Research Article
Volume 2 Issue 1 - 2018
Regulatory T cells and Transcription factor (FOXP3).
Medical Microbiology and Immunology -faculty of medecine -fayoum university- Egypt
*Corresponding Author: Abdelrahman Abdelmoktader, Medical Microbiology and Immunology -faculty of medecine -fayoum university- Egypt.
Received: February 27, 2018; Published: March 14, 2018
Abstract
Tregs, defined by the expression of CD4, CD25 and FOXP3. It is called scurf in, encode protein called foxp3 protein. Provides instructions for producing the forehead box P3 (Foxp3) protein. Which binds to specific regions of DNA and helps control the activity of genes that are involved in regulating the immune system
Keywords: CD4; CD25; FOXP3; Tregs; IPEX
Abbreviations: FOXp3: Forkhead box genes; Tregs: Regulatory T cells; IPEX syndrome: Immune dysfunction/Polyendocrinopathy/Enteropathy/X-linked; CD: cluster of differentiation IL: Interleukin; INF-y: interferon gamma; TNF-a: tumor necrosis factor alpha; TGF-B: tumor growth factor beta
Introduction
Regulatory T cells (Tregs)
Tregs, defined by the expression of CD4, CD25 and the transcription factor fork head box (FOXP3), have a central role in protecting an individual from autoimmunity and have been widely study in different autoimmune disorders [1].
Tregs, defined by the expression of CD4, CD25 and the transcription factor fork head box (FOXP3), have a central role in protecting an individual from autoimmunity and have been widely study in different autoimmune disorders [1].
Foxp3 (fork head box p3) gene
A member of a family of genes called FOX, it is called scurfin, encode protein called foxp3 protein. The human FOXP3 genes contains 12 exons, 4 domains, exon-intron boundaries are identical across the coding regions of the mouse and human genes. By genomic sequence analysis, the FOXP3 gene maps to the p arm of the X chromosome. It was identified during position cloning of Scurf in which is a gene responsible for X-linked autoimmune diseases in mice and humans [2-4].
A member of a family of genes called FOX, it is called scurfin, encode protein called foxp3 protein. The human FOXP3 genes contains 12 exons, 4 domains, exon-intron boundaries are identical across the coding regions of the mouse and human genes. By genomic sequence analysis, the FOXP3 gene maps to the p arm of the X chromosome. It was identified during position cloning of Scurf in which is a gene responsible for X-linked autoimmune diseases in mice and humans [2-4].
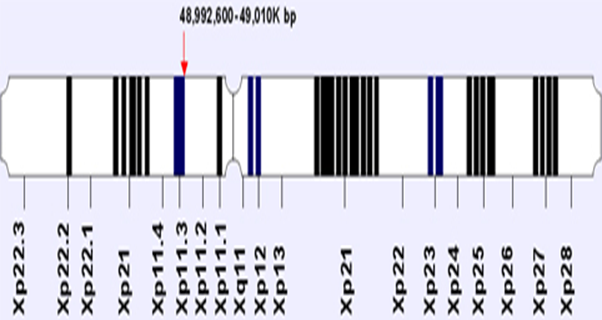
Figure 1: The location and orientation of FOXP3 gene on X chromosome. The
X-linked gene FOXP3 is a member of the fork head-box/winged-helix transcription
factor family. It was identified during position cloning of Scurf in, a gene responsible
for X-linked autoimmune diseases in mice and humans (2, 3, and 4).
FOXP3 function
It is a transcription factor, predominantly expressed in CD4+CD25+ Treg cells and is a master regulator for the development and function of Treg cells [5,6] The Foxp3 gene provides instructions for producing the Foxp3 protein. The Foxp3 protein binds to specific regions of DNA and helps control the activity of genes that are involved in regulating the immune system. On the basis of this role, the Foxp3 protein is called a transcription factor.
It is a transcription factor, predominantly expressed in CD4+CD25+ Treg cells and is a master regulator for the development and function of Treg cells [5,6] The Foxp3 gene provides instructions for producing the Foxp3 protein. The Foxp3 protein binds to specific regions of DNA and helps control the activity of genes that are involved in regulating the immune system. On the basis of this role, the Foxp3 protein is called a transcription factor.
This protein is essential for the production and normal function of regulatory T cells, which play an important role in preventing autoimmunity. The Foxp3 protein is found primarily in an immune system gland called the thymus, where regulatory T cells are produced. Mutations of Foxp3 gene in human are responsible for severe autoimmune disease, called IPEX syndrome [3]. Because deletion of Foxp3, in mature Treg cells, resulted in the loss of their suppressive function in vivo [7]. Exactly, Foxp3 amplifies and fixes pre-established molecular features of Treg cells, and solidifies Treg cell lineage stability [8]. Given the importance of Foxp3 in Treg cell development and function.
IPEX-syndrome accompanies autoimmune disease in multiple endocrine organs (e.g., type I diabetes and thyroiditis), inflammatory bowel disease (IBD), severe allergy including allergic dermatitis and food allergy, and fatal infection [9]. The indispensable role of Foxp3 for the control of these autoimmune and inflammatory disorders underlines the crucial importance of naturally arising Foxp3+CD4+ Tregs for self-tolerance and immune homeostasis. In mice, a Foxp3 mutation (a frameshift mutation that result in protein lacking the forkhead domain) is responsible for 'Scurfy', an X-linked recessive mouse mutant that results in lethality in hemizygous males 16 to 25 days after birth. [4] These mice have overproliferation of CD4+ T-lymphocytes, extensive multiorgans infiltration, and elevation of numerous cytokines.
Retroviral transduction or transgenic expression of Foxp3 in CD25−CD4+ T cells or CD8+ T cells is able to convert them to Treg-like suppressive T cells. for example, Foxp3-transduced CD25−CD4+ T cells are able to suppress proliferation of other T cells in vitro and inhibit the development of autoimmune disease and IBD in vivo (5,6). Foxp3 transduction suppresses IL-2 production but upregulates the expression of Treg-associated molecules, such as CD25, CTLA-4 and GITR, in conventional T cells (4).
Collectively, Foxp3 is a ‘master control gene’ for Treg development and function, especially for their suppressive function [11].
Genome-wide screening has revealed that Foxp3 up- or down regulates the expression of hundreds of genes by directly binding to their genomic loci [12]. Amongst these, a number of molecules crucial to Treg function are directly regulated by Foxp3. For example, Foxp3 directly binds to the promoter regions of IL-2 and CTLA-4 and co-operates with other transcriptional factors such as NFAT o down- or up-regulate their expression, respectively [13].
In animal studies, Tregs that express Foxp3 are critical in the transfer of immune tolerance, especially self-tolerance, so that hopefully in the future this knowledge can be used to prevent transplant graft rejection. [5,10] The induction or administration of Foxp3 positive T cells has, in animal studies, led to marked reductions in autoimmune disease severity in models of diabetes, multiple sclerosis, asthma, inflammatory bowel disease, thyroiditis and renal disease. [14]. these discoveries give hope that cellular therapies using Foxp3 positive cells may, one day, help overcome these diseases.
Factors control Foxp3 gene transcription
Many cytokines and factors regulate Foxp3 gene transcription as production of IL-4 following TCR stimulation up regulates GATA-3 expression (the master Th2 transcription factor), which can bind to the Foxp3 promoter region and suppress gene expression expression [15]. TGF-B signaling prevents IL-4 production, thereby inhibiting Gata3 expression and consequent binding to the Foxp3 promoter.
Many cytokines and factors regulate Foxp3 gene transcription as production of IL-4 following TCR stimulation up regulates GATA-3 expression (the master Th2 transcription factor), which can bind to the Foxp3 promoter region and suppress gene expression expression [15]. TGF-B signaling prevents IL-4 production, thereby inhibiting Gata3 expression and consequent binding to the Foxp3 promoter.
In murine T cells IL-4, IFN- y activates Stat1 that also prevents Foxp3 gene expression. However, in human T cells, IFN- y and IL-27 induced Stat1 can actually amplify TGF-B mediated Foxp3 gene expression. In addition, the human Foxp3 gene has a Stat1 binding element within proximal region of the promoter [16]. Thus, more thorough studies are required to clarify these results and define the effects of Th1-cytokines on Foxp3 gene transcription. The inflammatory cytokine IL-6 is well known to inhibit Foxp3 expression by promoting acquisition of the Th17 cell fate, in naïve T cells [17]. Foxp3 also promotes the expression of Foxp3 itself [13,18]. 1,25(OH)2VD3 can promote FOXP3 expression in CD4+ T cells via Binding to Vitamin D Response Elements in Its Conserved Non coding Sequence Region [19].
Conclusion
Tregs, defined by the expression of CD4, CD25 and the transcription factor foxp3. Foxp3 gene maps to the p arm of the X chromosome, and is a master regulator for the development and function of Treg cells provides instructions for producing the Foxp3 protein. The Foxp3 protein binds to specific regions of DNA and helps control the activity of genes that are involved in regulating the immune system. Mutations of Foxp3 gene in human are responsible for IPEX syndrome.
References
- Miyara M and Sakaguchi S. “Human FoxP3 (+) CD4 (+) regulatory T cells: their knowns and unknowns”. Immunology & Cell Biology 89.3 (2011): 346-351.
- Chatila TA., et al. “JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome”. Journal of Clinical Investigation 106.12 (2000): 75-81.
- Bennett Craig L., et al. “A rare polyadenylation signal mutation of the FOXP3 gene (AAUAAA→AAUGAA) leads to the IPEX syndrome”. Immunogenetics 53.6 (2001): 435-439.
- Wildin RS., et al. “Neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy”. Nature genetics 27.1 (2001): 18-20.
- Fontenot JD., et al. “Foxp3 programs the development function of CD4+CD25+ regulatory T cells”. Nature Immunology 4.4 (2003): 330-336.
- Khattri R., et al. “An essential role for Scurfin in CD4+CD25+ T regulatory cells”. Nature Immunology 4.4 (2003) (2003): 337-342.
- Williams LM and Rudensky AY. “Maintenance of the Foxp3-dependent Developmental program in mature regulatory T cells requires continued expression of Foxp3”. Nature Immunology 8.3 (2007): 277-284.
- Gavin MA., et al. “Foxp3-dependent programme of regulatory T-cell differentiation”. Nature 445.7129 (2007): 771-775.
- Wildin RS., et al. “Clinical and molecular features of the immunodysregulation, Polyendocrinopathy, enteropathy, X linked (IPEX) syndrome”. Journal of Medical Genetics 39.8 (2002): 537-345.
- Hori S., et al. “Control of regulatory T cell development by the transcription factor Foxp3”. Science 299.5609 (2003): 1057-1061.
- Daniel J Campbell and Meghan A Koch. “Phenotypic and functional specialization of FOXP3+ regulatory T cells”. Nature Reviews Immunology 11.2 (2011): 119-130.
- Marson A., et al. “Foxp3 occupancy and regulation of key target genes during T-cell stimulation”. Nature 445.7130 (2007): 931-935.
- Rudra D., et al. “Runx–CBFbeta complexes control expression of the transcription factor Foxp3 in regulatory T cells”. Nature Immunology 10.11 (2009): 1170-1177.
- Suri-Payer E and Fritzsching B. “Regulatory T cells in experimental autoimmune disease”. Seminars in Immunopathology 28.1 (2006): 3-16.
- Mantel PY., et al. “GATA3- driven Th2 responses inhibit TGF-beta1-induced FOXP3 expression and the formation of regulatory T cells”. PLOS Biology 5.12 (2007): 329.
- Ouaked N., et al. “Regulation of the foxp3 gene by the Th1 cytokines: the role of IL-27-induced STAT1”. Journal of Immunological Methods 182.2 (2009): 1041-1049.
- Bettelli E., et al.“Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells”. Nature 441.7090 (2006):235-238.
- Kitoh A., et al. “Indispensable role of the Runx1–Cbfbeta transcription complex for in vivo-suppressive function of FoxP3+ regulatory T cells”. Immunity 31.4 (2009): 609-620.
- Hu Zeng and Hongbo Chi. “Metabolic control of regulatory T cell development and function”. Trends in Immunology 36.1 (2015): 1-12.
Citation:
Abdelrahman Abdelmoktader. “ Regulatory T cells and Transcription factor (FOXP3).” Clinical Biotechnology and Microbiology
2.1 (2018): 298-301.
Copyright: © 2018 Abdelrahman Abdelmoktader. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.






























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.