Research Article
Volume 1 Issue 5 - 2017
Potentials of Jatropha carcus, Racinnus communis, Azadrichta indica and Lagenaria siceraria, de-oiled Cakes for Bioethanol Production
1Department of Microbiology, Faculty of Science, Usmanu Danfodiyo University, Sokoto
2Department of Veterinary Anatomy, Usmanu Danfodiyo University, Sokoto
2Department of Veterinary Anatomy, Usmanu Danfodiyo University, Sokoto
*Corresponding Author: Bello A, Department of Veterinary Anatomy, Usmanu Danfodiyo University, Sokoto.
Received: November 14, 2017; Published: November 30, 2017
Abstract
The potentials of Jatropha carcus, Racinnus communis, Azadrichta indica, Lagenaria siceraria, seed cakes were examined as a raw material for bioethanol production. the hydrolysis of the substrates by Aspergillus niger and fermentation inoculated by the Sacromyces cerevisiae, Baker’s yeast and mixture of s. cerevisiae and Baker’s yeast at different pH 6,7 and 8 and were incubated anaerobically at different temperature of 30°C 40°C ,50°C. However, the maximum bioethanol production was observed by Jatropha curcus, (42.62g/L) at pH 8 under fermentative organism of mixture of s. cerevisiae and Baker’s yeast at temperature 50°C. The least production was also observed from substrate Azadrichta indica (21.14 g/L) at pH 6 and temperature 30°C under fermentative organism Mixture of s. cerevisiae and Baker’s yeast. The results showed promising by the substrates with high potentials for bioethanol production shown by the substrates with high potentials for bioethanol production shown by the substrates that no significant differences in term of bioethanol production.
Keywords: Jatropha carcus; Racinnus communis; Azadrichta indica; Lagenaria siceraria; de-oiled Cakes; Bioethanol Production
Introduction
In 1925, Henry ford quoted ethyl alcohol, ethanol as ‘‘The fuel of the future’’ He further more stated, “The fuel of the future is going to come from apples, weeds, saw-dust almost anything. There is fuel in every bit of vegetable matter that can be fermented”. Today Henry ford’s futuristic vision significance can easily understood (Anujkumar., et al. 2007). The increasing demand for ethanol for various industrial purposes such as alternative source of energy, industrial solvents, cleansing agents, and preservatives has necessitated increased production of this alcohol.
Ethanol production is usually accomplished by chemical synthesis of petrochemical substrate and microbial conversion of carbohydrates present in an agricultural product. Increased yields of ethanol production by microbial fermentation depend on the use of ideal microbial strain, appropriate fermentation substrates and suitable process technology (Brooks, 2008). In the current time, the importance of alternative energy sources has become more necessary not only due to the continue depletion of limited fossil fuel stock but also for safe and better environment (Wayman, 1999).
In this work, renewable cellulosic wastes (cakes) a product of oil extraction were selected for the production of bioethanol fuel. Locally isolated microorganisms, cellulose producing fungi Aspergillus niger, ethanol producing Sacchromyces cerevisiae and backers yeast were employed for saccharification and fermentation respectively. Substrates were enzymatically saccharified by using A. Niger followed by addition of S. cerevisiae and Baker’s yeast for fermentative production of bioethanol. The samples were subjected to different pH values and temperatures respectively.
The main advantage of Bioethanol are that the fuel is renewable and that is no net contribution to greenhouse gas emission (unlike fossil fuels) this is due to the fact that the biomass cultivated for bioethanol is able to re-fix (by photosynthesis) the carbon dioxide produced during bioethanol production and combustion. (Walker 2010). The worldwide attention on biofuels is because of the perceived multiple benefits associated with their use. Energy security and reduced Green House Gas Emissions are the main benefits but other advocated advantage includes: waste reduction, improved local air quality, improved vehicle performance and enhanced rural development. The byproduct cake which is a waste product of Biodiesel plant can be used as a good raw-materials for ethanol production and also solve self-disposal of byproduct, it also encourage the plantation and development of biodiesel industries as two important Biofuels, Biodiesel and Bioethanol can be produced from the price of only one raw-material.
This study tends to investigate the Biofuel potentials of residual cakes from Jatropha carcus, Racinnus communis, Azadrichta indica, Lagenaria siceraria for possible bio-ethanol production. The objectives includes, determine the reducing sugar from the substrates, quantify the concentration of bio-ethanol generated from the substrates and to determine the effect of pH value (6, 7, 8) and temperature ranges (30, 40, 50°C) for bioethanol production from the substrates.
Materials and Methods
The materials used in this research work includes; Cakes extracted from seeds of Jatropha curcas, Lagenaria siceraria (calabash seed), Racinus communis (castor oil seed), Azadrachta indica (Neem seed), test tubes, conical flasks, measuring cylinder, filter paper, spectrophotometer, UV-vis spectrophotometer, pH meter, glass slide, cover slip, incubator, wire loop and autoclave.
Sample Collection
The de-oilseed’s cake from the substrates was collected after extraction of oil using sohxlet extraction from the extraction plant in Sokoto energy research center, U.D.U.S.
The de-oilseed’s cake from the substrates was collected after extraction of oil using sohxlet extraction from the extraction plant in Sokoto energy research center, U.D.U.S.
Sample Processing
This was carried out in accordance with the method described by Afuya, (1990). The samples were sun-dried, further crushed into powder form, using pestle and mortar, the samples were kept in polyethene bags for further analysis.
This was carried out in accordance with the method described by Afuya, (1990). The samples were sun-dried, further crushed into powder form, using pestle and mortar, the samples were kept in polyethene bags for further analysis.
Media Preparation
The media used in this work were Sabouround Dextrox Agar (SDA) and Potato Dextrox Agar (PDA). The media were prepared according to the manufacturer’s instructions and procedure of Oyeleke and Manga, (2008).
The media used in this work were Sabouround Dextrox Agar (SDA) and Potato Dextrox Agar (PDA). The media were prepared according to the manufacturer’s instructions and procedure of Oyeleke and Manga, (2008).
Isolation of Microorganisms
The organism: Aspergillus niger was isolated from soil collected from university Garden, U.D.U.S, the sample was serially diluted as described by Oyeleke and Manga, (2008). Dilution factor of 106 was then transferred into Petridis containing PDA media and incubated at room temperature for five days. Colonies suspected to be Aspergillus niger based on colonial characteristics were further sub cultured on PDA and examined microscopically.
The organism: Aspergillus niger was isolated from soil collected from university Garden, U.D.U.S, the sample was serially diluted as described by Oyeleke and Manga, (2008). Dilution factor of 106 was then transferred into Petridis containing PDA media and incubated at room temperature for five days. Colonies suspected to be Aspergillus niger based on colonial characteristics were further sub cultured on PDA and examined microscopically.
Sacchromyces cereviciae was isolated from sob drink, the drink was left open for two days. 0.2 ml of the sample was spread on the surface of the prepared PDA plate and incubated at room temperature for 4 days. Colonies suspected to be Sachromyces cereviciae based on colonial characteristics were further subcultured on PDA. The isolate was examined microscopically and identified by comparing their characteristics with those of known texa using the scheme of domsch and grams (1970) and biochemical test as described by Oyeleke and Manga, (2008). The yeast called baker’s yeast was purchased from Sokoto central market. It is yeast produced industrially for industrial purpose especially in bread production.
Pre-treatment
Thirty grams (30g) of the each sample were soaked in water and left overnight to remove dirty and excess oil. The oil and dirty substances floating at the surface and was gently separated from the cakes by sieving and dried to completely remove the moisture for further analysis.
Thirty grams (30g) of the each sample were soaked in water and left overnight to remove dirty and excess oil. The oil and dirty substances floating at the surface and was gently separated from the cakes by sieving and dried to completely remove the moisture for further analysis.
Hydrolysis
Thirty grams (30g) of each pre-treated samples were put into 500ml conical flask, 300ml of distilled water was added to each conical flask and then allowed to absorbed water in order to obtain homogeneous mixture. The conical flask were covered with cotton wool, wrapped with aluminium foil and sterilized at 121°C for 15 minutes using autoclave. The sterilized conical flask were allowed to cool and inoculated with Aspergillus niger and incubated at 37°C for 5days for hydrolysis to take place. Humprey and Caritas, (2007).
Thirty grams (30g) of each pre-treated samples were put into 500ml conical flask, 300ml of distilled water was added to each conical flask and then allowed to absorbed water in order to obtain homogeneous mixture. The conical flask were covered with cotton wool, wrapped with aluminium foil and sterilized at 121°C for 15 minutes using autoclave. The sterilized conical flask were allowed to cool and inoculated with Aspergillus niger and incubated at 37°C for 5days for hydrolysis to take place. Humprey and Caritas, (2007).
Fermentation
The fermentation of the hydrolyzed samples was carried out by the method described by Humprey and Caritas, (2007). 10 ml each of hydrolyzed substrate was put into 12, 100 ml conical flask each. The pH of each substrate was subjected at 6, 7, and 8 respectively. The pH values for all the four 4 substrates was maintained at 6 acidic form, 7 at neutral stage and 8 elevated using potassium hydroxide (KOH) and hydrochloric acid (HCl). The conical flasks containing substrates were allowed to cool at room temperature and inoculated with Sacchromyces cereviciae for all the samples and were kept in pH 6, 7, and 8.
The fermentation of the hydrolyzed samples was carried out by the method described by Humprey and Caritas, (2007). 10 ml each of hydrolyzed substrate was put into 12, 100 ml conical flask each. The pH of each substrate was subjected at 6, 7, and 8 respectively. The pH values for all the four 4 substrates was maintained at 6 acidic form, 7 at neutral stage and 8 elevated using potassium hydroxide (KOH) and hydrochloric acid (HCl). The conical flasks containing substrates were allowed to cool at room temperature and inoculated with Sacchromyces cereviciae for all the samples and were kept in pH 6, 7, and 8.
However, the same procedures were used for all the substrates with baker’s yeast and subsequently all the substrates with the mixture of baker’s yeast and Sacchromyces cereviciae. The same procedure was applied for the substrates at temperatures 30°C, 40°C, and 50°C. All the flasks containing the substrates were covered with cotton wool, wrapped with aluminium foil, and autoclaved at 121°C for 15 minutes. After autoclaved, the flasks was allowed to cool to room temperature and then inoculated with sachromyces cerevisiea, the flasks 6,7, and 8 were incubated anaerobically at 37°C while the same procedure was done and incubated at different temperature 30, 40, and 50°C. The same procedure was applied for the other substrates.
Fractional Distillation
This was carried out according to the method described by Oyeleke and Jubrin (2009). The fermented broths were dispensed into round bottom flask fixed to distillation column enclosed on running tap water. Conical flasks were fixed to the other end of the distillation column to collect the distillate. A heavy mantle with the temperature adjusted at 780C was used to heat the round bottom flask containing the fermented broth. i.e. Jatropha curcas with Sacchromyces cerevicae, Azadrachta indica with Sacchromyces cereviciae, Raccinus communis with Sacchromyces cerevicae. However, the same procedures were used for all the substrates with baker’s yeast and subsequently all the substrates with the mixture of baker’s yeast and Sacchromyces cereviciae.
This was carried out according to the method described by Oyeleke and Jubrin (2009). The fermented broths were dispensed into round bottom flask fixed to distillation column enclosed on running tap water. Conical flasks were fixed to the other end of the distillation column to collect the distillate. A heavy mantle with the temperature adjusted at 780C was used to heat the round bottom flask containing the fermented broth. i.e. Jatropha curcas with Sacchromyces cerevicae, Azadrachta indica with Sacchromyces cereviciae, Raccinus communis with Sacchromyces cerevicae. However, the same procedures were used for all the substrates with baker’s yeast and subsequently all the substrates with the mixture of baker’s yeast and Sacchromyces cereviciae.
Determination of Reducing Sugar
After hydrolysis the samples were filtered using white filter paper to remove the unhydrolysed materials. The reducing sugar for all the substrates were detected using Benedict’s reagents and spectrometer which was applied to obtained the concentrations of sugar in each sample. The determination of reducing sugar was obtained by adding 2 ml of Benedict’s solution in 5 ml of each filtered substrates in test tubes; the mixture was boiled in water bath for 10minutes. Portion of the mixture from each test tube was used for spectrometry and the concentration of reducing sugar was determined based on Beer’s Lambert law as shown blow.
After hydrolysis the samples were filtered using white filter paper to remove the unhydrolysed materials. The reducing sugar for all the substrates were detected using Benedict’s reagents and spectrometer which was applied to obtained the concentrations of sugar in each sample. The determination of reducing sugar was obtained by adding 2 ml of Benedict’s solution in 5 ml of each filtered substrates in test tubes; the mixture was boiled in water bath for 10minutes. Portion of the mixture from each test tube was used for spectrometry and the concentration of reducing sugar was determined based on Beer’s Lambert law as shown blow.
Where, conc. of Standard is given as 5.5 Mmol/l calibrated Absorbance of glucose standard is = 0.263
Determination of Bioethanol Concentration
After fermentation for 5 days the bioethanol produced by each substrate was determined using UV-spectrophotometer machine, the samples was calibrated and ran with at wavelength of 1000 nm to 250 nm and concentration of bioethanol produced was obtained
After fermentation for 5 days the bioethanol produced by each substrate was determined using UV-spectrophotometer machine, the samples was calibrated and ran with at wavelength of 1000 nm to 250 nm and concentration of bioethanol produced was obtained
Results and Discussion
Concentration of reducing sugar was determined from different substrates in this work as shown in Table 1. This reducing sugar defends on the type and nature of the substrate that determined the concentration of bioethanol to be produced. Concentration of bioethanol at different temperature and pH values was also determined using different fermentative organisms as indicated in Figures 1-9s.
The temperature values used are 30°C, 40°C and 50°C while the pH values are 6, 7 and 8. Fermentative Microorganisms include Sacchromyces cerevisiae, Baker’s yeast and mixture of both Sacchromyces cerevisiae and Baker’s yeast. At 30°C bioethanol concentration was recorded by varying pH values (6, 7 and 8) and fermentation organisms (Sacchromyces cerevisiae Baker’s yeast and mixture of both Sacchromyces cerevisiae and Baker’s yeast). The procedure was repeated by changing the temperature value to 40°C and 50°C.
| Samples | Absorbance of Substrate | Conc. of reducing sugar mol/L |
| Jatropha carcus | 1.841 ± 0.21 | 38.50 ± 1.02 |
| Lageneria siceraria | 1.684 ± 0.02 | 35.22 ± 0.04 |
| Racinnu scommunis | 1.687 ± 0.01 | 35.28 ± 0.12 |
| Azadrichta indica | 1.694 ± 0.05 | 35.43 ± 1.20 |
Table 1: Shows the absorbance and concentration of reducing sugars of the substrates calibrated at
standard of glucose. It indicates that Jatropha has the highest value and the list is from Calabash.
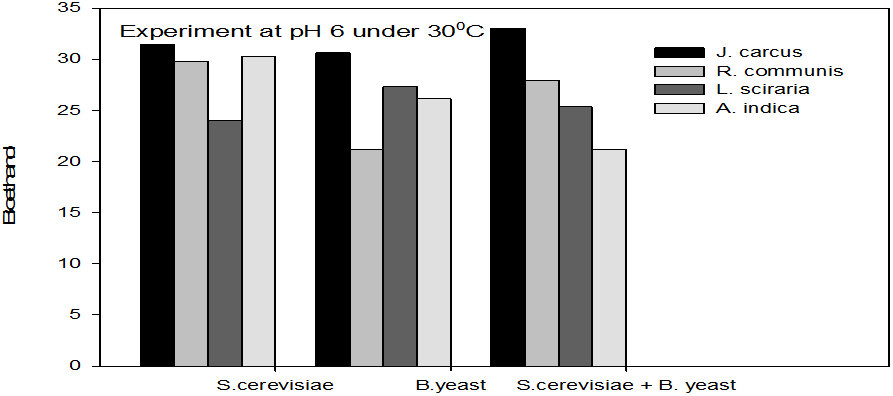
Figure 1: Concentrations of Bioethanol (mol/L) generated from cakes hydrolysate of J. curcus, R. cuminis,
L. siceraria, A. indica using S. cervise, B. yeast and mixture of S. cervise and B. yeast at pH 6 and 30°
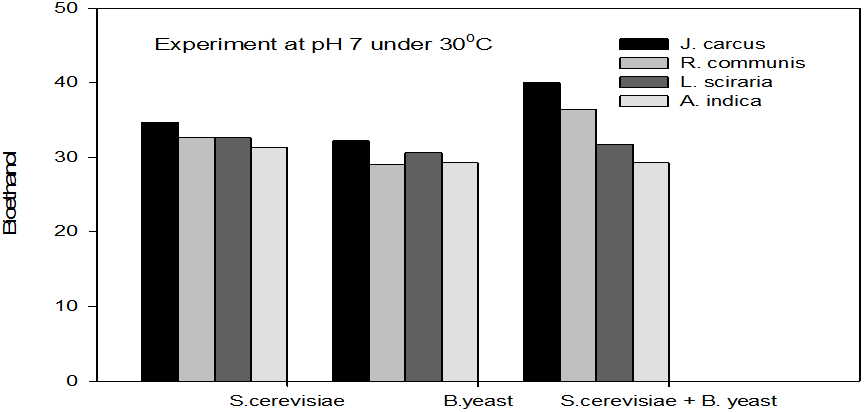
Figure 2: Concentrations of Bioethanol (mol/L) generated from cakes hydrolysate of J. curcus, R. cuminis,
L. siceraria, A. indica using S. cervise, B. yeast and mixture of S. cervise and B. yeast at pH7 and 30°
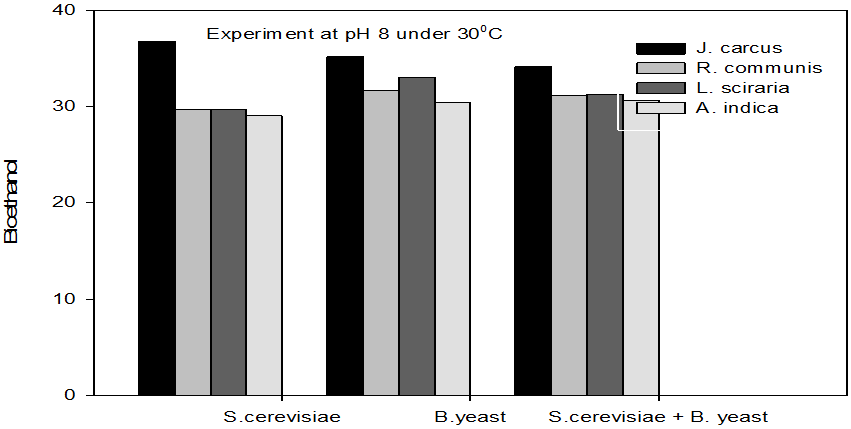
Figure 3: Concentrations of Bioethanol (mol/L) generated from cakes hydrolysate of J. curcus, R. cuminis,
L.siceraria, A. indica using S. cervise, B. yeast and mixture of S. cervise and B. yeast at pH8 and 30°
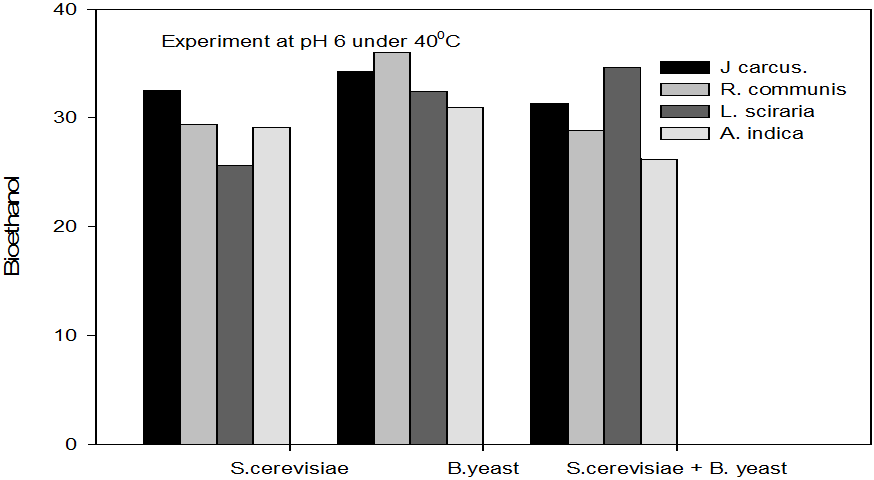
Figure 4: Concentrations of Bioethanol (mol/L) generated from cakes hydrolysate of J. curcus, R. cuminis,
L.siceraria, A. indica using S. cervise, B. yeast and mixture of S. cervise and B. yeast at pH 6 and 40°
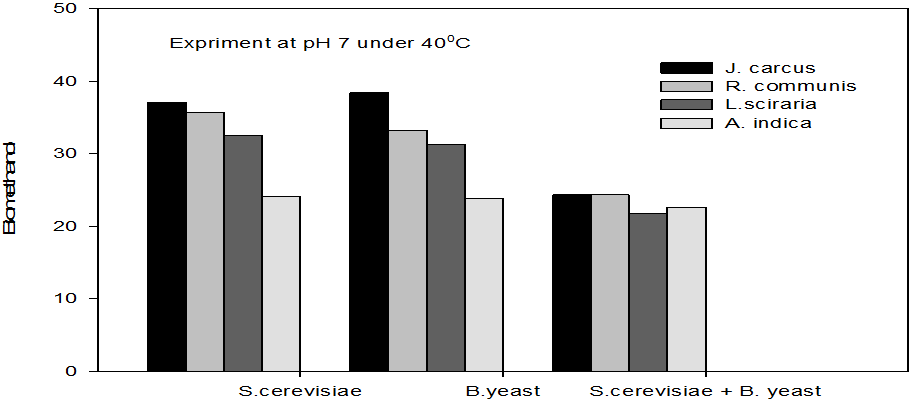
Figure 5: Concentrations of Bioethanol (mol/L) generated from cakes hydrolysate of J. curcus, R. cuminis,
L.siceraria, A. indica using S. cervise, B. yeast and mixture of S. cervise and B. yeast at pH7 and 40°
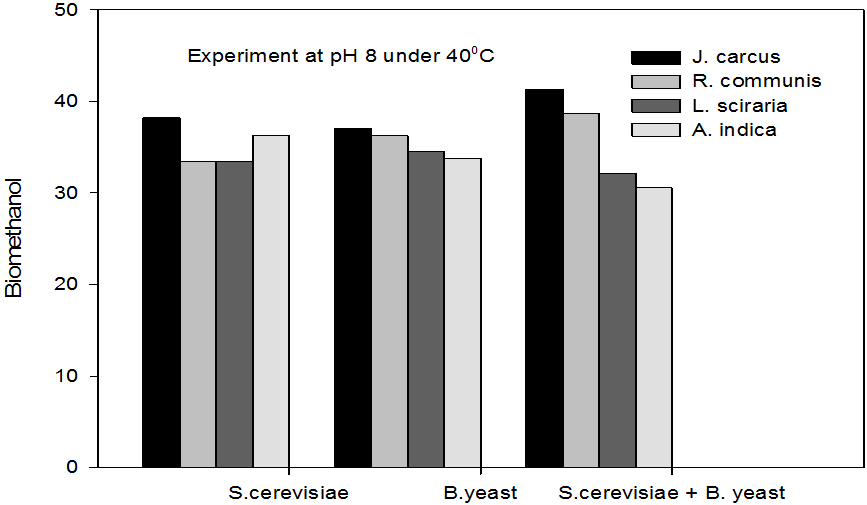
Figure 6: Concentrations of Bioethanol (mol/L) generated from cakes hydrolysate of J. curcus, R. cuminis,
L.siceraria, A. indica using S. cervise, B. yeast and mixture of S. cervise and B. yeast at pH8 and 40°
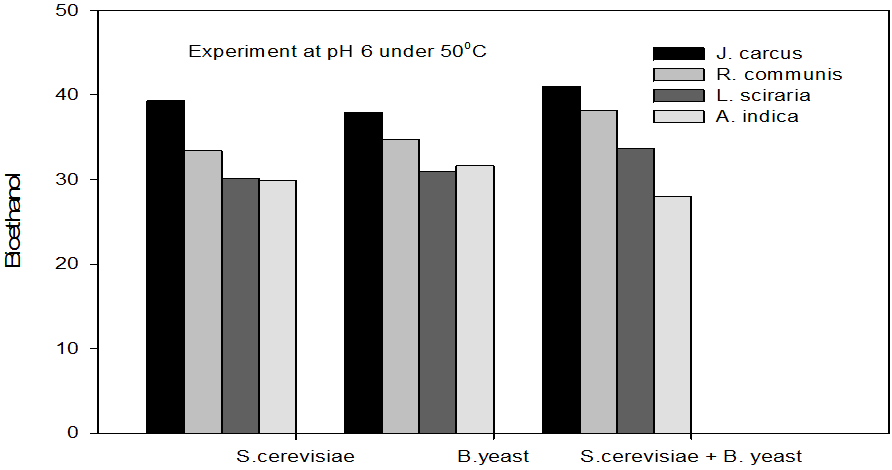
Figure 7: Concentrations of Bioethanol (mol/L) generated from cakes hydrolysate of J. curcus, R. cuminis,
L.siceraria, A. indica using S. cervise, B. yeast and mixture of S. cervise and B. yeast at pH 6 and 50°
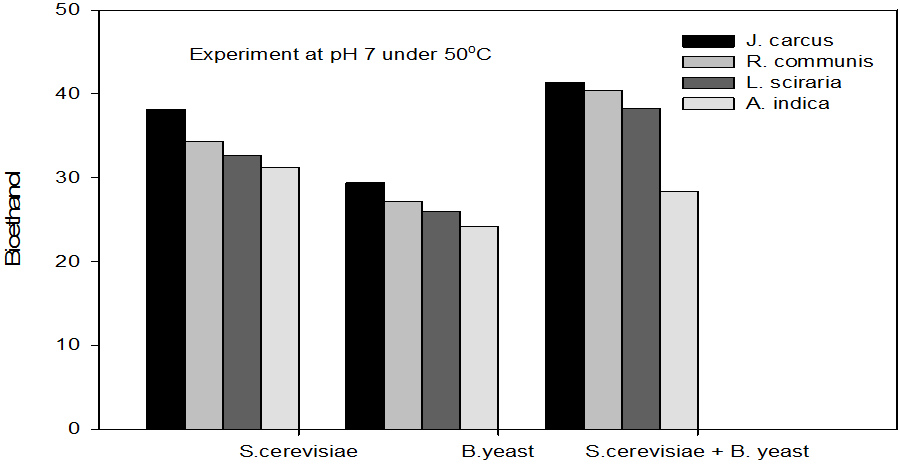
Figure 8: Concentrations of Bioethanol (mol/L) generated from cakes hydrolysate of J. curcus, R. cuminis,
L.siceraria, A. indica using S. cervise, B. yeast and mixture of S. cervise and B. yeast at pH 7 and 50°
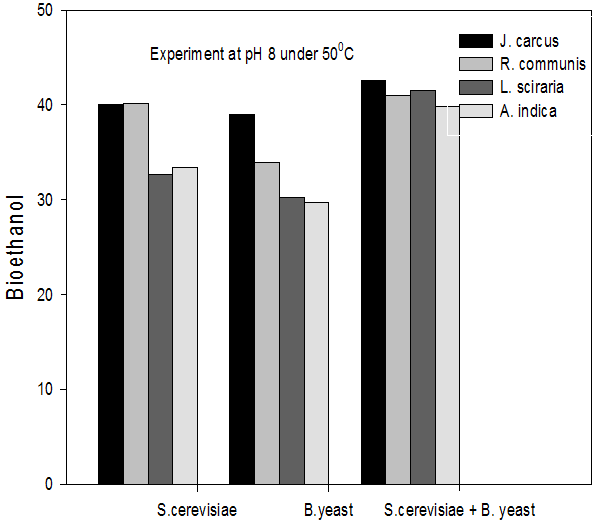
Figure 9: Concentrations of Bioethanol (mol/L) generated from cakes hydrolysate of J. curcus, R. cuminis,
L.siceraria, A. indica using S. cervise, B. yeast and mixture of S. cervise and B. yeast at pH8 and 50°
Discussion
In the present study, fuel ethanol was produced by fermentation of cellulosic substrates. The abundantly and cheaply available cellulosic substrate cakes (wastes) after extraction of oil from Jatropha curcas, Ricinnus communis, Azadrachta indica and Lagenaria siceraria (Calabash) were utilized. The production of bioethanol under different pH, temperature and fermentative agents indicated variation.
The quantity of bioethanol produced from the fermentation by Saccharomyces crevasse, Baker’s yeast and mixture of S. cerevisiae with Baker’s yeast were presented in figure however, the results obtained showed that Jatropha curcas (cake) produced highest bioethanol (42.62 g/l) at pH 8 under fermentation organisms mixture of S. cerevisae and Baker’s yeast at temperature range of 50°C as shown in Figure 9. The least bioethanol was observed from Azadrachta indica (Neem) (21.14g/l at pH6, temperature 30oC under fermentation by mixture of S. cerevisiae and Baker’s yeast as shown in Figure 4.
From the results, it showed that Aspergillus niger was able to hydrolyzed the cake from Jatropha curcas than the other substrates in to simple sugars which further fermented by the mixture of S. cerevisiae and Baker’s yeast in yielding maximum bioethanol. This result is in agreement with the report by Oyeleke and Jibrin (2009) reported maximum ethanol from Guinea Corn husk (26.83g/l) and Millet husk (18.31g/l) using A. niger and Z. mobilis as fermentation agents simultaneously. The maximum quantity of bioethanol produced (42.62g/l) by Jatropha curcas as a result of fermentation by mixture of S. cerevisiae and Baker’s correspond to that of olivera., et al. 2005 who reported highest quantity of bioethanol from Cassava waste using Z. mobilis as fermentation agent. It also corresponds to 27.7g/l reported by Lekneth., et al. (1994) on Sweet sorghum.
This implies that yeast is a good fermentation agent of cellulosic material in the production bioethanol. However, the maximum quantity of bioethanol produced by Jatropha waste in this work (42.62 g/l) is higher than that of Guinea corn husk (26.83 g/l) and Millet husk (18.31 g/l) as reported by Oyeleke and Jibrin, (2009). It was also higher than the (31.33 g/l) reported by This could be due to high content of simple sugar in Jatropha that could be fermented to ethanol than other substrates used in this work.
From the figures, there is no significant difference in the bioethanol concentration by all the four substrates since they produced remarkable quantity of bioethanol but Jatropha curcas’s cake produced higher quantity and by the mixture of S. cerevicae and Baker’s yeast. This may be due to the higher presence of alcohol dehydrogenase (ADH) in the mixture which appears to facilitate fermentation with higher ethanol production as reported by sharma., et al. 2007.
This implies that, the more the Alcohol dehydrogenase in the fermentation agent the more it ferments the substrate that gives bioethanol as product. All the substrates in this work shows remarkable production at temperature 5°C Which was consistent with the findings by el rufa'i., et al. (1992) the optimum maximum ethanol production from the beets molasses at varied temperatures, yielded high ethanol (41.22 g/l) at 45 and 50,. this could be attributed to the ability of the substrates to undergo fermentations and further breaks the complex sugar in to more simpler one sufficiently and effectively at the temperature range of 50°c.
Conclusion
Jatropha curcas, Ricinnus communis, Azadrachta indica and Lagenaria siceraria (Calabash) oil seed cakes has been found to be good raw material for cellulosic ethanol production. The pre-treatment and hydrolysis of oil seed cakes to convert the cellulose in to reducing sugar has shown positive results. However, it can be concluded that oil seed’s cake from these substrates has capability to undergo enzymatic hydrolysis and fermentation for the production of bioethanol.
All the substrates were found to be good source of cellulose and can be utilized for cellulosic ethanol production. In the future, the cakes which is a waste by-product of biodiesel plant can be used as a good raw-materials for ethanol production and also solve the problem of self-disposal of byproduct. Further improvement in the process can also be carried out to enhance the bioethanol productivity. It can also encourage the plantation and development of biodiesel industries, as two important biofuels, biodiesel and bioethanol can be produced from the price of only one raw-material.
References
- Anujkumar., et al. “Economics of Environmental Technologies; an appraisal”. Biotechnology and Molecular Biology Review 2.1 (2017): 14-32.
- Brooks AA. “Ethanol Production Potentials of Local Yeast Strains Isolated from the Banana Feels”. African Journal of Biotechnology 7.20 (2008): 3749-3752.
- El-Rufai., et al. “Some physiological parameters for ethanol production from beet molasses by saccharomyces cerevisiae”. Bioscience technology 42.3 (1992):183-189.
- Hurprey CN and Caritas UD. “Optimization of Ethanol Production from Garcinia kola (Bitter cola) Pulp Agro-waste” African Journal of Biotechnology 6.17 (2007): 2033-2037.
- Lekneth., et al. “Bioethanol Technology Review” Journal of Biotechnology 16 (1994): 983-988.
- Ofuya and Nwajuiba. “Microbial Degradation and Utilization of Cassava Peels” World Journal of Microbiology and Biotechnology 6 (1990): 144-148.
- Olivera ME., et al. “Ethanol as fuel; Energy, carbondioxide balances and Ecological footprint”. Bioscience 55.7 (2005): 593-602.
- Oyeleke, S.B and Jibrin, N.M “Production of Bioethanol from Guinea Corn Husk and Millet Husk”. African Journal of Microbiology Research 3.4 (2009): 147-152.
- Oyeleke SB and Manga SB “Essentials of Laboratory Practical in Microbiology” Tobest Publishers, Minna, Nigeria (2008): 36-67.
- Sharma N., et al. “Optimization of fermentation parameters for production of ethanol from kinnow waste and banana peels by simultaneous saccharification and fermentation”. Indian Journal of Microbiology 47.4(2007): 310-316.
- Wayman CE. “Potentials Synergies and Challenges in Refining Cellulosic Biomass to fuels, Chemicals, and Power”. Biotechnology Progress 19 (2001): 284-286.
- Wright JD. Ethanol from Lignocelluloses. An overview; Energy Program 8 (2001): 71.
Citation:
Bello A and Baki AS. “Potentials of Jatropha carcus, Racinnus communis, Azadrichta indica and Lagenaria siceraria, de-oiled
Cakes for Bioethanol Production”. Clinical Biotechnology and Microbiology 1.5 (2017): 189-197.
Copyright: © 2017 Bello A and Baki AS. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.