Research Article
Volume 2 Issue 1 - 2018
T Lymphocytes Response to Caecal Coccidiosis in Broilers Infected with Exo and Endogenous Stages of Eimeria Tenella
1Department of Veterinary Parasitology and Entomology, Faculty of Veterinary Medicine, University of Jos, Nigeria
2Department of Veterinary Parasitology and Entomology, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria, Nigeria
3Department of Veterinary Medicine, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria, Nigeria
4Department of Theriogenology and Production, Faculty of Veterinary Medicine, University of Jos, Nigeria
5Department of Veterinary Biochemistry and Physiology, Faculty of Veterinary Medicine, University of Jos, Nigeria
6Central Diagnostic Laboratory, National Veterinary Research Institute, Vom, Nigeria
2Department of Veterinary Parasitology and Entomology, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria, Nigeria
3Department of Veterinary Medicine, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria, Nigeria
4Department of Theriogenology and Production, Faculty of Veterinary Medicine, University of Jos, Nigeria
5Department of Veterinary Biochemistry and Physiology, Faculty of Veterinary Medicine, University of Jos, Nigeria
6Central Diagnostic Laboratory, National Veterinary Research Institute, Vom, Nigeria
*Corresponding Author: Kaze Paul Davou, Department of Veterinary Parasitology and Entomology, Faculty of Veterinary Medicine, University of Jos, Nigeria.
Received: January 12, 2018; Published: January 24, 2018
Abstract
The present study reveals the proliferation of cytokines in treated and non- treated broilers consisting of IFN- γ, IL-1, IL-2, IL-4, IL-6, TNF and TGF. The CD4 count in the treated and non- treated broilers orally administered with various developmental stages of the parasite reached a peak on day 10 at primary; secondary and day 24 at tertiary infections. There was significant difference in the CD4 cell count between groups of the infected broiler chickens (p < 0.05). The current study observed a relationship between the different developmental stages of the parasite and lymphocytes response. Broiler chickens infected with sporulated oocyst (sporozoites) and merozoites treated and non- treated gave high CD4 T- lymphocyte numbers than the other groups throughout the experimental periods.
Keywords: Developmental stages; Parasite; Primary; Secondary; Tertiary Infections; Broiler Chickens; Lymphocytes; Sporulated oocyst (sporozoite); Merozoites; Treated and Non-Treated; Experimental periods
Abbreviations: IFN- γ - Interferon gamma; IL – Interleukins; TNF – Tumor necrotic factor; TGF- Transforming growth factor; CD – Cell of differentiation
Introduction
Eimeria tenella is the most prevalent and pathogenic coccidian resulting in morbidity and mortality, causing serious economic losses to the poultry industry worldwide. Immunocompetence is a key factor in evolutionary process given that parasitism and disease are strong forces promoting genetic variability (Halmilton and Zuk, 1982, May and Anderson (1983). A decrease in the immune response when experimentally exposed to parasites has been interpreted as a depletion of resources devoted to immune stimulation (Johnsen and Zuk, 1999).
However, an increase in the immune response after parasite infection is considered to be due to the stimulation of host immunological activity (Christe., et al. 2002, Horak., et al. 2006). In birds, the most abundant and studied T- lymphocyte subsets are CD4, CD5 and CD8 as they played a role in active defense against infections (Berndt and Methner, 2001, Davidson., et al. 2008). CD4 subset is implicated in the secretion of active substances such as cytokines, interferon and interleukin-6 (Berndt., et al. 2006) and commonly used to evaluate the health status of infected birds with infections. This study aim is to determine the T-lymphocyte response in broilers infected with Exo and endogenous stages of Eimeria tenella.
Materials and Methods
Study area
The experimental settings was at the PETCA building, Anguldi, 5 kilometers from the National Veterinary Research Institute, Vom, Jos Plateau State, Nigeria, where the laboratory work was carried out. The Jos Plateau lies on the pre-cambian from the cambian to jurasic northern Nigeria crystalline complex in central Nigeria. Its average elevation is about 1,250 m above mean sea level. The state is bounded on the north and west by Kaduna plains (on the average of 600 m above mean sea level) and on the south by Benue plains (on the average of 700 m above mean sea level), (PADP, 2002). Geographically, the Jos Plateau is located between latitude 08°24'N and longitude 008°32' and 010°38' east.
The experimental settings was at the PETCA building, Anguldi, 5 kilometers from the National Veterinary Research Institute, Vom, Jos Plateau State, Nigeria, where the laboratory work was carried out. The Jos Plateau lies on the pre-cambian from the cambian to jurasic northern Nigeria crystalline complex in central Nigeria. Its average elevation is about 1,250 m above mean sea level. The state is bounded on the north and west by Kaduna plains (on the average of 600 m above mean sea level) and on the south by Benue plains (on the average of 700 m above mean sea level), (PADP, 2002). Geographically, the Jos Plateau is located between latitude 08°24'N and longitude 008°32' and 010°38' east.
The land surface of Jos Plateau consists of plains, hills, depressions and todes of various forms, shapes and sizes. It is a major tourist centre in Nigeria with agriculture as the main occupation of the people. The high altitude confers on the Plateau lower temperature than those encountered elsewhere in Nigeria except the Obudu and Mambilla Plateau. The dry season is determined by the north easterly tropical continental air masses known as harmattan (from October – April) and the wet season is the most tropical maritime air masses from May – September.
The average annual rainfall is about 1,100 mm and is evenly distributed. Another element of climate is temperature December and January experience temperatures of below 15°C. During February and March, the temperature rises again about 25°C. Most of the human activities are mining and agriculture involving rearing of chickens in both the rural and urban areas for subsistency and income (PADP, 2002).
Experimental birds
Four hundred (400) day-old broilers (marshal breed) were purchased from ECWA farms, Jos, brooded and used for the study. The birds were randomly distributed into six different groups of 40 each, in a clean wire cage (n= 40). At two weeks old, each group was again subdivided into two, treated and non-treated, of twenty broilers (n=20) each. The birds were kept in a clean building, and the legs banded or labelled under strict biosecurity measures. Feed (Broiler starter, Grand cereals and oil mills, PLC, Zawan, and Jos-Plateau, Nigeria) and water were provided adlibitum. The birds were vaccinated with Newcastle disease vaccine (La-Sota) at day 21 and Gomboro disease vaccines at days 14 and 28.
Four hundred (400) day-old broilers (marshal breed) were purchased from ECWA farms, Jos, brooded and used for the study. The birds were randomly distributed into six different groups of 40 each, in a clean wire cage (n= 40). At two weeks old, each group was again subdivided into two, treated and non-treated, of twenty broilers (n=20) each. The birds were kept in a clean building, and the legs banded or labelled under strict biosecurity measures. Feed (Broiler starter, Grand cereals and oil mills, PLC, Zawan, and Jos-Plateau, Nigeria) and water were provided adlibitum. The birds were vaccinated with Newcastle disease vaccine (La-Sota) at day 21 and Gomboro disease vaccines at days 14 and 28.
Experimental infection of broilers with infective materials and monitoring
The experimental birds, except the control were orally given primary and secondary challenge infections with the various developmental stages of Eimeria tenella, respectively at week 2 and 3 while at week 5 of age, all birds were infected the sporulated oocyst of the parasite (Table 1). Each group was subdivided into Treated (n = 20) and Non- Treated (n = 20). In each infected group, birds in one of the subdivisions were treated with amprolium 250 WSPR Holland was administered in drinking water at a concentration of 250 mg/1 (0.025%) for a period of 5 days as prescribed by the Manufacturer at the appearance of visible clinical signs.
The experimental birds, except the control were orally given primary and secondary challenge infections with the various developmental stages of Eimeria tenella, respectively at week 2 and 3 while at week 5 of age, all birds were infected the sporulated oocyst of the parasite (Table 1). Each group was subdivided into Treated (n = 20) and Non- Treated (n = 20). In each infected group, birds in one of the subdivisions were treated with amprolium 250 WSPR Holland was administered in drinking water at a concentration of 250 mg/1 (0.025%) for a period of 5 days as prescribed by the Manufacturer at the appearance of visible clinical signs.
To obtain serum, blood samples were collected from the experimental birds using the method described by Talebi and Mulcahy (1995). Briefly, 1ml of blood sample was obtained from the wing vein of each bird using 20 gauge needle (Becton Dickson co., Plymouth, UK) into a 2ml vacutainer. Samples were obtained on days 2, 4, 6, 8, and 10 after primary and secondary infections, and on days 5, 7, 11,14, 17, 20 and 24 after tertiary infection (Rose and Hasketh, 1982). The blood which had been allowed to clot for 1 hour at room temperature, was left over night at 4°C and then centrifuge at 800g for 5 minutes. The serum samples were thereafter heated at 56°C for 30 minutes to inactivate the compliment before storage at -20°C. All sera were analyzed with the developed ELISA Triplicate
| Group Treatment And No. of birds |
Infection type/ Age of bird | |||
| I°/wk 2 | 2°/wk 3 | 3°/wk 2 challenge with virulent E. tenella | ||
| I | T (n = 20) | 105 USO | 105 USO | 105 SO |
| NT (n = 20) | 105 USO | 105 USO | 105 SO | |
| II | T (n = 20) | 105 SO | 105 SO | 105 SO |
| NT (n = 20) | 105 SO | 105 SO | 105 SO | |
| III | T (n = 20) | 105 SCZ | 105 SCZ | 105 SO |
| NT (n = 20) | 105 SCZ | 105 SCZ | 105 SO | |
| Iv | T (n = 20) | 105 MRZ | 105 MRZ | 105 SO |
| NT (n = 20) | 105 MRZ | 105 MRZ | 105 SO | |
| V | T (n = 20) | 105 GMT | 105 GMT | 105 SO |
| NT (n = 20) | 105 GMT | 105 GMT | 105 SO | |
| VI | 0 | 0 | 0 | |
KEY; USO-Unsporulated oocyst
1°- primary infection
SO- Sporulated oocyst
2°- Secondary infection
SCZ- Schizoites
3°- Tertiary infection
WK-Week
T –Treated
NT – Non treated
MRZ- Merozoites
GMT- Gametocytes
Table 1: Experimental infection of broilers with developmental stages of Eimeria tenella.
1°- primary infection
SO- Sporulated oocyst
2°- Secondary infection
SCZ- Schizoites
3°- Tertiary infection
WK-Week
T –Treated
NT – Non treated
MRZ- Merozoites
GMT- Gametocytes
Table 1: Experimental infection of broilers with developmental stages of Eimeria tenella.
Determination of Immunity Conferred on Birds by Eimeria tenella Developmental Stages.
This was done by measurement of immune bodies using Lymph proliferation assay or Non-radioactive assay and Flow cytometric analysis
This was done by measurement of immune bodies using Lymph proliferation assay or Non-radioactive assay and Flow cytometric analysis
Lymphocyte proliferation studies
The lymphocyte proliferation assay is widely used to evaluate cell-mediated immunity in normal and disease states in chickens (Pfohl., et al.1997; Miyamoto., et. al. 2002). The spleen collected from each of the scarified broilers (four per group) at the end of each infection were crushed by pressing on fine mesh Petri dishes containing PBS and glass beads. The suspension was then passed through nylon cell strainer (70 µm; Becton, Dickson, Lincoln Park, NJ).
The lymphocyte proliferation assay is widely used to evaluate cell-mediated immunity in normal and disease states in chickens (Pfohl., et al.1997; Miyamoto., et. al. 2002). The spleen collected from each of the scarified broilers (four per group) at the end of each infection were crushed by pressing on fine mesh Petri dishes containing PBS and glass beads. The suspension was then passed through nylon cell strainer (70 µm; Becton, Dickson, Lincoln Park, NJ).
The filterate was centrifuged at 250 g for 10 minutes at 4oC and the sediment containing the spleen cells was collected for the study. One hundred microliter of splenic cell suspension containing 5×105 cells was placed in each of 96-well sterile culture plate (Corning, NY) containing 100 μl of complete Rose Park Memorial Institute (RPMI) media containing various concentrations (0.1, 1.0, 10 or 20 μg/ml). of concanavalin A (Con-A, Sigma, MO). The plates were incubated for 48 hours at 37°C in a 5% CO2, 95% humidity incubator (Ansar., et al. 1994).
Colorimetric analysis
After 48 hours of culture of the splenic cells of the orally infected broiler chickens, Resazurin or Alamar Blue TM (Accumed International, Westlake, OH, from Biosource/Tago Immunochemicals, Camars, CA) was added at 20 μl/well, and absorbance value were read at wavelengths of 570 nm (reduced state) and using an optical density Colorimeter Plate Reader (Molecular Devices, Menlo Park, CA) 24h after the addition of Alamar Blue. Purple colour was observed on the proliferated lymphocytes (Ansar., et al. 1994).
After 48 hours of culture of the splenic cells of the orally infected broiler chickens, Resazurin or Alamar Blue TM (Accumed International, Westlake, OH, from Biosource/Tago Immunochemicals, Camars, CA) was added at 20 μl/well, and absorbance value were read at wavelengths of 570 nm (reduced state) and using an optical density Colorimeter Plate Reader (Molecular Devices, Menlo Park, CA) 24h after the addition of Alamar Blue. Purple colour was observed on the proliferated lymphocytes (Ansar., et al. 1994).
Flow cytometric analysis
Whole blood (20 µl) from each group of treated and non-treated broilers orally infected with the parasite stages was added into a test tube, cells were stained with fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD90.2 (Thy 1.2), isotype anti-rat IgG2a, κ control antibodies; and kept in the dark for 15 minutes at room temperature. These stained cells were analyzed using CD4/CD4% PARTEC CYFLOW-Cyflow counter 2010, USA (Ansar and Sriranganathan, 1994).
Whole blood (20 µl) from each group of treated and non-treated broilers orally infected with the parasite stages was added into a test tube, cells were stained with fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD90.2 (Thy 1.2), isotype anti-rat IgG2a, κ control antibodies; and kept in the dark for 15 minutes at room temperature. These stained cells were analyzed using CD4/CD4% PARTEC CYFLOW-Cyflow counter 2010, USA (Ansar and Sriranganathan, 1994).
Data Analyses
The data were subjected to statistical analysis using Analysis of variance (ANOVA) by SPSS version 20. Data was expressed as (mean standard error of the mean (mean S.E.M). Values of p < 0.05 was considered significant.
The data were subjected to statistical analysis using Analysis of variance (ANOVA) by SPSS version 20. Data was expressed as (mean standard error of the mean (mean S.E.M). Values of p < 0.05 was considered significant.
Results
Lymphoproliferation studies in the experimental broiler chickens
Lymphoproliferation assay showed the proliferation of protective cytokines in the spleen of broilers infected with the various developmental stages of the parasite. Groups I (infected with unsporulated oocyst) and III (infected with schizonts) did elicit lymphocytes proliferation (IFN- γ, IL-1, IL-2, IL-4, IL-6 and TNF) at primary and secondary infections respectively. Groups II and IV revealed the proliferation of Interferon (IFN- γ), Interleukins (IL-1, IL-2, IL-4, IL-6), Turmor necrotic factor (TNF), Transforming growth factor (TGF), respectively at primary-secondary-tertiary infections, while Group V (infected with gametocytes) showed IL-1, IL-2 and IL-4 at primary and secondary infections. However, groups I, III and V showed proliferation of IFN- γ, IL-1, IL-2, IL-4, IL-6, TNF and TGF respectively at tertiary infection. The control showed no lymphocytes proliferation.
Lymphoproliferation assay showed the proliferation of protective cytokines in the spleen of broilers infected with the various developmental stages of the parasite. Groups I (infected with unsporulated oocyst) and III (infected with schizonts) did elicit lymphocytes proliferation (IFN- γ, IL-1, IL-2, IL-4, IL-6 and TNF) at primary and secondary infections respectively. Groups II and IV revealed the proliferation of Interferon (IFN- γ), Interleukins (IL-1, IL-2, IL-4, IL-6), Turmor necrotic factor (TNF), Transforming growth factor (TGF), respectively at primary-secondary-tertiary infections, while Group V (infected with gametocytes) showed IL-1, IL-2 and IL-4 at primary and secondary infections. However, groups I, III and V showed proliferation of IFN- γ, IL-1, IL-2, IL-4, IL-6, TNF and TGF respectively at tertiary infection. The control showed no lymphocytes proliferation.
In the study, the number of CD4 count increased post infection in treated and non-treated broilers orally administered with various developmental stages of the parasite, reaching a peak at day 10 ((groups I – 198.0 x 103 µl, 165.3 x 103 µl; 200.0 x 103 µl, 156 x 103 µl and 196.7 x 103 µl, 173.3 x 103 µl ; II – 199.0 x 103 µl, 186.0 x 103 µl ; 197.0 x 103 µl, 192.7 x 103 µl and 200.0 x 103 µl, 194 x 103 µl; III – 198 x 103 µl, 153.3 x 103 µl ; 200.0 x 103 µ,l 160.0 x 103 µl and 188.7 x 103 µl, 166.7 x 103 µl ; IV – 193.3 x 103 µl, 183 x 103 µl; 198.7 x 103 µl, 183.3 x 103 µl and 190 x 103 µl , 188.0 x 103 µl ; V – 200.0 x 103 µl, 198.0 x 103 µl ; 187.3 x 103 µl , 174 x 103 µl and 188.7 x 103 µl, 175.3 x 103 µl respectively) of primary and secondary infections and day 24 of tertiary infection (Tables 2.1, 2.2 and 2.3).
There was significant difference in the CD4 cell count among different groups of the experimental broilers (p < 0.05). CD4 levels were higher in the treated than the non-treated broilers at primary infection (Figure 1). The levels of CD4 cells increases rapidly in the non-treated birds at secondary infection, showing a non-significant difference in the CD4 levels in all the groups treated and non-treated (Figure 2). Groups II and IV of the non-treated birds had higher CD4 levels than groups I, III and V at both secondary and tertiary infections (Figures 2 and 3). There was significant difference in CD4 subset between groups of the study birds (p < 0.05). The current study observed a relationship between the different developmental stages of the parasite and immune responses (humoral and lymphocytes responses). Broilers infected with sporulated oocysts (sporozoites) and merzoites yielded high CD4 T- lymphocyte numbers than the other groups, throughout the experiment periods (Tables 2.1, 2.2 and 2.3).
| Group and Non-treated | Time (days) | ||||
| 2 | 4 | 6 | 8 | 10 | |
| I | 133.3 ± 58.6 | 158.3 ± 61.1 | 143.3 ± 57.7 | 133.3 ± 61.1 | 163.3 ± 57.7 |
| II | 120.0 ± 65.6 | 180.0 ± 58.9 | 139.3 ± 57.7 | 131.3 ± 60.8 | 186.0 ± 85.4 |
| III | 143.3 ± 84.7 | 147.3 ± 84.7 | 140.3 ± 85.8 | 133.3 ± 85.5 | 153.3 ± 76.4 |
| IV | 126.7 ± 55.1 | 166.0 ± 50.7 | 110.7 ± 53.5 | 146.0 ± 63.9 | 183.7 ± 90.2 |
| V | 144.0 ± 95.5 | 170.0 ± 85.4 | 146.7 ± 76.4 | 153.3 ± 68.1 | 180.0 ± 85.4 |
| VI | 134.7 ± 102.5 | 198.0 ± 86.6 | 195.0 ± 86.6 | 198.0 ± 86.6 | 198.0 ± 86.6 |
| Treated | |||||
| I | 198.7 ± 91.4 | 200.0 ± 117.9 | 160.0 ± 52.9 | 195.3 ± 95.1 | 199.0 ± 95.4 |
| II | 146.0 ± 87.0 | 200.0 ± 86.8 | 162.7 ± 83.6 | 168.0 ± 95.4 | 199.0 ± 95.4 |
| III | 150.0 ± 90.4 | 200.0 ± 86.6 | 160 ± 96.0 | 166.7 ± 83.3 | 198.0 ± 86.6 |
| IV | 163.0 ± 88.9 | 198.7 ± 94.3 | 171.0 ± 86.6 | 177.0 ± 88.9 | 193.3 ± 90.7 |
| V | 135.0 ± 91.7 | 200.0 ± 91.7 | 155.3 ± 96.5 | 169.7 ± 99.9 | 200.0 ± 86.6 |
Table 2.1: CD4 Lymphocytes subset counts (cells/103 µl)) in experimental broilers orally infected with E. tenella developmental stages at primary infection treated and non-treated.
No significant difference was observed across the days for both treated and non-treated (p > 0.05). However, comparison of treated and non-treated showed significant difference (p < 0.05) for all the days.
| Non-treated | days 2 | 4 | 6 | 8 | 10 |
| I | 140.0 ± 60.8 | 144.0 ± 62.9 | 140.0 ± 40.0 | 154.0 ± 64.1 | 156.7 ± 70.1 |
| II | 183.3 ± 82.5 | 185.3 ± 86.0 | 187.3 ± 90.7 | 189.3 ± 95.0 | 192.7 ± 84.7 |
| III | 143.3 ± 62.5 | 145.3 ± 63.6 | 146.7 ± 64.3 | 170.0 ± 50.7 | 160.0 ± 69.3 |
| IV | 180.0 ± 85.4 | 168.0 ± 76.9 | 178.7 ± 81.0 | 177.3 ± 86.7 | 183.3 ± 85.8 |
| V | 166.7 ± 76.4 | 170.7 ± 77.1 | 172.0 ± 76.9 | 166.0 ± 73.5 | 174.0 ± 78.1 |
| VI | 183.3 ± 104.1 | 200.0 ± 86.6 | 200.0 ± 86.6 | 200.0 ± 86.6 | 200.0 ± 86.6 |
| Treated | |||||
| I | 200.0 ± 86.6 | 196.0 ± 99.1 | 190.0 ± 96.4 | 199.3 ± 95.3 | 200.0 ± 86.6 |
| II | 196.7 ± 95.2 | 188.7 ± 90.3 | 194.0 ± 91.2 | 197.3 ± 95.0 | 197.0 ± 96.0 |
| III | 200.0 ± 86.6 | 192.7 ± 90.7 | 196.0 ± 92.6 | 200.0 ± 86.6 | 200.0 ± 86.6 |
| IV | 193.3 ± 95.0 | 195.3 ± 95.1 | 196.0 ± 95.2 | 196.0 ± 91.1 | 198.7 ± 94.5 |
| V | 180.0 ± 85.4 | 184.0 ± 85.9 | 186.0 ± 83.1 | 182.0 ± 81.6 | 187.3 ± 81.8 |
Table 2.2: CD4 Lymphocytes subset counts (cells/103 µl) in experimental broilers orally infected with E. tenella developmental stages at secondary infection treated and non-treated.
No significant difference was observed across the days for both treated and non-treated groups (p > 0.05). However, comparison of treated and non-treated showed significant difference (p < 0.05) for all the days.
| Group and Non-treated | Time (days) | ||||||
| 5 | 7 | 11 | 14 | 17 | 20 | 24 | |
| I | 133.3 ± 57.7 | 140.0 ± 60.8 | 140.0 ± 69.3 | 156.7 ± 67.0 | 160.7 ± 70.4 | 169.3 ± 76.8 | 173.3 ± 77.7 |
| II | 193.3 ± 90.7 | 194.7 ± 95.6 | 194.7 ± 77.1 | 186.0 ± 88.5 | 194.0 ± 87.8 | 191.3 ± 93.6 | 194.3 ± 85.6 |
| III | 165.3 ± 56.6 | 166.0 ± 28.6 | 134.0 ± 58.0 | 168.7 ± 46.3 | 138.7 ± 46.3 | 151.3 ± 66.9 | 166.7 ± 76.4 |
| IV | 186.7 ± 90.2 | 180.7 ± 85.9 | 189.3 ± 82.4 | 174.3 ± 79.3 | 171.0 ± 78.6 | 174.0 ± 81.3 | 188.0 ± 90.3 |
| V | 140.0 ± 60.8 | 146.7 ± 64.5 | 148.0 ± 64.5 | 171.3 ± 79.1 | 191.0 ± 41.4 | 174.0 ± 77.8 | 175.3 ± 81.1 |
| VI | 200.0 ± 86.6 | 200.0 ± 86.6 | 200.0 ± 86.6 | 200.0 ± 86.6 | 200.0 ± 86.6 | 200.0 ± 86.6 | 200.0 ± 86.6 |
| Treated | |||||||
| I | 194.0±95.5 | 195.3 ± 86.2 | 196.7 ± 95.2 | 198.0 ± 95.3 | 197.3 ± 98.0 | 192.7 ± 90.7 | 196.7 ± 89.5 |
| II | 196.3 ± 87.4 | 199.3 ± 95.3 | 214.0 ± 68.2 | 196.0 ± 84.9 | 198.7 ± 90.8 | 197.3 ± 98.0 | 200.0 ± 86.6 |
| III | 186.7 ± 81.4 | 178.7 ± 94.2 | 190.7 ± 88.9 | 194.0 ± 87.8 | 166.7 ± 76.4 | 165.3 ± 71.8 | 188.7 ± 90.3 |
| IV | 194.0 ± 87.8 | 195.3 ± 95.1 | 198.0 ± 97.5 | 170.7 ± 77.1 | 180.7 ± 85.9 | 186.7 ± 81.4 | 190.7 ± 97.0 |
| V | 182.0 ± 83.1 | 180.7 ± 85.9 | 179.3 ± 79.2 | 206.0 ± 45.9 | 184.7 ± 86.3 | 154.0 ± 66.9 | 188.7 ± 81.7 |
Table 2.3: CD4 Lymphocytes subset counts (cells /103 µl) in experimental broilers orally infected with E. tenella developmental stages at tertiary infection treated and non-treated.
No significant difference was observed across the days for both treated and non-treated (p > 0.05). A comparison of treated and non-treated showed significant difference (p < 0.05).
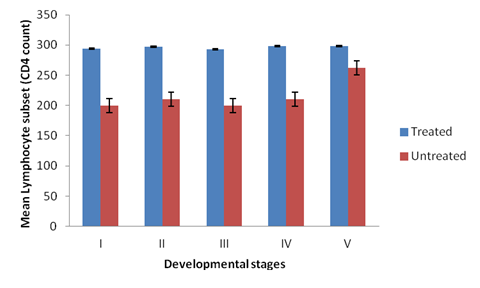
Figure 1: CD4 Levels in the plasma of broilers treated and non-treated
infected with the developmental stages of the parasite at primary infection.
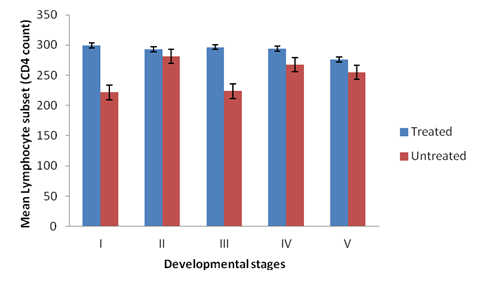
Figure 2: CD4 levels in the plasma of broilers treated and non-treated infected
with the developmental stages of the parasite at secondary infection.
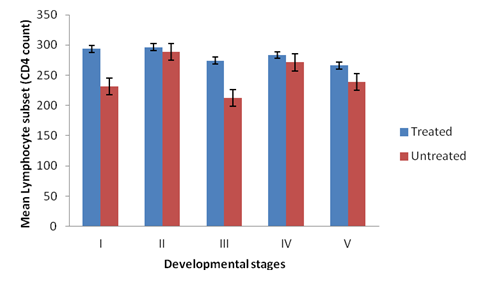
Figure 3: CD4 levels in the plasma of broilers treated and non-treated infected
with the developmental stages of the parasite at tertiary infection.
Discussion
The cytokines generally reported in this study include IFN- γ, IL-1, IL-2, IL-4, IL-6, TNF and TGF confirmed the findings of Oldham (2009) and Gadde., et al. (2011). The study also showed that infection of broilers with various developmental stages of Eimeria tenella elicited both cellular. Homologous and heterologous challenges of the birds with the various developmental stages of the parasite at primary, secondary and tertiary infection levels stimulated the secretion and proliferation of lymphocytes.
This is in concordance with the findings of Chapman., et al. (2005) who reported that the primary infection with Eimeria tenella oocyst induced complete protection against homologous challenges The findings from the study revealed the immunogenicity of the developmental stages of the Eimeria tenella and this is consistent with the reports of Molloy., et al. (2008) and Chow., et al. (2011) who stated that the surface antigens (SAGs) of the different Eimeria tenella stages were capable of initiating humoral and cytokines responses in birds.
The study reported a strong immune responses (humoral and cell mediated) in broilers infected with sporulated oocyst (sporozoite) and merozoites. This is similar to finding of Rose and. Hesketh (1987), Jenkins., et al. (1991), Lillehoj (1998), Kiani and Farhang (2008) and Molloy., et al. (2008). The strong immune response can be attributed to the relative invasive features of the parasite in nature due to proteins released from micronemes which are important for host binding and invasion.
The rhoptry proteins secreted during invasion to form the parasitephorous value within which the parasite resides. Also, the possession of glicosulphosphatidylinositol (G.P.I) –linked surface antigens (SAGs) may mediate binding to the host (Taberes., et al. 2004). However, this study is inconsistent with work of Onaga and Nakamiura (2003). The present study revealed an increase in the number of CD4 cells at day 10 post primary infection in both treated and non-treated broilers as shown by Lillehoj (1998).
Our study also recorded high numbers of CD4 cells after secondary infection of the birds at day 10 as against day 6 recorded by Lillehoj, (1998). This difference may be due to differences in the age of the broilers, the strain of the parasite used or the genetic background of the birds (Bucy., et al. 1998). The present study showed the expression of CD4 cells subset by blood lymphocytes of broilers infected with the various developmental stages of Eimeria tenella. This may indicate the induction of adaptive immune response to the infection (Gadde., et al. 2013).
The CD4 cell count expresses the numerical reactions of the broilers to oral administration of the different developmental stages of Eimeria tenella, revealing the stimulation of the immune system. This agrees with the reports of Hong., et al. (2006) and Lemus., et al. (2010). The study also revealed that the number of CD4 cells were significantly higher in the treated, than non-treated broilers. This is consistent to the finding of Hong., et al. (2006) who reported higher number of CD4 lymphocytes in infected birds treated than the non-treated ones.
The present study also demonstrated that the CD4 lymphocytes count increased at the different periods of infection with the various developmental stages of the parasite in both the treated and non- treated broilers. This is similar to the study of Bassey., et al. (1996) who observed that the CD4 changes follow the phases of the parasite cycle for the Eimeria species considered. There was also no significant difference in the CD4 cells count in both the treated and non-treated birds at secondary and tertiary infections. This is in accordance with the reports of Bassey., et al. (1996) in infections of birds with Eimeria tenella. In summary, this work add to our understanding of the ability of the various developmental stages of Eimeria tenella to induce immune responses in the chicken.
Conclusion
The following can be concluded from the results obtained:
- An immune response against ceacal coccidiosis could be established by immunization with Eimeria tenella-specific sporulated oocyst (sporozoites) and merozoites as well as other stages in birds of less than four weeks old.
- The prominent cytokines detected in the infected broilers were IFN- γ, IL-2, IL-4, IL-6, TNF and TGF, while the immunoglobulins are IgG or IgY.
- Circulating CD4 lymphocytes subset count increased with the duration of infection.
References
- Ansar AS and Sriranganathan N. “Differential effects of dexamathasone on the thymus and spleen: alterations in programmed cell death, lymphocyte subsets and activation of T cells”. Immunopharmacology28.1 (1994): 55-66.
- Ansar AS., et al. “A new rapid and non-radioactive assay to monitor and determine the proliferation of lymphocytes: An alternative to [3H] thymidine incorporation assay”. Journal of Immunological Methods 170.2(1994): 211-224.
- Bassey, M., et al. “Changes in intestinal intra-epithelial and systematic T-cell subpopulations after an Eimeria infection in chickens: Comparative study between Eimeria acervulina and Eimeria tenella”. Veterinary Research 27.4 (1996): 503-514.
- Berndt A and Methner U. “Gamma/delta T cell response of chicken after oral administration of attenuated and non-attenuated Salmonella typhimurium strains”. Veterinary Immunology and Immunopathology 78.2 (2OO1): 143-161
- Berndt, A., et al. “Circulating γð T cells in response to Salmonella enteric Scrovar Enteritidis Exposure in chickens”. Infection and Immunity74.7 (2006): 3967-3978.
- Bucy RP., et al. “Avian T cells expressing gama delta receptor localized in spleenic sinusoids and intestinal epithelium”. The Journal of Immunology141.7(1998): 2200-2205.
- Chapman HD., et al. “Acquisition of immunity of to Eimeria Maxima in newly hatched chickens given 100 oocyst”. Avian Diseases 49.3 (2005): 426-429.
- Chow YP., et al. “Immunogenic Eimeria tenella glycosylphosphatidylinositol-anchored surface antigens (SAGs) induce inflammatory responses in avian microphages”. PLos ONE 6 (2011):0025233.
- Christe P., et al. “Genetic and environmental components of phenotypic variation in immune response and body size of a colonial bird, Delichon urbica (the house martin)”. Heredity 85 (2002): 75-83.
- Davidson F., et al. “Avian Immunology”. Academic press, Elsevier Ltd. London (2008):
- Gadde, U., et al. “Acquisition of immunity to the protozoan Eimeria adenoeides in turkey poults and cellular responses to infection”. Poultry science 92.12 (2013): 3149-3157.
- Gadde U., et al. “Cellular immune responses, chemokines and cytokine profiles in turkey poults following infection with the intestinal parasite Eimeria adenoeides”. Poultry Science90.10 (2011): 2243-2250.
- Hamilton WD and Zuk M. “Heritable true fitness and bright birds: a role for parasite?” Science 218.4570 (1982): 384-387.
- Hong YH., et al. “Analysis of chicken cytokine and chemokine gene expression following Eimeria acervulina and Eimeria tenella infections”. Veterinary Immunology and Immunopathology114.3-4 (2006): 209-223.
- Horak P., et al. “Antioxidant protection, carotenoids and the cost of immune challenge in greenfinches”. Journal of Experimental Biology 209 (2006): 4329-4338.
- Jenkins MC., et al. “X irradiation of Eimeria tenella oocysts provides direct evidence that sporozoite invasion and early schizont development induce a protective immune responses”. Infection and Immunology 59.11 (1991): 4042-4048.
- Johnsen TS and Zuk M. “Parasites and tradeoffs in the immune response of female red jungle fowl”. Oikos 86.3 (1999): 487-492.
- Kiani R and Farhang HH. “Development of an ELISA test for serological diagnosis of coccidial infections and studying of resistance against coccidiostats based on flock history”. Asian Journal of Biological Sciences 1(2008): 77-83.
- Lemus J., et al. “Responses of circulating T-lymphocytes to a coccidian Infection: Insights from Parasitization-Vaccination experiment”. Functional Ecology 24.3 (2010): 638–645.
- Lillehoj HS and Kyeong S Chung “Postnatal development of T-Lymphocytes subpopulations in the intestine intraepithelium and lamina propria in chickens”. Veterinary Immunology and Immunopathology31.3-4 (1998): 347-360.
- Miyamoto T., et al. “Lymphocyte proliferation response during Eimeria tenella infection assessed by a new, reliable, Nonradioactive Colomrimetric assay”. Avian Disease 46 (2002): 10-16.
- Molloy JB., et al. “Antibody response against endogenous stages of an attenuated Eimeria tenella”. Veterinary Parasitology 154.3-4 (2008): 306-313.
- Oldham D. “Current concept in immunology”. In principles of cancer biotherapy, springer Publishing house, Heidelbery, London (2009): 85-99.
- Onaga H and Nakamiura T. “An Enzyme linked immunosorbent assay with recombinant merozoite protein as an antibodies of Eimeria necatrix”. Avian Diseases 47.2 (2003): 309-318.
- “Plateau Agricultural Development Programme (PADP)”. Annual Report (2002):
- Pfohl JL., et al. “Development of a highly, quantitative resproducible assay for determination of chicken T cell growth biological activity”. Poultry Science76.10 (1997): 1379-1386.
- Rose ME and Hesketh P. “Eimeria tenella: Effects of immunity on sporozoites within the lumen of the small intestine”. Experimental Parasitology 63. 3 (1987): 337-344.
- Taberes E., et al. “Eimeria tenella sporozoites and merozoites differentially express glycosulphatdylinosol-anchored variant surface proteins”. Molecular Biochemistry and Parasitology 135.1 (2004): 123-132.
- Talebi A and Mulcahy J. “Correlation between immune responses and oocyst production in chickens monospecifically infected with Eimeria maxima”. Avian Pathology 24.3 (1995): 485-495.
Citation:
Kaze Paul Davou., et al. “T Lymphocytes Response to Caecal Coccidiosis in Broilers Infected With Exo and Endogenous
Stages of Eimeria Tenella”. Clinical Biotechnology and Microbiology 2.1 (2018): 274-283.
Copyright: © 2018 Kaze Paul Davou., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.






























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.