 10.2 software for spatial analysis. The trap sites were viewed in an ArcMap [Figure 2]. The map shows ranges of numbers of Mastomys caught per trap sites. The size of the red dots reflects the number of Mastomys caught per trap sites (green dots). The map also shows that Mastomys are evenly distributed within the study sites.
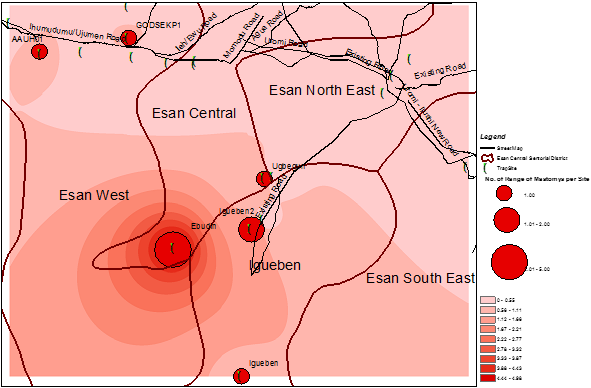
Interpolation methods showed varying numbers of the rodent vector within the sites where human cases were reported [Figure 2-5]. In order to improve accuracy, a kriging technique was carried out after IDW. The results still show varying sizes of the rodent vector across the study sites. A very important feature observed is that the greater the intensity of the colour, the higher the number of the Mastomys predicted. Using the data from a previous study (34), the interpolation shows that more Mastomys were located away from the cities where a majority of the cases occurred [Figure 5]. Human cases occurred more in the urban area while a higher proportion of the rodent vector was estimated to be located in the rural area.
From a visual inspection of both IDW and Kriging maps, we observed that the area of highest density of Mastomys was Ebudin village of the Essen central Area of the District. The Eastern part of the study area is free, but moving towards the South-Western part, there seems to be more concentration of the rodent vector.
| Location ID | X coordinate | Y coordinate | Sex | Frequency (n = 11) |
Trap date |
| Ekpoma 1 | 6.141630 | 6.749690 | Male | 1 | 31/3/14 |
| AAU Hostel, Ekpoma | 6.077360 | 6.739560 | Female | 1 | 11/4/14 |
| Ebudin | 6.173660 | 6.592180 | Female | 5 | 09/5/14 |
| Ugbegun | 6.239660 | 6.645050 | Female | 1 | 09/5/14 |
| Igueben | 6.223070 | 6.497900 | Female | 1 | 07/5/14 |
| Igueben 2 | 6.230390 | 6.607250 | Female | 2 | 07/5/14 |
Table 1: Geographical Coordinates (in Decimal Degrees) of Location of Trapped Mastomys Rodents within the Location of Lassa Fever Cases in the Study Area.
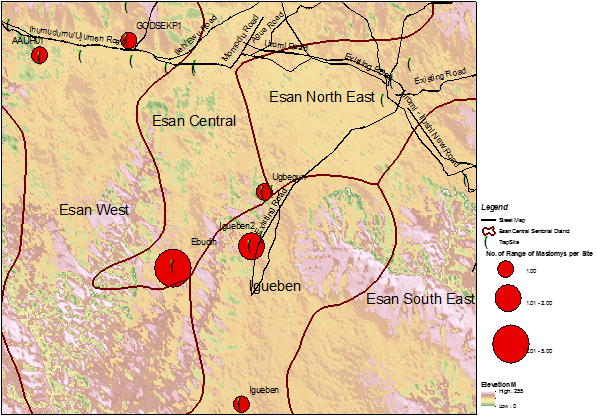
Figure 2: GIS map showing 19 trap sites in the study area (trap period was from March-May 2014) (Distribution of Mastomys rodents is shown as red dots of varying sized).
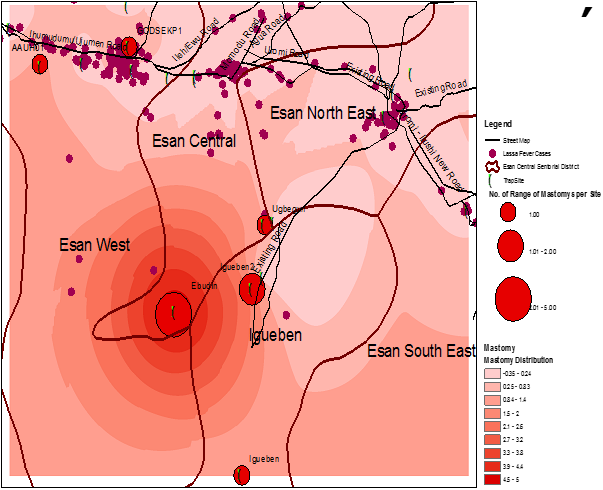
Figure 5: Spatial distribution of predicted Mastomys rodent activity and locations of Lassa fever cases in the study area.
Discussion
Ecological Niche of Mastomys rodents
Several studies agree that Mastomys rodent is the usual or the only rodent vector for the transmission of Lassa fever [20-22, 24-27] The presence of the rodent vector is responsible for maintaining a viral circulation within the human population. [35] Results of rodent trapping also supports the findings that Mastomys is the rodent vector for Lassa fever transmission in Edo State, Nigeria.
Several studies agree that Mastomys rodent is the usual or the only rodent vector for the transmission of Lassa fever [20-22, 24-27] The presence of the rodent vector is responsible for maintaining a viral circulation within the human population. [35] Results of rodent trapping also supports the findings that Mastomys is the rodent vector for Lassa fever transmission in Edo State, Nigeria.
However, the results of our interpolations methods suggest that the majority of the cases of human transmission in Ekpoma might have been as a result of human to human transmission. This is because areas where previous studies have identified geographical hotspot of Lassa fever, trap result yielded more Rattus rates than Mastomys. Whereas the very rural areas where the cases are not abundant have the highest proportion of Mastomys rat. One possible explanation for the difference in the location of the rodent vector and the location of previous outbreaks is the role of rapid urbanization, poor environmental hygiene and poor housing play in the transmission chain. Another explanation is the feeding habit of Mastomys; the rodent vector could travel as far as 500 miles in search for food and shelter during seasonal population expansions or migration. [2] It is possible that the rodents migrate to the sub-urban areas during feeding, and back to their usual dwelling in the rural community. Mastomys could also change their usual place of habitation several times in their lifespan. [2] So, the prediction of more abundance of the rodent vector art sites not known for a higher proportion of Lassa fever cases could be explained by the behaviour of the rodent itself. We do not, however, rule out the possibility of under-reporting of the human cases from the sites that have more rodents.
From the total trap result, we observed that Mastomys rodents are tolerable animals. Their low territoriality makes it possible for other small rodents like Rattus Rattus, Mus Musculus, Shrews, and Praomys to live within the same ecological niche. Rodent studies in West Africa, specifically implicated Mastomys natalensis as the specie among all other species of Mastomys as the usual vector of Lassa fever. [22,25,36-39] Some other species have been found to be predominant in certain geographies either as the only specie or with other species of Mastomys. There are up to 12 members of the genus Mastomys which may be responsible for Lassa fever transmission at certain locations. [26] This particular study did not aim at chromosomal analyses to differentiate each of the captured Matomys rodents; our concern was to investigate for the presence of the multimammate rat in an environment known to be endemic of the disease and to estimate or predict other locations where they could be found.
Spatial prediction methods used in this study have been used for the control of epidemics of infectious diseases. [40-44] Unique to our approach, is the use of two similar methods in one study and the application of a previous spatial map in visualizing the relationship between our interpolation result and the result of a previous study. The previous study [34] was carried out by the same authors, in the same period and at the same location. This initial study focused on the geographical hotspots and clusters of Lassa fever in the Edo Central District of Nigeria, whereas our current study focuses on the identification of the rodent vector within the endemic area.
Inverse distance weighted methods are spatial interpolation tools that could be utilized in estimating the number of Mastomys rodents in an environment known for Lassa fever outbreaks. Once a particular number of the rodent vector is captured within a Geo-coded area, with the use of a GIS software, it is possible to estimate the relative abundance of the rodent vector in other unmeasured areas. This approach is a very economical approach for field surveillance and for clinical studies in Lassa fever, especially in the face of dwindling funds or research or for control of the disease. We also considered the inferiority of IDW to kriging in determining the accuracy of our estimation; that is why we performed a kriging technique. We also used the kriging technique to predict the relative abundance of the rodent vector in an already mapped location for geographical hotspots and clusters of the disease. The main reason behind our estimation was because of the inability to sample all locations. There were environmental challenges such as inaccessible roads, bad weather condition and uncooperative residents.
Limitations of the Approach
Estimation in itself is a limitation because it does not really describe the actual relative abundance and location of Mastomys within the study area. We have tried to improve our level of accuracy by repeating the estimation result of IDW with kriging.
Estimation in itself is a limitation because it does not really describe the actual relative abundance and location of Mastomys within the study area. We have tried to improve our level of accuracy by repeating the estimation result of IDW with kriging.
Another limitation of our study is the small sample size of rodents used for the prediction. We believe future studies could improve on our study by increasing the sample size and the number of measured points.
Using a retrospective human case data for a prospective rodent study might blur the true picture of the link between the present location of Mastomys and the human cases. It is possible that prospective human and rodent studies could improve the results of this study.
Conclusion
Lassa fever is endemic in West Africa, and Mastomys rodent is the vector for the disease transmission. In Edo State, Nigeria, where the disease is endemic, Mastomys rodents are found at the location where a higher proportion of the human cases have been reported in the State. However, there is a prediction of a relative abundance of the rodent vector towards the South-Western part of the Edo Central Senatorial District of Edo State. These areas are not known as hotspot areas for the disease.
Declaration
The authors have no conflict of interest in this study.
The authors have no conflict of interest in this study.
References
- Branco LM., et al. “Lassa Virus-like Particles displaying all major immunological determinants as a vaccine candidate for Lassa hemorrhagic fever”. Virology Journal 7 (2010): 279.
- Bonner PC., et al. “Poor Housing Quality Increases Risk of Rodent Infestation and Lassa fever in Refugee Camps of Sierra Leone”. The American Journal of Tropical Medicine and Hygiene77.1 (2007): 169-175.
- Casey DE., et al. “Epidemiological aspects of the 1970 epidemic, Jos, Nigeria”. Transactions of the Royal Society of Tropical Medicine and Hygiene 66.3 (1972): 402-408.
- Frame JD., et al. “Lassa fever, a new virus disease of man from West Africa. Clinical description and pathological findings”. American Tropical Medicine and Hygiene19.4 (1970): 670- 676.
- Monath TP., et al. “Lassa fever in the Eastern Province of Sierra Leone, 1970-1972. Clinical observations and virological studies on selected hospital cases”. The American Journal of Tropical Medicine and Hygiene 23.6 (1974): 1140- 1149.
- Monath TP., et al. “A hospital epidemic of Lassa fever in Zorzor, Liberia, March- April 1972”. The American Journal of Tropical Medicine and Hygiene 22.6 (1973): 773-779.
- “Communicable Disease Surveillance Centre: Lassa fever imported to England”. Communicable disease report CDR weekly 10 (2000): 99.www.virologyj.com/content/8/1/205.
- Schmitz H., et al. “Monitoring of clinical and laboratory data in two cases of imported lassa fever”. Microbes and Infection 4.1 (2002): 43-50.
- Veldkamp PJ and Schippers EF. “A man with fatal Lassa fever following a stay in Sierra Leone”. Nederlands Tijdschrift voor Geneeskunde 146.46 (2002): 2201-2204.
- Haas.WH., et al. “Imported Lassa fever in Germany; Surveillance and management of contact persons”. Clinical Infectious Diseases 36.10 (2003): 1254- 1258.
- “Center for Disease Control and Prevention (CDC): Imported Lassa fever- New Jersey”. Morbidity and Mortality Weekly Report 53 (2004): 894-897.
- Kitching A., et al. “A fatal case of Lassa fever in London, January 2009”. Euro surveillance 14.6 (2009): 19117.
- Amorosa V., et al. “Imported Lassa fever, Pennsylvania, USA, 2010”. Emerging Infectious Diseases 16.10 (2010):1598-1600.
- Richmond JK and Baglole DJ. “Lassa fever: epidemiology, clinical features, and social consequences”. British Medical Journal 327.7426 (2003): 1271-1275.
- McCormick JB., et al. “A prospective study of the epidemiology and ecology of Lassa fever”. The Journal of Infectious Diseases 155.3 (1987): 437-444.
- Fisher – Hoch SP., et al. “Review of Cases of nosocomial Lassa fever in Nigeria: the high price of poor medical practice”. British Medical Journal 311.7009 (1995): 857-859.
- Mertens PE., et al. “Clinical presentation of Lassa fever during the hospital epidemic at Zorzor, Liberia, March- April 1972”. The American Journal of Tropical Medicine and Hygiene 22.6 (1973): 780-784.
- Bausch DG., et al. “Lassa fever in Guinea: I. Epidemiology of human disease and clinical observations”. Vector-Borne and Zoonotic Diseases 1.4 (2001): 269-281.
- Adewuyi GM., et al. “Lassa fever: Another infectious disease”. African Journal of Clinical and Experimental Microbiology 10.3 (2009): 144-155.
- Monath TP., et al. “Lassa virus isolation from Mastomys natalensis rodents during an epidemic in Sierra Leone”. Science185.4147 (1974): 263-265.
- Wulff H., et al. “Recent isolation of Lassa virus from Nigerian rodents”. Bulletin World Health Organization 52.4-6 (1975): 609-613.
- Fichet- Calvet E., et al. “Fluctuation of abundance and Lassa virus prevalence in Mastomys natalensis in Guinea West Africa”. Vector-Borne and Zoonotic Diseases7.2 (2007):119-128.
- Lecompte E., et al. “Genetic identification of Kodoko Virus, a novel arena virus of the African Pigmy mouse (Mus Nannomy’s minutoides) in West Africa”. Virology 364.1 (2007): 178-183.
- Monath TP. “Lassa fever: Review of epidemiology and epizootiology”. Bulletin of the World Health Organization 52.4-6 (1975): 577-592.
- Cummins D., et al. “Lassa fever encephalopathy: clinical and laboratory findings”. The American Journal of Tropical Medicine and Hygiene 95.3 (1992): 197-201.
- Lecompte E., et al. “Mastomys natalensis and Lassa fever, West Africa”. Emerging Infectious Diseases 12.12 (2006): 1971-1974.
- Ford CE and Hamerton JC. “A cholchicine, hypotonic citrate, squash sequence for mammalian chromosomes”. Stain Technology 31.6 (1956): 247-251.
- Yan L., et al. “Landscape elements and Hantaan virus–related hemorrhagic fever with renal syndrome, People’s Republic of China”. Emerging Infectious Diseases 13.9 (2007): 1301-1306.
- Peterson AT., et al. “Geographic potential for outbreaks of Marburg hemorrhagic fever”. The American journal of tropical medicine and hygiene 75.1(2006): 9-15.
- Morrison AC., et al. “Defining challenges and proposing solutions for control of the virus vector Aedes aegypti”. PLOS Medicine 5.3(2008): e68.
- Racloz V., et al. “Surveillance of dengue fever virus: a review of epidemiological models and early warning systems”. PLOS Neglected Tropical Diseases 6.5(2012): e1648.
- Esri. ArcGIS 10. How IDW works. http://www.esri.com/en/arcgisdesktop/10.0/help/index.htm#//009z00000076000000.htm
- Esri. ArcGIS 10. How Kriging works http://www.esri.com/en/arcgisdesktop/10.0/help/index.htm#//009z00000076000000.htm
- Ike C and Asogun D. “Detection of clusters and geographical hotspots for Lassa fever in Edo Central Senatorial District of Nigeria: a step into a Nation-wide mapping of Lassa fever. [Poster]”.
- Adrian Q. N. Mylne., et al. “Mapping the zoonotic niche of Lassa fever in Africa”. Transactions of the Royal Society of Tropical Medicine and Hygiene 109.8 (2015): 483-492.
- Keenlyside RA., et al. “Case-control study of Mastomys natalensis and humans in Lassa virus-infected households in Sierra Leone”. The American Journal of Tropical Medicine and Hygiene32.4(1983):829-837.
- Mc Cormick JB. “Epidemiology and control of Lassa fever”. Current Topics in Microbiology and Immunology 134 (1987): 69-78.
- Lukashevich LS., et al. “Lassa virus activity in Guinea: distribution of human anti-viral antibody defined using enzyme-linked immunosorbent assay with recombinant antigen”. Journal of Medical Virology 40.3(1993): 210- 217.
- Granjol L., et al. “Systematics of the genus Mastomys (Rodentia; Muridae)”. The Belgian Journal of Zoology 127.1 (1997): 7-18.
- Bhunia GS., et al. “Spatial and temporal variation and hotspot detection of kala-azar disease in Vaishali district (Bihar), India”. BMC infectious diseases 13.1 (2013): 64.
- Naish S., et al. “Spatio-temporal patterns of Barmah Forest virus disease in Queensland, Australia”. PloS one 6.10(2011): e25688.
- Martínez-López B., et al. “Identifying equine premises at high risk of introduction of vector-borne diseases using geo-statistical and space-time analyses”. Preventive veterinary medicine 100.2 (2011): 100-108.
- Khormi HM and Kumar L. “Examples of using spatial information technologies for mapping and modelling mosquitoborne diseases based on environmental, climatic, socioeconomic factors and different spatial statistics, temporal risk indices and spatial analysis: a review”. Journal of Food, Agriculture & Environment 9.2 (2011): 41-49.
- Moise IK and Kalipeni E. “Applications of geospatial analysis to surveillance data: a spatial examination of HIV/AIDS prevalence in Zambia”. GeoJournal 77.4(2012): 525-540.
Citation:
Ike CG., et al. “Determination of the Presence and Spatial Prediction of Mastomys Rodents in a Lassa fever Endemic Zone of
Nigeria”. Clinical Biotechnology and Microbiology 1.2 (2017): 60-69.
Copyright: © 2017 Ike CG., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.






























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.