Research Article
Volume 2 Issue 3 - 2018
Antibody Response to Respiratory Syncytial Virus Antigen Is Modulated by Artesunate
1Senior Lecturer and Group Leader, Department of Pharmaceutical Microbiology and Biotechnology, Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka, Enugu State, Nigeria
2Graduate Student, Department of Pharmaceutical Microbiology and Biotechnology, Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka, Enugu State, Nigeria
3Graduate Student, Department of Pharmaceutical Microbiology and Biotechnology, Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka, Enugu State, Nigeria
4Senior Lecturer, Department of Pharmaceutical Technology and Industrial Pharmacy, Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka, Enugu State, Nigeria
5Graduate Student, Department of Pharmaceutical Microbiology and Biotechnology, Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka, Enugu State, Nigeria
6Graduate Student, Department of Pharmaceutical Microbiology and Biotechnology, Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka, Enugu State, Nigeria
2Graduate Student, Department of Pharmaceutical Microbiology and Biotechnology, Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka, Enugu State, Nigeria
3Graduate Student, Department of Pharmaceutical Microbiology and Biotechnology, Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka, Enugu State, Nigeria
4Senior Lecturer, Department of Pharmaceutical Technology and Industrial Pharmacy, Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka, Enugu State, Nigeria
5Graduate Student, Department of Pharmaceutical Microbiology and Biotechnology, Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka, Enugu State, Nigeria
6Graduate Student, Department of Pharmaceutical Microbiology and Biotechnology, Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka, Enugu State, Nigeria
*Corresponding Author: Damian Chukwu Odimegwu, Department of Pharmaceutical Microbiology and Biotechnology, Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka, Enugu State, Nigeria.
Received: May 12, 2018; Published: May 31, 2018
Abstract
Background:The negative-sense, single-stranded RNA virus of the genus pneumovirus, known as respiratory syncytial virus (RSV) is the leading cause of viral lower respiratory tract illness in infants worldwide. There is presently no approved vaccine against RSV due to several challenges including inadequate protection. Adjuvants may help alleviate these challenges. Artesunate, the potent antimalarial agent, with known anti-inflammatory and immunomodulatory effect is hypothesized to improve immune response to RSV antigen.
Objectives: To evaluate the adjuvant effect of artesunate to respiratory syncytial virus antigen.
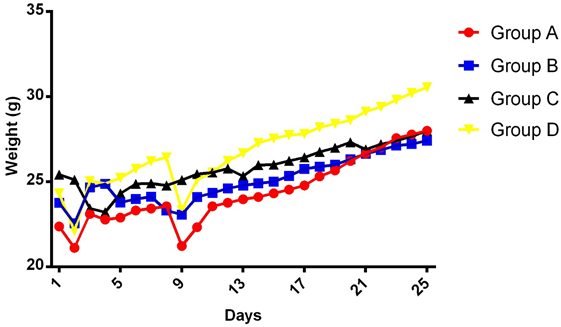
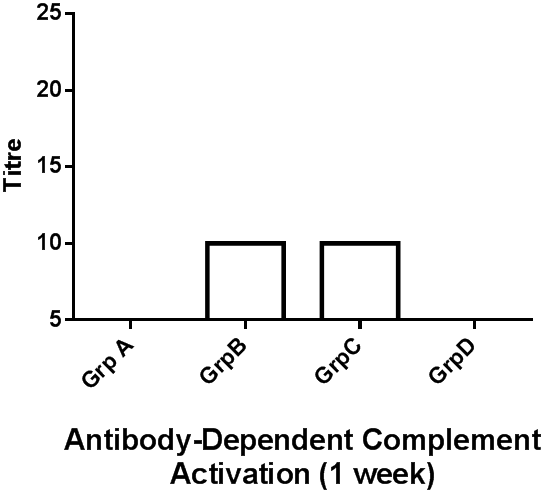
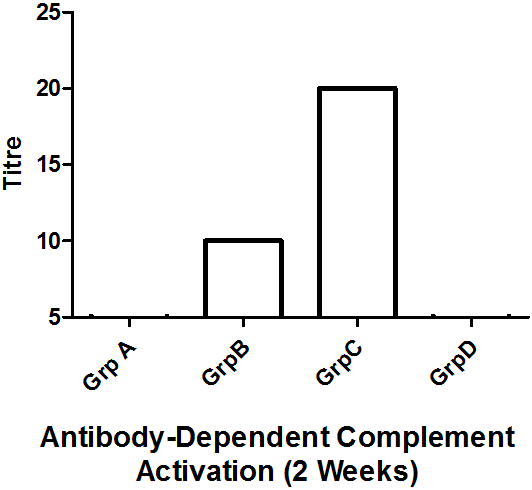
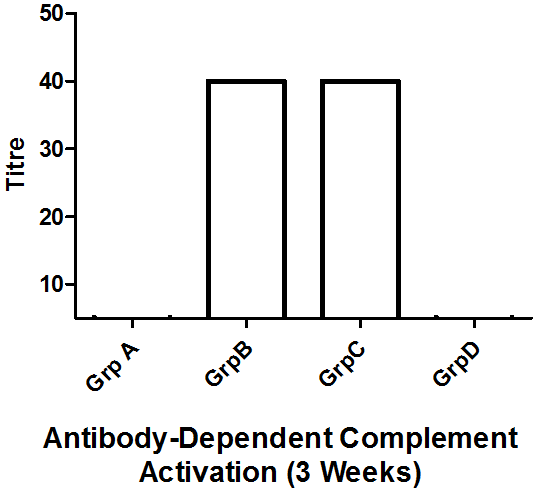
Results: Antibody-dependent complement activation assay revealed that mice treated with RSV antigen alone (Group B) or RSV antigen plus artesunate (Group C) both elicited antibody production at week 1, 2, and 3 respectively, with a progressive response rate for Group C. Periodic weight assessmentrevealed that only Group C recorded slight weight loss meanwhile other group had a more pronounced weight loss at day 2 and 9.
Conclusion: altogether, the results obtained suggest that artesunate displays a positive up-regulating effect on the vaccination outcome of the RSV antigen, and can be further developed for possible utility as adjuvant for RSV vaccine.
Keywords: Respiratory syncytial virus (RSV); RSV vaccine; Artesunate; Adjuvant; Immune system; Antibody
Introduction
Respiratory syncytial virus (RSV) which came to limelight in 1956 is the leading cause of viral lower respiratory tract illness worldwide (Collins and Graham, 2008). The Centers for Disease Control consider RSV to be the "most common cause of bronchiolitis and pneumonia in children under 1 year of age in the United States" (CDC, 2008). About 3.4 million hospitalizations and more than 200,000 deaths are recorded globally due to this virus infection (Nair., et al. 2010). This alarming death calls for a means of preventing the disease. Hence, the extensive studies of the epidemiology, clinical manifestations, diagnostic techniques, animal models and the immunobiology of RSV infection. Despite all these studies, there is yet no convincing, safe and licensed vaccine made available (Graham, 2011; Borchers, 2013) which is due to several challenges peculiar to RSV namely; the young age of infection; the multiple mechanisms RSV uses to evade innate immunity; the lack of durable protective immunity induced by natural infection; a legacy of vaccine-enhanced disease dating back to clinical trials done in the 1960s with a formalin-inactivated whole virus vaccine; and animal models not faithfully replicating the pathogenesis of human RSV (Graham, 2011). Suitable immunological adjuvants can go a long way in facilitating effective vaccine formulation as adjuvant can help increase the response to the vaccine in the pediatric population, by increasing mean antibody titers and/or the fraction of subjects that become protectively immunized and equally increasing seroconversion rates in these infants/children (Beran, 2008; Podda, 2001). Adjuvant can also be used to achieve qualitative alteration of immunological response such as promoting the types of immunity not effectively generated by the non-adjuvanted antigens thus adding adjuvant to RSV vaccine can help overcome the problem of RSV using multiple mechanism to evade innate immunity as the adjuvant can alter the breadth, affinity or specificity of the immune response (Khurana., et al. 2010; Malherbe., et al. 2008). This study therefore, focused on a means of modulating the immune system to respond adequately to RSV vaccine. Artesunate, a derivative of artemisinin was evaluated for its adjuvant effect to the RSV vaccine. Artemisinin itself is a sesquiterpene lactone bearing a peroxy group isolated from Artemisia annua, a herb that has been used traditionally in China for the treatment of malaria. Artemisinin and its derivatives are currently considered the most effective drug in treating cerebral malaria and chloroquine resistant falciparum malaria (White, 2008; van Hensbroek., et al. 1996) It is also recognized as the “best hope for the treatment of malaria” by the World Health Organization because of its effectiveness, nonresistant characteristics, and minimal side effects (White, 2008; van Hensbroek., et al. 1996). The active metabolite of artemisinin is dihydroartemisinin (DHA).
Currently, artemisinin derivatives used in clinical treatment include DHA, artemether, artesunate, and arteether. Besides the outstanding antimalarial activity, artemisinin and its derivatives also possess anti-inflammatory and immunomodulatory activities and are experimentally used to treat autoimmune diseases, such as Systemic Lupus Erythematosus, Rheumatoid Arthritis, and Multiple Sclerosis (Shi., et al. 2015). Artemisinin derivatives perform immunomodulatory functions in these diseases primarily through inhibiting pathogenic T cell activation, suppressing B cells activation and antibody production, and expanding regulatory T cells (Shi., et al. 2015). Artesunate, as an anti-malarial agent acts by suppressing the production or activity of antioxidant enzyme in the erythrocyte, causing lysis of parasitic cell due to the highly reactive free oxygen radicals. It also demonstrates its immunosuppressive traits towards different immune components mainly by regulating the cellular proliferation and cytokine release (Shi., et al. 2015). Thus artesunate which has anti-malarial, anti-inflammatory and immunomodulatory potential is worth evaluating to ascertain its relationship with RSV vaccine since it might present as a possible adjuvant to RSV vaccine through further developments.
Materials and Method
Mice
Twenty-four (24) pathogen-free Mus musculus between 6-8 Weeks old (20-35kg) of both sexes obtained from the animal house of the department of Pharmacology and Toxicology, University of Nigeria, Nsukka were used. The animals were housed under normal lighting condition. They were fed with standard pellet and had unrestricted access to clean drinking water for 1 week duration so as to allow the animals acclimatize to their new environment.
Twenty-four (24) pathogen-free Mus musculus between 6-8 Weeks old (20-35kg) of both sexes obtained from the animal house of the department of Pharmacology and Toxicology, University of Nigeria, Nsukka were used. The animals were housed under normal lighting condition. They were fed with standard pellet and had unrestricted access to clean drinking water for 1 week duration so as to allow the animals acclimatize to their new environment.
Preparation of Artesunate solution
A solution of artesunate was prepared by weighing accurately a 100mg of pure artesunate powder (Jawa Pharmaceutical, Nigeria) using the sensitive digital weighing balance, and then pouring it into 2080µl volume of DMSO. The mixture was shaken very well so as to get a uniform solution.
A solution of artesunate was prepared by weighing accurately a 100mg of pure artesunate powder (Jawa Pharmaceutical, Nigeria) using the sensitive digital weighing balance, and then pouring it into 2080µl volume of DMSO. The mixture was shaken very well so as to get a uniform solution.
Vaccine reconstitution and Vaccination
A 2ml volume of distilled water was introduced into the dry powder Bottle containing the respiratory syncytial virus antigen to get a cloudy solution. The mice were grouped into 4 groups of 6 animals each. The animals in each group were weighed accurately using a digital sensitive balance and the values recorded. Different groups were given different treatments as follows:
Group A received 20µl DMSO only
Group B received 20µl of the reconstituted RSV antigen
Group C received 20µl of the reconstituted RSV antigen plus solution of artesunate (20 µl)
Group D received 20µl of the solution of artesunate powder
All samples were administered intraperitoneally. The vaccination procedure was repeated once after 1 week. The blood of the mice was collected at 1, 2 and 3 weeks post-vaccination and the sera employed for antibody-dependent complement activation assay.
A 2ml volume of distilled water was introduced into the dry powder Bottle containing the respiratory syncytial virus antigen to get a cloudy solution. The mice were grouped into 4 groups of 6 animals each. The animals in each group were weighed accurately using a digital sensitive balance and the values recorded. Different groups were given different treatments as follows:
Group A received 20µl DMSO only
Group B received 20µl of the reconstituted RSV antigen
Group C received 20µl of the reconstituted RSV antigen plus solution of artesunate (20 µl)
Group D received 20µl of the solution of artesunate powder
All samples were administered intraperitoneally. The vaccination procedure was repeated once after 1 week. The blood of the mice was collected at 1, 2 and 3 weeks post-vaccination and the sera employed for antibody-dependent complement activation assay.
Blood Collection
The animals were anesthetized with diethyl ether, following standard procedure as demonstrated by Hoff (2000). An approximate 1 ml of blood was collected from the retro-orbital plexus of the mice by intraocular puncture. The collected blood was centrifuged using a standard centrifuge at a speed of 5000 revolutions per minutes for 10 minutes. Using a sterile 1 ml syringe, the serum was collected and transferred into a fresh sample bottle and labeled appropriately. It was then stored at (-2oC) for future analysis, this was repeated for all the mice.
The animals were anesthetized with diethyl ether, following standard procedure as demonstrated by Hoff (2000). An approximate 1 ml of blood was collected from the retro-orbital plexus of the mice by intraocular puncture. The collected blood was centrifuged using a standard centrifuge at a speed of 5000 revolutions per minutes for 10 minutes. Using a sterile 1 ml syringe, the serum was collected and transferred into a fresh sample bottle and labeled appropriately. It was then stored at (-2oC) for future analysis, this was repeated for all the mice.
Antibody-Specific Complement Activation Assay
Complement activation assay was conducted following established protocol (Serion, 2010). Briefly, RSV antigen is mixed with the test sera containing specific antibodies or no antibodies. Complement is added and the entire set-up in 96-well plate incubated overnight in veronal buffer to facilitate formation of immune complexes. Next day, the haemolytic system (composed of antibody-coated sheep erythrocytes) (Serion, Würzburg Germany) is added to set-up. In the absence of specific antibodies immune complexes, the complement components remain in their non-fixed state but if otherwise they will be fixed. Sedimentation of sheep erythrocytes indicate fixing of complement, and vice versa.
Complement activation assay was conducted following established protocol (Serion, 2010). Briefly, RSV antigen is mixed with the test sera containing specific antibodies or no antibodies. Complement is added and the entire set-up in 96-well plate incubated overnight in veronal buffer to facilitate formation of immune complexes. Next day, the haemolytic system (composed of antibody-coated sheep erythrocytes) (Serion, Würzburg Germany) is added to set-up. In the absence of specific antibodies immune complexes, the complement components remain in their non-fixed state but if otherwise they will be fixed. Sedimentation of sheep erythrocytes indicate fixing of complement, and vice versa.
Periodic weight monitoring
Periodic weight monitoring was also carried out after immunization for 25 days using the digital sensitive weighing balance.
Periodic weight monitoring was also carried out after immunization for 25 days using the digital sensitive weighing balance.
Results
The result of the periodic weight monitoring revealed weight loss at day 2 immediately after immunization in all groups (Figure 1) except the RSV antigen plus artesunate group C which had it weight loss on days 3 to 4. This is followed by a progressive increase in weight in all groups until the mice were sacrificed. Except for the decrease noticed on day 9 across the groups with the exception of the RSV antigen plus artesunate group C whose slight decrease was noticed on days 13 and 21.
The compliment activation assay revealed antibody presence in groups B and C only (Figures 2, 3, 4). And only the antibody produced in group C increases in a geometric progression.
Discussion
Respiratory syncytial virus (RSV) is a common virus that infects the linings of the airways (the nose, throat, windpipe, bronchi and bronchioles). The virus infects adults and children, but children often have more severe disease with RSV than adults. The virus is so widespread that almost all children have had a RSV infection by the time they are 2-3 years old (Collins and Graham, 2008; Chu., et al. 2013). The prevalence of this viral infection calls for a means of preventing it. Vaccination is one peculiar means of preventing diseases. However, the vaccine for this vaccination is not yet available due to several challenges. A suitable adjuvant could help avert or overcome these challenges. Artesunate was tested as a possible adjuvant using the compliment fixation assay. Briefly, the complement system is a biochemical cascade of the innate immune system that helps clear pathogens within host organism. Activation of this system leads to cytolysis, chemotaxis, opsonization and inflammation (Nesargikar., et al. 2012). Three biochemical pathways activate the complement system namely, the classical complement pathway, the alternate complement pathway and the mannose binding lectin pathway. The classical complement pathway typically requires antibodies for activation and is a specific immune response. Therefore, our study was designed to assess antibody response to RSV antigen in the presence or absence of immune-modulatory and potential adjuvant function of Artesunate.
In our assay, the serum antibodies could fix complement and as such the complement fixation assay demonstrates the presence of specific antigens and antibody. This would be of utmost utility not only systemically, but also in nasopharyngeal secretions where RSV and other respiratory pathogens could easily reside as their first port of entry (Abdul-Lateef., et al. 2016; Odimegwu., et al. 2013; Openshaw and Tregoning, 2005). Thus the immune complexes formed from the complement fixation engages complement present in the set-up thereby removing them. This removal and ensuing absence of free complement in the system means that the sheep erythrocytes (srbc) remain intact as complement is no longer available to react with the haemolytic system [srbAb-srbAg] (inhibition of hemolysis) (Serion, 2010). On the other hand, haemolysis ensues if complement becomes accessible. This was consistent with our study at the different titre recorded for the different samples. Thus, in this present study it has been demonstrated that the immune system can be modulated following the vaccination of mice with a Respiratory syncytial virus antigen plus artesunate (Figures 2, 3 & 4). Geometric increase in immune response was recorded in the RSV antigen-artesunate-treated group C relative to controls unlike the RSV antigen-alone group B which did not show antibody increase between the 1 and 2 week, however, after 3 weeks antibody responses was upregulated in this group B to the same levels as group C (Figure 4). The antibody produced in the group B and C suggests that the RSV antigen administered is immunogenic, and the addition of artesunate did not limit the immunogenicity but rather appeared to have potentiated it as seen by the geometric progression of antibody produced over time. This perhaps reflects the efficacy of combining the two agents. The absence of antibody in the DMSO-treated group was expected since DMSO is not known to be an immunomodulatory agent. Also, the absence of RSV-specific antibodies in the artesunate-treated group D is expected since artesunate acting alone should not lead to RSV antibody production.
Animal weight monitoring could furnish information regarding the health status of the animals used in experiment especially if infection or disease states are involved. However, physiological changes due to administered immunogens or substances could also instigate such changes (Odimegwu., et al. 2008; 2011). From our periodic weight monitoring results it may be possible that the observable weight change in the RSV antigen-artesunate combination group C at days 3, 4, 13 and 21 could be due to the treatment-induced physiological changes in the mice, although weight loss was equally noticed in the control groups at days 2 and 9 for unexplanable reason. The humoral immune response studied vis-à-vis complement activation while it measures small titre ranges demonstrates the immunomodulatory effect of artesunate and perhaps suggests other possibilities when administered in the context of vaccinations. This again may require further specific evaluations to define the robust relationship between artesunate and RSV vaccine. Moreover, that artesunate may become a subject of an interaction relationship with vaccines is not in doubt given the perfusive nature of malarial with artemisins-based therapies as mainline treatment options. Therefore, our modest immune modulatory analyses around the classical complement cascades constitute a measure of steps taken towards defining this relationship.
Contribution to knowledge: In general the contribution of our findings to the body of knowledge is notable. Since the immunological goal for vaccine development is to induce effective neutralizing antibody to prevent infection, and one of the major challenges to RSV vaccine development has been inadequate immunological response. The discovery and development of an artesunate adjuvant could be a gateway towards vaccine development. This research work has demonstrated that artesunate can be used to modulate the immune system to respond adequately to RSV vaccine, hence suggesting a means to the development of an RSV vaccine.
Conclusion
The result of this study has evaluated the combined immunological efficacy of the Respiratory syncytial virus antigen and artesunate in mice. The outcome reveals that artesunate showed some adjuvancy effect, and could be further developed for use as adjuvant.
Acknowledgement
We acknowledge and thank the University of Nigeria for the enabling environment provided for this research to be carried out.
We acknowledge and thank the University of Nigeria for the enabling environment provided for this research to be carried out.
References
- Abdul-Lateef., et al. “Effected of Xylitol on Biofilm Formation and Growth of Streptococcus Pneumoniae Isolated from Children with Acute Otitis Media in Hilla Province/Iraq”. Australian Journal of Basic and Applied Sciences 10.1 (2017): 21-25.
- Beran J. “Safety and immunogenicity of a new hepatitis B vaccine for the protection of patients with renal insufficiency including pre-haemodialysis and haemodialysis patients”. Expert Opinion on Biological Therapy 8.2 (2008): 235-247.
- Borchers AT., et al. “Respiratory syncytial virus--a comprehensive review”. Clinical Reviews in Allergy & Immunology 45.3 (2013): 331-379.
- CDC. 2008. Respiratory Syncytial Virus. Center for Disease Control, Respiratory and Enteric Viruses Branch. October 17, 2008. Retrieved March 10 (2016).
- Chu HY., et al. “Molecular epidemiology of respiratory syncytial virus transmission in childcare”. Journal of Clinical Virology 57.4 (2013): 343-350.
- Collins PL and BS Graham., “Viral and host factors in human respiratory syncytial virus pathogenesis”. Journal of Virology 82.5 (2008): 2040-2055.
- Graham BS., et al. “Biological Challenges and Technological Opportunities for Respiratory Syncytial Virus Vaccine Development”. Immunological Reviews 239.1 (2011): 149-166.
- Hoff J. “Methods of blood collection in the mouse”. Laboratory animal29.10 (2000): 47-53.
- Khurana S., et al. “Vaccines with MF59 adjuvant expand the antibody repertoire to target protective sites of pandemic avian H5N1 influenza virus”. Science Translational Medicine 2. 15 (2010).
- Malherbe L., et al. “Vaccine adjuvants alter TCR-based selection thresholds”.Immunity 28.5 (2008): 698-709.
- Nair H., et al. “Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis”. Lancet 375.9725 (2010): 1545-1555.
- Nesargika PN., et al. “The Complement system: history, pathways, cascade and inhibitors’. European Journal of Microbiology and Immunology 2.2 (2012): 103-111.
- Odimegwu D., e t al. “Maternal Immunization with Genetic RSV Vaccines to Protect Newborns against Severe RSV Infections”. Conference proceedings: Federation of Clinical Immunological Societies 27-30 (2013): 2013.
- Odimegwu DC., et al. “Bioassay-tracked temperature-stress-induced chemical degradation of bio-active wound healing antibacterial extract of Dissotis theifolia stem present in a pharmaceutical cream and ointment”. International Journal of Applied Research in Natural Products4.1 (2011): 20-28.
- Odimegwu DC., et al. “Wound healing and antibacterial activities of the extract of Dissotis theifolia (Melastomatacea) stem formulated in a simple ointment base”. Journal of Medicinal Plants Research2.1 (2008): 11- 16.
- Openshaw PJM and J Tregoning. “Immune Responses and Disease Enhancement during Respiratory Syncytial Virus Infection”. Clinical Microbiology Reviews 18.3 (2005): 541-55.
- Podda A. “The adjuvanted influenza vaccines with novel adjuvants: Experience with the MF59-adjuvanted vaccine”.Vaccine 19.17 (2001): 2673-2680.
- Serion CFT Reagents: Reagents for complement fixation tests.
- Shi C., et al. “Anti-inflammatory and immune regulatory functions of artemisinin and its derivative”. Mediators of inflammation (2015): 7.
- Van Hensbroek., et al. “A trial of artemether or quinine in children with cerebral malaria’. The New England Journal of Medicine335.2 (1996): 69-75.
- White NJ. “Qinghaosu (artemisinin): the price of success”. Science320.5874 (2008): 330-334.
Citation:
Damian Chukwu Odimegwu., et al. “Antibody Response to Respiratory Syncytial Virus Antigen Is Modulated by Artesunate”.
Clinical Biotechnology and Microbiology 2.3 (2018): 364-370.
Copyright: © 2018 Damian Chukwu Odimegwu., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.






























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.