Research Article
Volume 2 Issue 4 - 2018
Synthesis, Characterization and Antimicrobial Investigation of Transition Metal Complexes by using Schiff Base Containing N-, O- Donor Atoms
Department of Chemistry, Government College University, Faisalabad-38000 Pakistan
*Corresponding Author: Syed Faheem Askari Rizvi, Department of Chemistry, Government College University, Faisalabad-38000
Pakistan.
Received: June 18, 2018; Published: June 29, 2018
Abstract
Schiff base was prepared by the condensation of 5-aminosalicylic acid and 2-hydroxy-3-methoxybenzaldehyde in methanol. A series of transition metal complexes of Cu (II), Cd (II), Co (II), Zn (II) and Ni (II) were synthesized by using Schiff base as ligand. Schiff base and metal complexes were characterized on the bases of melting point, solubility, colour of product and FTIR analysis. The ligand and metal complexes were screened for antibacterial analysis against two of which Gram positive bacteria (B. subtillus, S. aureus) and two Gram negative bacteria (E. coli, P. multocida) and antifungal analysis against Ganoderma lucidum, Alterharia lterhata, Penicillium notatum and Aspergillus flavus by using disc diffusion method.
Key Words: Schiff base; Metal Complexes; Transition Metals; Antibacterial; Antifungal activities
Introduction
The Schiff bases and their metal complexes have more importance recently because of their application as biological, biochemical, analytical, antimicrobial, anticancer, antibacterial, and antifungal and anti-tumor activity [1]. They have been studied as a class of ligands and are known to coordinate with metal ions through the azomethine nitrogen atom. The synthesis of transition metal complexes with Schiff base ligands are studied due to sensitivity, selectivity and synthetic flexibility towards metal atoms. They used as catalyst, in medicine like antibiotics and anti-inflammatory agents and in the industry as anticorrosion. In this paper we describe the behavior of the bidentate aromatic Schiff base ligand with various transition metal (II) ions [2]. Now a days, the use of Schiff bases as ligand is very common. They are used as intermediates for preparation of amino acid and for synthesis of complex with transition metals. Coordination of Schiff base to the metal takes place through the nitrogen atom of azomethine group and oxygen atom of the phenolic group. Schiff bases are widely used for metal complexation because of formation of stable complexes [3].
Many metallic elements are very important in living organism because they play a very important role in proper functioning of living organism. These metals are also called metals of life [4]. Four metals are very important these are sodium, magnesium, calcium and potassium. But transition elements i.e. chromium, manganese, iron, cobalt, nickel, copper and zinc are also very important in living organisms to perform their proper functioning [5]. In living organism, transition metals are present in very minute quantities usually at trace levels at molecular level these transition metals are also very important in the formation of Schiff base complexes [6]. From the nineteenth century, Schiff base metal complexes are well known in different field of life synthesis of Schiff base metal complexes are very important rather than Schiff bases in the development of coordination chemistry [7].
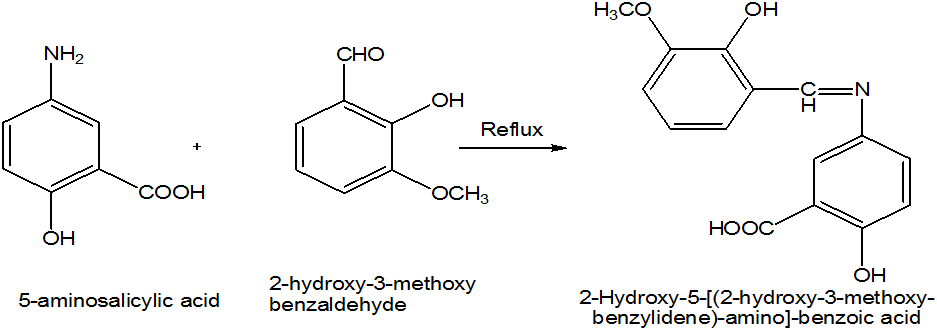
The aim of the present study was to prepare the Schiff bases by the condensation of 5-aminosalicylic acid and 2-hydroxy-3-methoxybenzaldehyde in methanol. These Schiff bases were used to synthesize the different transition metal complexes of Cu (II), Cd (II), Co (II), Zn (II) and Ni (II) as ligand. The characterization of these Schiff base and metal complexes were carried out on the bases of melting point, solubility, colour of product and FTIR analysis. The ligand and metal complexes were screened for antibacterial analysis against two of which Gram positive bacteria (B. subtillus, S. aureus) and two Gram negative bacteria (E. coli, P. multocida) and antifungal analysis against Ganoderma lucidum, Alterharia lterhata, Penicillium notatum and Aspergillus flavus by using disc diffusion method.
Experimental
Present work describes the synthesis of Schiff bases and their metal complexes. For the synthesis of these compounds, different glass wares are used which were oven and flame dried. All the commercially available reagents and chemicals were used as such without further purification. By using standard procedures solvents were dried, where necessary. Thin-layer chromatography was used to determine the progress and completion of reactions. In thin-layer chromatography (TLC) commercial aluminium backed Merck plates, coated with silica gel GF254 (0.25 mm thick), was used having a fluorescent indicator which was active at 254 nm wavelength. UV light at 254 nm was used to visualize the chromatograms.
Present work describes the synthesis of Schiff bases and their metal complexes. For the synthesis of these compounds, different glass wares are used which were oven and flame dried. All the commercially available reagents and chemicals were used as such without further purification. By using standard procedures solvents were dried, where necessary. Thin-layer chromatography was used to determine the progress and completion of reactions. In thin-layer chromatography (TLC) commercial aluminium backed Merck plates, coated with silica gel GF254 (0.25 mm thick), was used having a fluorescent indicator which was active at 254 nm wavelength. UV light at 254 nm was used to visualize the chromatograms.
Melting points were determined in open capillary tubes on an electro thermal (Griffin 1090) melting point. For the determination of IR, Perkin Elmer 1600-FT spectrometer and Matter son Satellite spectrometer fitted with a Spec arch Golden Gate ATR sampling platform were used and IR spectra were recorded on it in the range 4000-250 cm-1. The results of IR were reported in wavenumbers (cm-1). For IR analysis, samples were applied as solids or as neat liquids or in the form of KBr discs.
Synthesis of Schiff base 1
5-aminosalicylic acid (9.7 mmol) was dissolved in 15 ml methanol in beaker, (9.7 mmol) of 2-hydroxy-3-methoxybenzaldehyde was also dissolved in 15 ml methanol. Mix these two solutions in round bottom flask and 3-4 drops of acetic acid was also added. Reflux it for about two and half hours. Reddish orange colour precipitate appeared. Reaction mixture was cooled to room temperature and placed in refrigerator for about 24 hours. The precipitated solid was filtered and washed with methanol.
5-aminosalicylic acid (9.7 mmol) was dissolved in 15 ml methanol in beaker, (9.7 mmol) of 2-hydroxy-3-methoxybenzaldehyde was also dissolved in 15 ml methanol. Mix these two solutions in round bottom flask and 3-4 drops of acetic acid was also added. Reflux it for about two and half hours. Reddish orange colour precipitate appeared. Reaction mixture was cooled to room temperature and placed in refrigerator for about 24 hours. The precipitated solid was filtered and washed with methanol.
Synthesis of Metal Complexes
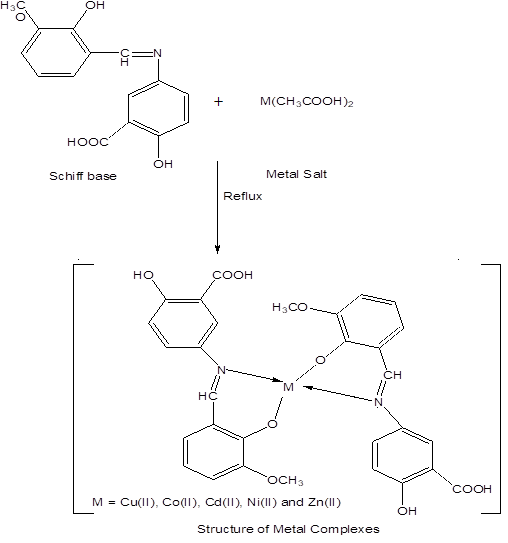
Schiff base (3.5 mmol) was dissolved in 10 ml ethanol in a beaker and metal salt (1.75 mmol) was dissolved in 10 ml ethanol in a beaker. Mix these two solutions in round bottom flask. Reflux it for four hours. Metal to ligand ratio was 1:2 (w/w). Coloured precipitates appear. Filter these precipitate and washed with ethanol.
Schiff base (3.5 mmol) was dissolved in 10 ml ethanol in a beaker and metal salt (1.75 mmol) was dissolved in 10 ml ethanol in a beaker. Mix these two solutions in round bottom flask. Reflux it for four hours. Metal to ligand ratio was 1:2 (w/w). Coloured precipitates appear. Filter these precipitate and washed with ethanol.
Antibacterial Assay by Disc Diffusion Method
Disc diffusion method was used to determine the antibacterial activities against two gram positive bacteria (B. subtillus, S. aureus) and two Gram negative bacteria (E. coli, P. multocida). Nutrient agar media was prepared by suspending the 28 g nutrient agar in one liter distilled water. The media was autoclaved for 15 min at 121°C. Nutrient media (100 mL) was taken in beaker, to the media 100 µL of inocula was added and mixed well. Transfer the mixture (media and inocula) to the petri plate. Sample (100 µL) was applied on each flat paper discs. These discs were laid on the growth medium having bacterial strain in petri plates and incubated it for 37°C for 24 hours for the growth of bacteria. The active samples inhibit the growth of bacteria and clear zone were formed. Zone reader was used to measure the zone of inhibition [8].
Disc diffusion method was used to determine the antibacterial activities against two gram positive bacteria (B. subtillus, S. aureus) and two Gram negative bacteria (E. coli, P. multocida). Nutrient agar media was prepared by suspending the 28 g nutrient agar in one liter distilled water. The media was autoclaved for 15 min at 121°C. Nutrient media (100 mL) was taken in beaker, to the media 100 µL of inocula was added and mixed well. Transfer the mixture (media and inocula) to the petri plate. Sample (100 µL) was applied on each flat paper discs. These discs were laid on the growth medium having bacterial strain in petri plates and incubated it for 37°C for 24 hours for the growth of bacteria. The active samples inhibit the growth of bacteria and clear zone were formed. Zone reader was used to measure the zone of inhibition [8].
Antifungal Assay by Disc Diffusion Method
Disc diffusion method was used to determine the antifungal activities against Ganoderma lucidum, Alterharia lterhata, Penicillium notatum and Aspergillus flavus. Potato dextrose agar media was prepared. The media was autoclaved for 15 min at 121°C. Potato dextrose agar media (100 mL) was taken in beaker, to the media 100 µL of inocula was added and mixed well. Transfer the mixture (media and inocula) to the petri plate. Sample (100 µL) was applied on each flat paper discs. These discs were laid on the growth medium having fungal strain in petri plates and incubated it for 28°C for 48 hours for the growth of fungus. The active samples inhibit the growth of fungus and clear zone were formed. Zone reader was used to measure the zone of inhibition [9].
Disc diffusion method was used to determine the antifungal activities against Ganoderma lucidum, Alterharia lterhata, Penicillium notatum and Aspergillus flavus. Potato dextrose agar media was prepared. The media was autoclaved for 15 min at 121°C. Potato dextrose agar media (100 mL) was taken in beaker, to the media 100 µL of inocula was added and mixed well. Transfer the mixture (media and inocula) to the petri plate. Sample (100 µL) was applied on each flat paper discs. These discs were laid on the growth medium having fungal strain in petri plates and incubated it for 28°C for 48 hours for the growth of fungus. The active samples inhibit the growth of fungus and clear zone were formed. Zone reader was used to measure the zone of inhibition [9].
Results and Discussion
The transition metal (II) complexes with bidentate aromatic Schiff base as ligand were prepared by stirring stoichiometric amounts of metal (II) salts and aromatic Schiff base derived from 5-aminosalicylic acid and 2-hydroxy-3-methoxybenzaldehyde. Analytical data and some Physical properties of the Schiff base as ligand and their metal complexes are listed in (Table 1).
| Sample code | R1 | R2 | Physical state | Molecular formula | Molecular mass (g/mol) | Melting Point (°C) | Yield (%) |
| Schiff base 1 | 5-aminosalicylic acid | 2-hydroxy-3-methoxybenz-aldehyde | Reddish orange solid | C15H13O5N | 287 | 207-211 | 71 |
| 1Cu | Schiff base 1 | Copper acetate | Greenish solid | C30H24O10N2Cu | 635.5 | 291-294 | 68 |
| 1Cd | Schiff base 1 | Cadmium acetate | Light orange Solid | C30H24O10N2Cd | 684.4 | 314-319 | 65 |
| 1Co | Schiff base 1 | Cobalt acetate | Dark yellowish Solid | C30H24O10N2Co | 631 | 301-304 | 71 |
| 1Zn | Schiff base 1 | Zinc acetate | Light yellowish Solid | C30H24O10N2Zn | 637.4 | 321-325 | 69 |
| 1Ni | Schiff base 1 | Nickel Chloride | Yellowish solid | C30H24O10N2Ni | 630 | 333-337 | 73 |
Table 1: Physical characteristics data of Schiff base and metal complexes.
The complexes were characterized by the usual methods melting point, colour of product, solubility and FTIR analysis. The complexes are stable in air and light and are soluble in organic solvents such as DMF and DMSO. (Table 2).
| Sample Code | Dist. Water | Methanol | Ethanol | Ethyl acetate | n-Hexane | CHCl3 | DMF | DMSO |
| Schiff base 1 | I | I | S | S | PS | S | S | S |
| 1Cu | I | I | I | PS | I | PS | S | S |
| 1Cd | I | PS | I | I | I | PS | S | S |
| 1Co | I | I | I | PS | I | S | S | S |
| 1Zn | I | I | PS | PS | I | PS | S | S |
| 1Ni | I | PS | I | PS | I | PS | S | S |
Table 2: Solubility of Schiff bases and complexes.
I = Insoluble
S = Soluble
PS = Partially soluble
I = Insoluble
S = Soluble
PS = Partially soluble
Antibacterial Activity of Schiff base and Complexes
The Schiff bases and complexes were screened for their antibacterial activity against four bacterial strains by using disc diffusion method. Two of which, gram positive bacteria (B. subtillus, S. aureus) and two gram negative bacteria (E. coli, P. multocida). Results are summarized in (Table 3). In order to compare the results, Ampicillin is used as standard control drug. It was observed from the tabulated data of that the antibacterial activity of the Schiff bases are lower than the standard drug Ampicillin and the complexes showed higher activity than that of Schiff bases which are used as ligands. From the results, it is clear that complexes showed more activity towards Gram positive bacteria such as B. subtillus and S. aureus as compare to Gram negative strains such as E. coli and P. multocida due to the fact that structures of cell wall of Gram negative bacteria’s are more complex as compare to Gram positive bacteria’s.
The Schiff bases and complexes were screened for their antibacterial activity against four bacterial strains by using disc diffusion method. Two of which, gram positive bacteria (B. subtillus, S. aureus) and two gram negative bacteria (E. coli, P. multocida). Results are summarized in (Table 3). In order to compare the results, Ampicillin is used as standard control drug. It was observed from the tabulated data of that the antibacterial activity of the Schiff bases are lower than the standard drug Ampicillin and the complexes showed higher activity than that of Schiff bases which are used as ligands. From the results, it is clear that complexes showed more activity towards Gram positive bacteria such as B. subtillus and S. aureus as compare to Gram negative strains such as E. coli and P. multocida due to the fact that structures of cell wall of Gram negative bacteria’s are more complex as compare to Gram positive bacteria’s.
| Sample No. | Bacterial inhibition zone (mm) | |||
| E. coli | S. aureus | B. subtilis | P. multocida | |
| Schiff base 1 | 14.5 | 15.5 | 22.5 | 16 |
| 1Cu | 17.5 | 21 | 24.5 | 20 |
| 1Cd | 22 | 30 | 29 | 24 |
| 1Co | 16 | 26 | 15.5 | 17 |
| 1Zn | 24.5 | 22 | 26 | 18 |
| 1Ni | 23 | 25.5 | 23 | 22.5 |
| Ampicillin | 32 | 30 | 31 | 29 |
Table 3: Antibacterial activity of Schiff base and Complexes.
Values are mean of three individual replicates
Concentration = 10 mg/ml of DMSO - = No activity
Values are mean of three individual replicates
Concentration = 10 mg/ml of DMSO - = No activity
Antifungal Activity of Schiff base and Complexes
In vitro tests for antifungal activity of Schiff bases and their complexes were screened against four fungal strains Ganoderma lucidum, Alterharia lterhata, Penicillium notatum and Aspergillus flavus by using disc diffusion method. Fluconazole was used as standard control drug for the determination of antifungal activity. The results of antifungal activity were shown in (Table 4). According to results, complexes (1Cd, 1Ni) show more activity against Ganoderma lucidum, Alterharia lternaria and Penicillium notatum. The increased activity of complexes may be due to coordination of Schiff base with transition metals [10].
In vitro tests for antifungal activity of Schiff bases and their complexes were screened against four fungal strains Ganoderma lucidum, Alterharia lterhata, Penicillium notatum and Aspergillus flavus by using disc diffusion method. Fluconazole was used as standard control drug for the determination of antifungal activity. The results of antifungal activity were shown in (Table 4). According to results, complexes (1Cd, 1Ni) show more activity against Ganoderma lucidum, Alterharia lternaria and Penicillium notatum. The increased activity of complexes may be due to coordination of Schiff base with transition metals [10].
| Sample No. | Fungal inhibition zone (mm) | |||
| G. lucidum | A. lternaria | P. notatum | A. flavus | |
| Schiff base 1 | 26 | 19.5 | 24 | 17.5 |
| 1Cu | 31 | 23 | 27 | 23.5 |
| 1Cd | 33 | 30 | 35.5 | 26 |
| 1Co | 29 | 28.5 | 19 | 18 |
| 1Zn | 26.5 | 22 | 27 | 21.5 |
| 1Ni | 28 | 17.5 | 29 | 24 |
| Fluconazole | 37 | 36 | 35 | 31 |
Table 4: Antifungal activity of Schiff base and Complexes.
Values are mean of three individual replicates
Concentration = 10mg/ml of DMSO - = No activity
Values are mean of three individual replicates
Concentration = 10mg/ml of DMSO - = No activity
Infrared Spectroscopy
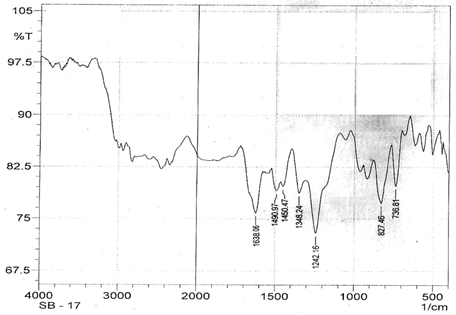
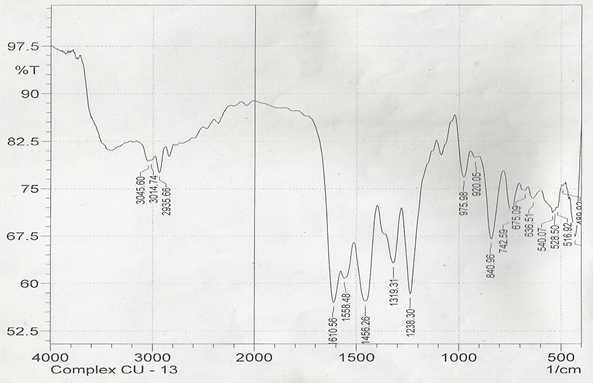
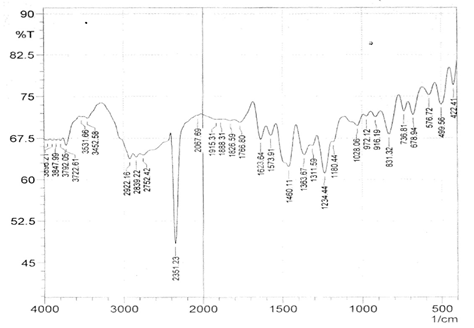
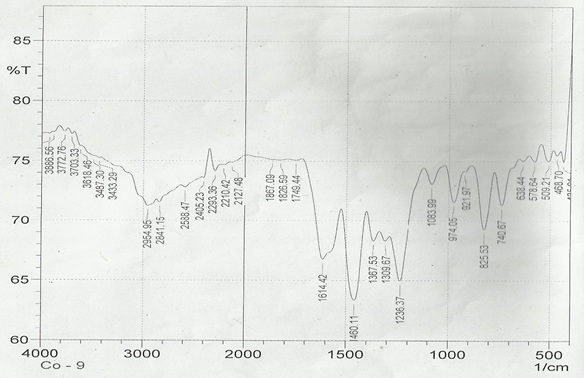
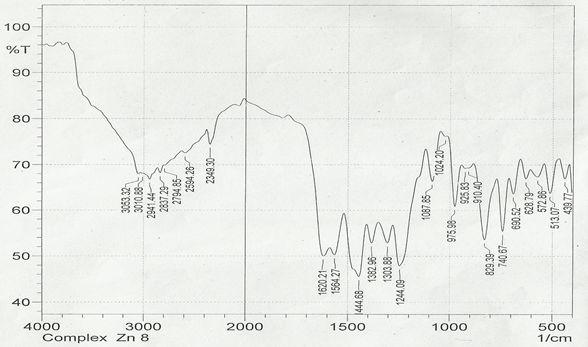
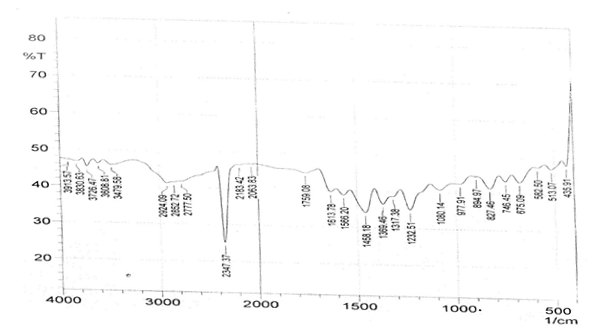
The infrared spectroscopy of Schiff base 1 and complexes (1Cu-1Ni) were recorded in the range 4000-250 cm-1 as KBr pellets. IR spectra of Schiff bases and their complexes showed remarkable broad bands as shown in (Table 5). The IR spectra provide valuable information regarding the nature of functional groups attached to ligand and metal atom in complexes. In order to study the bonding bond of Schiff base and metal complexes, IR spectrum of free ligand was compared with the spectra of complexes. A band in free Schiff base appeared at 1638.06 cm-1 was due to C=N vibrational frequency. The azomethine peak (1638.06 cm-1) in Schiff base 1 was shifted to lower value 1610.56-1623.64 cm-1 suggesting the coordination. The shifting of this group to lower frequency in the metal complexes when compared to free Schiff base, suggested the coordination of metal ion through nitrogen atom of azomethine group. It is expected that coordination of metal atom would reduce the electron density in the azomethine link and thus lower the –HC=N absorption. Peaks in the range 540.07-582.50 cm-1 in complexes were due to M-N bonding and peaks in the range 435.04-480.82 cm-1 in complexes were due to M-O bonding [11].
The infrared spectroscopy of Schiff base 1 and complexes (1Cu-1Ni) were recorded in the range 4000-250 cm-1 as KBr pellets. IR spectra of Schiff bases and their complexes showed remarkable broad bands as shown in (Table 5). The IR spectra provide valuable information regarding the nature of functional groups attached to ligand and metal atom in complexes. In order to study the bonding bond of Schiff base and metal complexes, IR spectrum of free ligand was compared with the spectra of complexes. A band in free Schiff base appeared at 1638.06 cm-1 was due to C=N vibrational frequency. The azomethine peak (1638.06 cm-1) in Schiff base 1 was shifted to lower value 1610.56-1623.64 cm-1 suggesting the coordination. The shifting of this group to lower frequency in the metal complexes when compared to free Schiff base, suggested the coordination of metal ion through nitrogen atom of azomethine group. It is expected that coordination of metal atom would reduce the electron density in the azomethine link and thus lower the –HC=N absorption. Peaks in the range 540.07-582.50 cm-1 in complexes were due to M-N bonding and peaks in the range 435.04-480.82 cm-1 in complexes were due to M-O bonding [11].
| Sample code | ʋ (C = N) (cm-1) | ʋ (-OCH3) (cm-1) | ʋ (M-N) (cm-1) | ʋ (M-O) (cm-1) |
| Schiff base 1 | 1638.06 | 1242.16 | - | - |
| 1Cu | 1610.56 | 1238.30 | 540.07 | 480.82 |
| 1Cd | 1623.64 | 1234.44 | 576.72 | 422.41 |
| 1Co | 1614.42 | 1236.37 | 578.64 | 435.04 |
| 1Zn | 1620.21 | 1244.09 | 572.86 | 439.77 |
| 1Ni | 1613.78 | 1232.51 | 582.50 | 435.91 |
Table 5: IR data of Schiff base and complexes.
Conclusion
Schiff base of 5-aminosalicylic acid, 2-hydroxy-3-methoxybenzaldehyde and its metal complexes of Cu (II), Cd (II), Co (II), Zn (II) and Ni (II) were synthesized and characterized by analytical and spectral techniques. These compounds were tested against different of bacterial and fungal strains and exhibited promising activity against all the tested microorganisms.
Acknowledgements
Authors are cordially thankful to Chairman, Department of Chemistry, Government College University, and Faisalabad-Pakistan for providing chemical of AR grade and instrumentations.
Authors are cordially thankful to Chairman, Department of Chemistry, Government College University, and Faisalabad-Pakistan for providing chemical of AR grade and instrumentations.
Conflict of Interest
Authors declare that they have no conflict of interest.
Authors declare that they have no conflict of interest.
References
- Mishra L and Sinha R. “Mononuclear and dinuclear ruthenium (III) (polypyridyl complexes containg 2, 6-bis (2-benzimidazolyl)-pyridine as coli and: Synthesis, spectroscopic and redox activity”. Indian Journal of Chemistry-Section 29 (2000): 1131-1139.
- Krishnan KK and Ummathur MB. “Metal complexes of 1-phenylammo-7-phenyl-4, 6-heptadiene-1, 3-dione and its Schiff's base”. Journal of Indian chemical Society 83 (2006): 663-669.
- Rishikesh P., et al. “Effect of chlorine substitution on triple state structure of thioxanthone: A time-resolved resonance Raman study”. Journal of Raman Spectroscopy44 (2013): 270-276.
- Saritha P., et al. “Synthesis and structural studies on divalent transition metal complexes of 5-acetyl 2, 4-dihydroxy acetophenone semicarbazone”. Journal of Indian Chemical Society 83 (2006): 1204-1209.
- Singh, BK., et al. “Spectroscopic characterization and biological activity of Zn(II), Cd(II), Sn (II) and Pb (II) complexes with Schiff base derived from pyrrole-2-carboxaldehyde and 2-amino phenol”. Polyhedron 76 (2009): 376-383.
- Shibuya Y., et al. “The copper (II) complex with two didentate Schiff base ligands. The unique rearrangement that proceeds under alcohol vapor in the solid state to construct non-inclusion structure”. Transition Metal Chemistry 37 (2008): 78-79.
- Roy S., et al. “Syntheses, characterization and X-ray crystal structures of Co (III) and Mn (II) complexes of pyrimidine derived Schiff base ligands”. Polyhedron27 (2008): 593-601.
- Sarker SD., et al. “Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals”. Methods 42 (2007): 321-324.
- Huynh QK., et al. “Isolation and characterization of a 30 kDa protein with antifungal activity from leaves of Engelmannia pinnatifida”. Journal of Biological Chemistry 316 (2001): 723-727.
- Gulcan M., et al. “Synthesis, characterization and antimicrobial activity of a new pyrimidine Schiff base and its Cu(II), Ni(II), Co(II), Pt(II) and Pd(II) complexes”. Turkey Journal of Chemistry 36 (2012): 189-200.
- Victory B., et al. “Synthesis and characterization of biologically active schiff base complexes of nickel and cobalt compounds”. Research Journal of Pharmaceutical Biological and Chemical Sciences 2 (2010): 324-328.
Citation:
Syed Faheem Askari Rizvi., et al. “Synthesis, Characterization and Antimicrobial Investigation of Transition Metal Complexes
by using Schiff Base Containing N-, O- Donor Atoms”. Clinical Biotechnology and Microbiology 2.4 (2018): 382-391.
Copyright: © 2018 Syed Faheem Askari Rizvi., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.






























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.