Editorial
Volume 2 Issue 4 - 2018
Morphology Response of Growth Regulators on Callus of Japonica Rice Through Anther Culture
University of Agricultural Sciences, GKVK, Bengaluru, India
*Corresponding Author: Avinash Sharma, University of Agricultural Sciences, GKVK, Bengaluru, India.
Received: June 04, 2018; Published: August 03, 2018
Abstract
The present investigation was conducted to demonstrate the effect of growth regulators on callus morphology of japonica rice varieties Azucena and Moroberekan through another culture. The rice panicles with a distance of 12-13 cm between flag leaf and subtending leaf for Azucena and 14-15 cm for Moroberekan were selected because at this stage of panicle development microspores were in the mid-uninucleate stage. The callus type, callus colour and callus growth were observed in 16 growth regulator treatments. The response of callus morphology was recorded between 8-20 weeks. The data of callus induction was computed with factorial completely randomized design (FCRD). In japonica rice variety Azucena, among 16 growth regulator treatments, all treatments were recorded compact calli except treatment T14. The treatments T1, T5, T6, T8, T10, T11, T15 and T16 were showed white calli and remained treatments were showed yellow calli. The treatment T10 (2, 4 - D 1 mg L-1 + NAA 1 mg L-1 + Kinetin 1 mg L-1) and T16 (2, 4 - D 2 mg L-1 + NAA 2 mg L-1+ Kinetin 1 mg L-1) were observed early callus morphology in 8 weeks. In japonica rice variety Moroberekan, all treatments were observed compact calli except treatment T5 and T11. The treatments T2, T3, T6, T8, T10, T12, T14 and T15 were showed white calli and remained treatments were showed yellow calli. The treatment T16 (2, 4-D 2 mg L-1 + NAA 2 mg L-1 + Kinetin 1 mg L-1) was observed early callus morphology in 7 weeks. Therefore, callus morphology is essential factor to the development of haploid plant through another culture.
Introduction
Rice (Oryza sativa L. 2n = 24) is a monocotyledonous albuminous seed that belongs family of grasses, Gramineae and genus Oryza. Rice is the second most widely consumed cereal in the world next to wheat. Over 90 per cent of rice is produced and consumed in Asia alone, which has half the world’s population. Rice contains 80% carbohydrates, 7-8% protein, 3% fat, and 3% fiber. Rice accounts for about 29.3 per cent and 29.9 per cent of caloric intake in Asia and India respectively. The area covered under rice in the world is 161 million hectares with a total production of 700 million tonnes and average productivity of 4.53 tonnes per hectare. India has the largest area (43.3 million hectares) under rice among the rice growing countries of the world, but ranks second in total production. In India annual production of rice is 105.48 million tonnes (FAOSTAT, 2016).
The cultivated species are Oryza sativa and Oryza glaberrima. Oryza sativa is grown all over the world while Oryza glaberrima has been cultivated in West Africa. Oryza sativa contains two major subspecies: Japonica or sinica and Indica. Japonicais a relatively short plant with narrow, dark green leaves, and the grains are short and sticky with low amylose content. Japonica varieties are usually cultivated in dry fields, in temperate East Asia, upland areas of Southeast Asia and high elevations in South Asia. Another subspecies is indica the plants of which tall with broad to narrow, light green leaves. The grains are long, slender, and non-sticky with high amylase content. Indica varieties are grown mostly in submerged conditions throughout tropical Asia.
It has been believed that callus induction is very difficult in monocot plants, because they are recalcitrant in vitro manipulation. Yel rice was the first in them which response positively to callus (Niizeki and Oono, 1968). Now a day’s information is available on callus induction and shoot, root differentiation from different explants in crop includes rice.
The principle is involved in production of callus textures through anther culture; Microspore cell is diverted to normal development and function of the pollen cell to become a male gamete to form a vegetative cell. Microspore vegetative cell produces callus textures that undergo somatic embryogenesis for the production of haploid plantlets. There are two types of androgenesis; direct androgenesis where the microspores behave like a zygote and undergo change to form embryoids which ultimately give rise to a plantlets and the other is indirect androgenesis, where the microspores divide repeatedly to form a callus tissue, which differentiates into haploid plantlets. The anther is produced two types of callus i.e. embryogenic callus and non-embryogenic callus. Calli that were creamy white, some compact, friable, nodular and globular are defined as embryogenic callus, which further developed into plantlets. Calli that is completely yellow or bright brown in colour with soft or compact in texture are defined as non embryogenic callus, which further couldn’t develop into plantlets. It was documented that embryogenic callus displayed higher frequency of plant regeneration than the non-embryo genic one (Thadavong., et al. 2002). Embryogenic callus is having high proliferation potential, small size, isodiametric, dense cytoplasm content with prominent enlarged nuclei and small vacuole (Tang., et al. 2011) while non embryogenic callus shows enlongated cells with large nuclei (Feher, 2005).
Several factors are effecting the callus texture derived from in vitro anther culture of rice are genotype, microspore developmental stages, types of growth regulator along with its concentrations, culture media and culture conditions (Rukmini., et al. 2016). Embryogenic calli induction and subsequent green plant regeneration are highly influenced by culture components of medium and genetic makeup of genotypes (Talebi., et al. 2007). A less number of perfect calluses are obtained low regeneration with influence of growth regulator concentrations and media (Silva and Ratnayake, 2009). Callus induction is critical stage where the regeneration of plants is highly dependent on the quality of callus. The embryogenic callus of Gramineae species has a relatively dry, compact and nodular appearance (Quiroz-Figueroa., et al. 2006) while in embryogenic rice colouration has been used as criterion to select embryogenic callus (Oinem and Kothari, 1995). The combination of auxin and cytokinin is known to affect where the regeneration of plants is highly dependent on the embryo genic calli (Rueb., et al. 1994). Texture and colours of the calli reflect their green plantlet re differentiation competences (Ambarwati., et al. 2009). With this introduction, the present experiment was demonstrated on effect of growth regulators into callus morphology of japonica rice varieties Azucena and Moroberekan through another culture.
Review of Literature
Plant tissue culture is a symphony of art and science, which develops genetic diversity, produces virus-free plants and improves micropropagation under aseptic conditions in the short term (Birch, 1997). Plant cells possess high plasticity potential for cell differentiation. Stresses such as pathogen infection or wounding may lead to the production of tumours or callus. The history of the first callus growth traced back to 1979 when neely described a massive and disorganized cell mass in debarked trees (Neely, 1979). Embryogenic calli, rather than direct tissues such as immature inflorescences, shoot spices, leaves and roots, is an effective and safe tool for regeneration of wild and modified plants invitro conditions (Benlioglu., et al. 2015). Interestingly, calli are divided to various subgroups according to their microscopic traits. For instance, calli with some organ regeneration are named embryonic, shooty or rooty calli, whereas calli without organ regeneration are called compact or friable callus (Ikeuchi., et al. 2013). Callogenesis and growth highly depend on genotype, basal salt mediums, plant growth regulators (PGRs), carbohydrate, explants and adjuvant materials (Pawar., et al. 2015). Additionally, media strength is another essential factor to regulate callus’ growth and regeneration (Din., et al. 2016). Several authors have been reported effect of different factors on callus morphology of rice i.e.
Plant tissue culture is a symphony of art and science, which develops genetic diversity, produces virus-free plants and improves micropropagation under aseptic conditions in the short term (Birch, 1997). Plant cells possess high plasticity potential for cell differentiation. Stresses such as pathogen infection or wounding may lead to the production of tumours or callus. The history of the first callus growth traced back to 1979 when neely described a massive and disorganized cell mass in debarked trees (Neely, 1979). Embryogenic calli, rather than direct tissues such as immature inflorescences, shoot spices, leaves and roots, is an effective and safe tool for regeneration of wild and modified plants invitro conditions (Benlioglu., et al. 2015). Interestingly, calli are divided to various subgroups according to their microscopic traits. For instance, calli with some organ regeneration are named embryonic, shooty or rooty calli, whereas calli without organ regeneration are called compact or friable callus (Ikeuchi., et al. 2013). Callogenesis and growth highly depend on genotype, basal salt mediums, plant growth regulators (PGRs), carbohydrate, explants and adjuvant materials (Pawar., et al. 2015). Additionally, media strength is another essential factor to regulate callus’ growth and regeneration (Din., et al. 2016). Several authors have been reported effect of different factors on callus morphology of rice i.e.
Genotype of donor plant
The genotype has a strong effect on a pollen development formation. Genotype is also an important factor in addition to plant growth regulators (Abe and Futsuhara, 1986). Not only have the species within a genus but also the cultivars of the same species often showed different response. The donor plant is provided diversity into callus type and colour in authentic media. Several workers reported callus morphology from donated plant of i.e., Aly., et al. (1998) resulted compact, white/creamy colour to friable white from anthers of japonica genotypes Giza 171, 172, 173, 175, 177 and 178 in N6 media. The F1 hybrids of S × MA92 was founded compact and yellow calli in N6 media (Trejo-Tapia., et al. 2002). Bangladeshi rice cultivar BRRI Dhan-29 was produced compact and white calli in defined media (Shahnewaz., et al. 2003). The material of japonica variety Taipei 309 was founded white and yellow calli in N6 media (Biswas and Mandal. 2007). Ambarawati., et al. (2009) recorded compact and white milky calli from interspecific cross of recurrent parent and a wild rice in N6 media. F1 plant anthers of OMCS2000/OM4900OM5930/OM4900OM5992/OM4900OM3536/OM4900 were derived compact and yellow calli in N6 media (Tran and Nguyen, 2011). The sterilized explants of Biris rice were produced compact or friable and white or yellow calli in standard media (Libin., et al. 2012). The anthers of japonica rice varieties Azucena were recorded White and compact calli in N6 media (Dalpat, 2013). The anther of japonica rice varieties Azucena or Moroberekan was viewed white and compact calli in N6 media (Prabhu, 2013). Multiple callus type and callus colour were observed from Oryza sativa L. cultivar Swarna in defined media (Sukhla., et al. 2014). The anthers of japonica rice variety Moroberekan and japonica rice variety Azucena were recorded yellow or friable callus and white or compact callus in N6 media (Archana, 2015). F1 and BC1F1 of Dular × IR58025eB rice were noticed compact and creamy white calli in N6 media (Kaushal., et al. 2016). Rout., et al. (2016) reported compact and yellow calli from anthers of long duration rice hybrid CRHR32.
The genotype has a strong effect on a pollen development formation. Genotype is also an important factor in addition to plant growth regulators (Abe and Futsuhara, 1986). Not only have the species within a genus but also the cultivars of the same species often showed different response. The donor plant is provided diversity into callus type and colour in authentic media. Several workers reported callus morphology from donated plant of i.e., Aly., et al. (1998) resulted compact, white/creamy colour to friable white from anthers of japonica genotypes Giza 171, 172, 173, 175, 177 and 178 in N6 media. The F1 hybrids of S × MA92 was founded compact and yellow calli in N6 media (Trejo-Tapia., et al. 2002). Bangladeshi rice cultivar BRRI Dhan-29 was produced compact and white calli in defined media (Shahnewaz., et al. 2003). The material of japonica variety Taipei 309 was founded white and yellow calli in N6 media (Biswas and Mandal. 2007). Ambarawati., et al. (2009) recorded compact and white milky calli from interspecific cross of recurrent parent and a wild rice in N6 media. F1 plant anthers of OMCS2000/OM4900OM5930/OM4900OM5992/OM4900OM3536/OM4900 were derived compact and yellow calli in N6 media (Tran and Nguyen, 2011). The sterilized explants of Biris rice were produced compact or friable and white or yellow calli in standard media (Libin., et al. 2012). The anthers of japonica rice varieties Azucena were recorded White and compact calli in N6 media (Dalpat, 2013). The anther of japonica rice varieties Azucena or Moroberekan was viewed white and compact calli in N6 media (Prabhu, 2013). Multiple callus type and callus colour were observed from Oryza sativa L. cultivar Swarna in defined media (Sukhla., et al. 2014). The anthers of japonica rice variety Moroberekan and japonica rice variety Azucena were recorded yellow or friable callus and white or compact callus in N6 media (Archana, 2015). F1 and BC1F1 of Dular × IR58025eB rice were noticed compact and creamy white calli in N6 media (Kaushal., et al. 2016). Rout., et al. (2016) reported compact and yellow calli from anthers of long duration rice hybrid CRHR32.
Growth Regulators
Growth regulator plays significant function to rice callus morphology. Its concentration with or without combination decides type of callus as well as callus colour in defined media. The effect of specific growth regulator combination and specific single growth regulator concentration produces perfect rice callus morphology. These findings were reported by many authors i.e., Shahnewaz., et al. (2003) viewed embryogenic calli from rice cultivar BRRI Dhan-29 into 2 mg L-1 2, 4-D + 2.5 mg L-1 NAA + 0.5 mg L-1 Kinetin. Japonica genotype Taraori Basmati was produced embryogenic calli from 0.5 mg L-1 NAA (Bidhan and Mandal, 2005). The anthers of japonica genotype Chhomrong local and Chandan nath-3 were observed embryogenic calli into NAA 4 mg L-1 + Kinetin 2 mg L-1 (Sah, 2008). The embryogenic calli was found from rice variety Kurulu Thuda with accurate concentration of growth regulators (Silva and Ratnayake, 2009) The combination of 2.0 mg L-1 NAA + 0.5 mg L-1 kinetin was produced embryogenic calli from japonica genotype Dreamy 2/CaMsrB2-8-DH-7 (Park., et al. 2013). Ramachandra., et al. (2013) reported that the combination of 2, 4 - D + kinetin was more effective in producing an embryogenic calli. Vladislavovna (2014) recorded embryogenic calli from F2rice of Oryza sativa L. subspecies japonica with 2 mg L-1 2, 4-D. Kaushal., et al. (2014) reported embryogenic calli from F1 hybrid of 25eB x Dular into 2 mg L-1 2, 4-D + 0.5 mg L-1 Kinetin. The F1 hybrids of japonica × indica were noticed embryogenic calli from 2 mg L-1 2, 4-D + 0.5 mg L-1 Kinetin (Zin and Irie, 2017).
Growth regulator plays significant function to rice callus morphology. Its concentration with or without combination decides type of callus as well as callus colour in defined media. The effect of specific growth regulator combination and specific single growth regulator concentration produces perfect rice callus morphology. These findings were reported by many authors i.e., Shahnewaz., et al. (2003) viewed embryogenic calli from rice cultivar BRRI Dhan-29 into 2 mg L-1 2, 4-D + 2.5 mg L-1 NAA + 0.5 mg L-1 Kinetin. Japonica genotype Taraori Basmati was produced embryogenic calli from 0.5 mg L-1 NAA (Bidhan and Mandal, 2005). The anthers of japonica genotype Chhomrong local and Chandan nath-3 were observed embryogenic calli into NAA 4 mg L-1 + Kinetin 2 mg L-1 (Sah, 2008). The embryogenic calli was found from rice variety Kurulu Thuda with accurate concentration of growth regulators (Silva and Ratnayake, 2009) The combination of 2.0 mg L-1 NAA + 0.5 mg L-1 kinetin was produced embryogenic calli from japonica genotype Dreamy 2/CaMsrB2-8-DH-7 (Park., et al. 2013). Ramachandra., et al. (2013) reported that the combination of 2, 4 - D + kinetin was more effective in producing an embryogenic calli. Vladislavovna (2014) recorded embryogenic calli from F2rice of Oryza sativa L. subspecies japonica with 2 mg L-1 2, 4-D. Kaushal., et al. (2014) reported embryogenic calli from F1 hybrid of 25eB x Dular into 2 mg L-1 2, 4-D + 0.5 mg L-1 Kinetin. The F1 hybrids of japonica × indica were noticed embryogenic calli from 2 mg L-1 2, 4-D + 0.5 mg L-1 Kinetin (Zin and Irie, 2017).
Carbon Sources
Carbon source in the culture medium serves as source of energy for growth and development. In rice, sucrose has been found to be the most suitable carbon source because of their osmotic and nutritional effects (Powell, 1990). Opinions concerning the optimum sucrose concentration for anther culture development vary, but generally it is used at the concentration of 3-5 per cent (Raina., et al. 1987). Chen (1978) reported callus morphology with 6 per cent sucrose in the callus formation medium. Mercy and Zapata (1986) reported significant increase in anther callusing, in the media supplemented with 6 per cent and 12 per cent sucrose as compared to 2 per cent sucrose. However, some investigation feel that 6 per cent sucrose concentration is too high for callus growth and differentiation and consequently concentration of 4-5 per cent of sucrose give good callus induction and green plant formation in rice anther culture. High sucrose levels 6-17 per cent are required in those species (e.g., Gramineae, Cruciferae) in which mature pollen is shed in the tri-cellular condition (Dunwell and Thurling, 1985).
Carbon source in the culture medium serves as source of energy for growth and development. In rice, sucrose has been found to be the most suitable carbon source because of their osmotic and nutritional effects (Powell, 1990). Opinions concerning the optimum sucrose concentration for anther culture development vary, but generally it is used at the concentration of 3-5 per cent (Raina., et al. 1987). Chen (1978) reported callus morphology with 6 per cent sucrose in the callus formation medium. Mercy and Zapata (1986) reported significant increase in anther callusing, in the media supplemented with 6 per cent and 12 per cent sucrose as compared to 2 per cent sucrose. However, some investigation feel that 6 per cent sucrose concentration is too high for callus growth and differentiation and consequently concentration of 4-5 per cent of sucrose give good callus induction and green plant formation in rice anther culture. High sucrose levels 6-17 per cent are required in those species (e.g., Gramineae, Cruciferae) in which mature pollen is shed in the tri-cellular condition (Dunwell and Thurling, 1985).
Lentini., et al. (1995) reported well callus induction with highly recalcitrant genotypes of rice by replacing sucrose with maltose (351 mM). However, Sucrose rapidly breaks down to glucose and fructose, this fructose is toxic to the microspore. Whereas maltose yields two glucose units upon hydrolysis, so that it has been shown to be a superior carbon source this replaces the sucrose. Maltose has been demonstrated to androgenesis of japonica (Xie., et al. 1995). Bagheri., et al. (2009) resulted highest callus induction frequency 29.25% from rice genotype Rashti with 4% maltose.
Khatun., et al. (2012) tested the effect of 5 different media namely, N6, R2, SK3, He2, & MO19 media in 5 rice genotypes. It was observed that, on N6, He2, MO19 and R2 media, the androgenic response was very poor in comparison to SK3. They concluded that SK3 was the best induction medium for anther culture response in rice. The superior response of anthers in respect on callus induction in SK3 might be due to the presence of amino acids like L-proline and L-glutamine at a relatively higher concentration and substitution of maltose with sucrose.
Media and culture conditions
The continued division of the microspores for the formation of embryos or callus induction requires the presence of appropriate nutrients in the medium (Lenka and Reddy, 1994). Further, the effect of induction medium on the differentiation ability of calli is greater than the effect of the differentiation medium. It is critical to change the composition of the media or replenish them to keep the balance of micronutrients and maintain the pH. The most commonly used basal media for anther culture are N6 medium (Chu, 1978); modified MS medium (Murashige and Skoog, 1962); Nitsch and Nitsch (1969) medium and B5 medium (Gamborg., et al. 1968), but there are many others. Generally, half strength MS salt mixtures are suggested for the Solanaceae, and N6 medium for the cereals (Chu, 1978). Although efforts have been made in defining suitable medium for rice anther culture, results obtained, so far, are divergent. Rice anthers respond to many basic media such as B5 medium (Gamborg., et al. 1968), Nitsch and Nitsch (1969) medium, Miller medium (Wang., et al. 1974), N6 medium (Chu., et al. 1975), modified White's medium (Tsai and Lin, 1977), modified MS medium (Chen, 1977), LS and modified LS media (Chaleff and Stolarz, 1981). So far, N6 medium (Chu., et al. 1975) widely adapted for japonica rice anther culture. The main features of N6 medium are the growth and differentiations of rice pollen callus are influenced by major salts, especially by NH4+ salt. Lower concentration of NO3- ions was Beneficial for callus induction.
The continued division of the microspores for the formation of embryos or callus induction requires the presence of appropriate nutrients in the medium (Lenka and Reddy, 1994). Further, the effect of induction medium on the differentiation ability of calli is greater than the effect of the differentiation medium. It is critical to change the composition of the media or replenish them to keep the balance of micronutrients and maintain the pH. The most commonly used basal media for anther culture are N6 medium (Chu, 1978); modified MS medium (Murashige and Skoog, 1962); Nitsch and Nitsch (1969) medium and B5 medium (Gamborg., et al. 1968), but there are many others. Generally, half strength MS salt mixtures are suggested for the Solanaceae, and N6 medium for the cereals (Chu, 1978). Although efforts have been made in defining suitable medium for rice anther culture, results obtained, so far, are divergent. Rice anthers respond to many basic media such as B5 medium (Gamborg., et al. 1968), Nitsch and Nitsch (1969) medium, Miller medium (Wang., et al. 1974), N6 medium (Chu., et al. 1975), modified White's medium (Tsai and Lin, 1977), modified MS medium (Chen, 1977), LS and modified LS media (Chaleff and Stolarz, 1981). So far, N6 medium (Chu., et al. 1975) widely adapted for japonica rice anther culture. The main features of N6 medium are the growth and differentiations of rice pollen callus are influenced by major salts, especially by NH4+ salt. Lower concentration of NO3- ions was Beneficial for callus induction.
Many reports point out that media with a relatively high content of inorganic salts are more suitable for the differentiation of callus. The differentiation frequencies of rice pollen callus on different media were as follows: Modified N6 > MS > l/2MS > Miller > Nitsch. Addition of various concentrations of Na2Fe-EDTA, Na2Fe-EDTA or a combination of these two compound improved plantlet production in rice (Chen., et al. 1986).
Ogawa., et al. (1995) investigated nitrogen content of the induction medium and found that it also affected the callus induction and plant regeneration. Medium containing 20 mM KNO3 and 5 mM glutamine gave highest number of colonies which was 2 fold higher than the control medium containing 40 mM KNO3 and 2.5 mM (NH4)2SO4. Medium containing 20 mM KNO3 and 5 mM alanine resulted in the highest number of regenerating calli and green plantlets. Use of modified MS medium containing reduced nitrogen and 10 per cent (w/v) ficoll might favour microspore embryogenesis and green plant regeneration in rice (Datta., et al. 1990).
The nutrient medium not only provides nutrition to the microspores but also directs the pathway of embryo development. It is critical to change the composition of the media or replenish them to keep the balance of micronutrients and maintain the pH. The pH of the media, particularly liquid media, changes dramatically with time at the onset of embryo development (Datta and Wenzel, 1998).
Darz., et al. (1991) reported high frequency of callus induction obtained from 3 media, namely G1, Fj, and L8. G1 is a combination of medium B5 and N6. Fj and L8 have B5 and N6 as a basal medium, respectively. Karim., et al. (1986) tested 14 media for their suitability for rice anther culture of both indica and japonica cultivars and observed the best results from M10 medium. Cho and Zapata (1988) found modified Gamborg’s B5 liquid medium better for anther response to callus induction and MS medium for regeneration.
Materials and Methods
The current study on Influence of growth regulators on callus morphology of japonica rice through anther culture was carried out at the Plant Tissue Culture Laboratory, Department of Plant Biotechnology, University of Agricultural Sciences, GKVK Campus, and Bengaluru-560065 during the years 2013-2015. The material and methods used for the study are described in this chapter.
Plant material
Two japonica rice varieties Azucena and Moroberekan grown in the field were used as the source of explants. Their specific characteristics are presented in (Table 1). Recommended fertilizers and plant protection measures were adopted to raise healthy plants.
Two japonica rice varieties Azucena and Moroberekan grown in the field were used as the source of explants. Their specific characteristics are presented in (Table 1). Recommended fertilizers and plant protection measures were adopted to raise healthy plants.
| Genotype | Type | Features |
| Azucena | Japonica | Tropical and deep rooted, 140 days and 47.5 qha-1 yield. |
| Moroberekan | Japonica | Tropical, drought tolerant and blast resistance and 145 days. |
Table 1: Characteristics of the japonica rice varieties selected for the study.
Media preparation
Stock solutions (macro, micro, Fe-EDTA and vitamins) were prepared initially by dissolving the analytical grade chemicals in the required quantities in volumetric flasks using double distilled water. Fe-EDTA stocks were prepared and heated for a few minutes until it turned golden yellow and was then stored in a brown bottle and kept in refrigerator for further use. Composition of the modified N6 (Nitsch) medium used for callus induction is given in (Table 2).
Stock solutions (macro, micro, Fe-EDTA and vitamins) were prepared initially by dissolving the analytical grade chemicals in the required quantities in volumetric flasks using double distilled water. Fe-EDTA stocks were prepared and heated for a few minutes until it turned golden yellow and was then stored in a brown bottle and kept in refrigerator for further use. Composition of the modified N6 (Nitsch) medium used for callus induction is given in (Table 2).
| Constituents | Working conc. | Stock conc. |
| Macronutrients | (mg L-1) | (20X for 200 mL) |
| Potassium nitrate (KNO3) | 2830 | 11.320 g |
| Potassium phosphate (KH2PO4) | 400 | 1.600 g |
| Magnesium sulphate (MgSO4 ·7H2O) | 185 | 0.740 g |
| Calcium chloride (CaCl2 · 2H2O) | 166 | 0.646 g |
| Ammonium sulphate (NH4 SO4) | 463 | 1.852 g |
| Fe-EDTA | (mg L-1) | (100X for 100 mL) |
| Ferrous sulphate (FeSO4·7H2O) | 37.29 | 0.372 g |
| Na2.EDTA.2H2O | 27.85 | 0.278 g |
| Micronutrients | (mg L-1) | (100X for 100 mL) |
| Potassium iodide (KI) | 0.8 | 0.080 g |
| Boric acid (H3BO3) | 1.6 | 0.160 g |
| Manganese sulphate (MnSO4·H2O) | 3.33 | 0.333 g |
| Zinc sulphate (ZnSO4·7H2O) | 1.5 | 0.150 g |
| Cobalt chloride (CoCl2 · 6H2O) | 0.025 | 0.0025 g |
| Copper sulphate (CuSO4 · 5H2O) | 0.025 | 0.0025 g |
| Sodium molybdate (Na2MoO4·2H2O) | 0.25 | 0.025 g |
| Vitamins | (mg L-1) | (100X for 100 mL) |
| Thiamine HCl | 1.0 | 0.100 g |
| Pyridoxine HCl | 0.5 | 0.050 g |
| Nicotinic acid | 0.5 | 0.050 g |
| Glycine (free acid) | 2.0 | 0.200 g |
| The following are freshly added at the time of preparing medium Agar 0.8 per cent Maltose 3.0 per cent pH is adjusted to 5.8 |
||
Table 2: Composition of modified N6 (Nitsch) basal medium used for rice anther culture.
Preparation of modified N6 and MS medium from stock solutions
The nutrients required for the modified N6 medium and MS medium were pipette out in required quantities from the stock solution (macronutrients, micronutrients, Fe-EDTA and vitamins) along with the required amount of maltose 30 g L-1 for callus induction in modified N6 medium and sucrose 30 g L-1 for regeneration in MS medium. Growth regulator was added and specified final volume was made up with double distilled water. The pH was adjusted to 6 for modified N6 medium and 5.8 for MS medium using 0.1 N NaOH and 0.1 N HCl. The required quantity of Agar (8g L-1) was added and the mixture was then heated for dissolving the agar. Then the medium was transferred to conical flasks, making sure that each flask was filled with not more than half of its capacity to ensure proper autoclaving. The flasks were covered with aluminium foil and autoclaved at 121ºC temperature and 15 lbs. pressure for 15 min. After autoclaving, the media along with other materials (culture containers, parafilm etc.) were shifted to the laminar hood, which was priorly sterilized by UV for 15 min. After switching off the UV, the airflow and illumination were switched on. The floor of the cabinet and hands were swabbed with 70 per cent alcohol to ensure total sterility. The medium was poured into the Petri plates before it cooled down near the flames of a Bunsen burner. Then, the media was allowed to solidify.
The nutrients required for the modified N6 medium and MS medium were pipette out in required quantities from the stock solution (macronutrients, micronutrients, Fe-EDTA and vitamins) along with the required amount of maltose 30 g L-1 for callus induction in modified N6 medium and sucrose 30 g L-1 for regeneration in MS medium. Growth regulator was added and specified final volume was made up with double distilled water. The pH was adjusted to 6 for modified N6 medium and 5.8 for MS medium using 0.1 N NaOH and 0.1 N HCl. The required quantity of Agar (8g L-1) was added and the mixture was then heated for dissolving the agar. Then the medium was transferred to conical flasks, making sure that each flask was filled with not more than half of its capacity to ensure proper autoclaving. The flasks were covered with aluminium foil and autoclaved at 121ºC temperature and 15 lbs. pressure for 15 min. After autoclaving, the media along with other materials (culture containers, parafilm etc.) were shifted to the laminar hood, which was priorly sterilized by UV for 15 min. After switching off the UV, the airflow and illumination were switched on. The floor of the cabinet and hands were swabbed with 70 per cent alcohol to ensure total sterility. The medium was poured into the Petri plates before it cooled down near the flames of a Bunsen burner. Then, the media was allowed to solidify.
Anther culture
Standardization of culture media for callus induction
Selection of explants
Stage of panicle harvest
Panicles were harvested at the early flowering stage, when young panicles were still enclosed within the leaf sheath. Suitable period for panicle collection was from July-August (in the pots) and September-October (in the field). Panicles with a distance of 10-15 cm between the subtending leaf and the flag leaf were selected.
Standardization of culture media for callus induction
Selection of explants
Stage of panicle harvest
Panicles were harvested at the early flowering stage, when young panicles were still enclosed within the leaf sheath. Suitable period for panicle collection was from July-August (in the pots) and September-October (in the field). Panicles with a distance of 10-15 cm between the subtending leaf and the flag leaf were selected.
Cold pre-treatment
Panicles were collected between 6.00-9.00 am and washed with water and sprayed with 70 per cent ethanol. These panicles were sealed in a polyethylene bag and were wrapped in aluminum foil. Cold pre-treatment was given by placing them in refrigerator at 5°C for 8 days.
Panicles were collected between 6.00-9.00 am and washed with water and sprayed with 70 per cent ethanol. These panicles were sealed in a polyethylene bag and were wrapped in aluminum foil. Cold pre-treatment was given by placing them in refrigerator at 5°C for 8 days.
Pollen growth stage
Spikelets were selected from three parts (top, middle and basal) of each panicle and anthers stained with acetocarmine and observed under a light microscope to identify the pollen development stage.
Spikelets were selected from three parts (top, middle and basal) of each panicle and anthers stained with acetocarmine and observed under a light microscope to identify the pollen development stage.
Sterilization and inoculation of the explant
Necessary precautions were taken to avoid contamination. On the day of inoculation, selected panicles were taken out the refrigerator and sterilized with 70 per cent alcohol for 20 seconds followed by 0.2 per cent HgCl2 for 10 minutes in laminar air flow chamber. After 10 minutes, the sterilant was drained off and the panicles were thoroughly rinsed with sterile distilled water for 3-4 times. After that, the anthers were isolated from spikelet avoiding any mechanical damage followed by inoculation on petridish (60 mm x 15 mm) containing 10 mL of N6 solid basal medium.
Necessary precautions were taken to avoid contamination. On the day of inoculation, selected panicles were taken out the refrigerator and sterilized with 70 per cent alcohol for 20 seconds followed by 0.2 per cent HgCl2 for 10 minutes in laminar air flow chamber. After 10 minutes, the sterilant was drained off and the panicles were thoroughly rinsed with sterile distilled water for 3-4 times. After that, the anthers were isolated from spikelet avoiding any mechanical damage followed by inoculation on petridish (60 mm x 15 mm) containing 10 mL of N6 solid basal medium.
Treatments for callus induction
Treatment for callus induction was done using modified N6 as a basal medium with different concentrations of 2, 4-D, NAA and Kinetin (Table 3).
Treatment for callus induction was done using modified N6 as a basal medium with different concentrations of 2, 4-D, NAA and Kinetin (Table 3).
| Treatment | 2,4-D (mg L-1) | NAA (mg L-1) | Kinetin (mg L-1) |
| T0 (Control) | 0 | 0 | 0 |
| T1 | 1 | 0 | 0.5 |
| T2 | 1 | 0 | 1 |
| T3 | 2 | 0 | 0.5 |
| T4 | 2 | 0 | 1 |
| T5 | 0 | 1 | 0.5 |
| T6 | 0 | 1 | 1 |
| T7 | 0 | 2 | 0.5 |
| T8 | 0 | 2 | 1 |
| T9 | 1 | 1 | 0.5 |
| T10 | 1 | 1 | 1 |
| T11 | 1 | 2 | 0.5 |
| T12 | 1 | 2 | 1 |
| T13 | 2 | 1 | 0.5 |
| T14 | 2 | 1 | 1 |
| T15 | 2 | 2 | 0.5 |
| T16 | 2 | 2 | 1 |
Table 3: Growth regulator treatments used to induce callus from anthers.
Culture conditions for callus induction
All the cultures were incubated in dark condition in a culture room maintained at 23 ± 2°C, with a relative humidity of 50-60 per cent and 16 hour photoperiod at a photon flux density of 3000 lux from white cool fluorescent tubes.
All the cultures were incubated in dark condition in a culture room maintained at 23 ± 2°C, with a relative humidity of 50-60 per cent and 16 hour photoperiod at a photon flux density of 3000 lux from white cool fluorescent tubes.
Observations
The cultures were observed frequently and the contaminated plates were removed. After 10-20 weeks of culture the following observations were recorded.
The cultures were observed frequently and the contaminated plates were removed. After 10-20 weeks of culture the following observations were recorded.
- Number of anthers inoculated
- Number of anthers producing callus
- Weeks taken for callusing
- Colour of the callus: White or Yellow
- Friability of the callus: Compact or Friable
- Callus growth: - = No callus, + = Poor callus growth, ++ = Moderate callus growth
Statistical analysis
All the experiments were conducted in the plant tissue culture laboratory, under uniform condition of temperature, humidity and light. For each treatment used in the experiment, three replications were maintained and data were analyzed by Factorial Completely Randomized Design (FCRD) method for callus induction.
All the experiments were conducted in the plant tissue culture laboratory, under uniform condition of temperature, humidity and light. For each treatment used in the experiment, three replications were maintained and data were analyzed by Factorial Completely Randomized Design (FCRD) method for callus induction.
ANOVA structure for genotype and treatment interaction
| Source of variance | Degrees of freedom | MSS |
| Replications | r-1 | Mr |
| Treatments | t-1 | Mt |
| Varieties | v-1 | Mv |
| Varieties × treatments | (v-1) (t-1) | Mvt |
| Error | (rtv-1)- {[(v-1) (t-1)]- (v-1) -(t-1)} | Me |
| Total | rtv-1 |
Experimental Results
Androgenic callus induction
Stage of panicle harvest
Panicles with a distance of 12-13 cm for Azucena and 14-15 cm for Moroberekan between the flag leaf and subtending leaf were selected (Figure 1). These panicles were collected and kept in refrigerator for cold pretreatment at 4°C for 8 days. In order to identify the stage of pollen development, anthers of Azucena and Moroberekan were stained with acetocarmine and observed under light microscope. The spikelets in the middle and bottom position of panicle which contained mid uninucleate stage were selected for culturing (Figure 2).
Androgenic callus induction
Stage of panicle harvest
Panicles with a distance of 12-13 cm for Azucena and 14-15 cm for Moroberekan between the flag leaf and subtending leaf were selected (Figure 1). These panicles were collected and kept in refrigerator for cold pretreatment at 4°C for 8 days. In order to identify the stage of pollen development, anthers of Azucena and Moroberekan were stained with acetocarmine and observed under light microscope. The spikelets in the middle and bottom position of panicle which contained mid uninucleate stage were selected for culturing (Figure 2).

Figure 1: Stage of panicle harvest in Azucena and Moroberekan varieties. Distance between
flag leaf and subtending leaf was 12.4 cm in Azucena and 14 cm in Moroberekan.
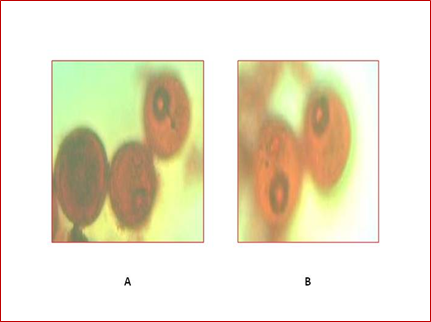
Figure 2: Pollen grains at mid uninucleate developmental stage observed
under light microscope at 40X, in Azucena (A) and Moroberekan (B).
Effect of growth regulator on callus morphology of japonica rice varieties Azucena and Moroberekan the calli properties of japonica rice varieties Azucena and Moroberekan observed significant differences with effect of growth regulator treatments. The data was presented in (Table 4). In japonica variety Azucena, among 16 growth regulator treatments, the treatments T0 (control), T2 and T9 were not produced callus texture, callus colour or callus growth. All treatments were recorded compact calli except treatment T14. The treatments T1, T5, T6, T8, T10, T11, T15 and T16 were showed white calli and remained treatments were showed yellow calli. The response of callus morphology was recorded between 8-20 weeks. The treatment T10 (2, 4-D 1 mg L-1 + NAA 1 mg L-1 + Kinetin 1 mg L-1) and T16 (2, 4-D 2 mg L-1 + NAA 2 mg L-1+ Kinetin 1 mg L-1) were observed early callus morphology in 8 weeks whereas, the treatment T1 (2, 4-D 1 mg L-1 + Kinetin 0.5 mg L-1) was observed late callus morphology in 13 weeks (Figure 5, Figure 3).
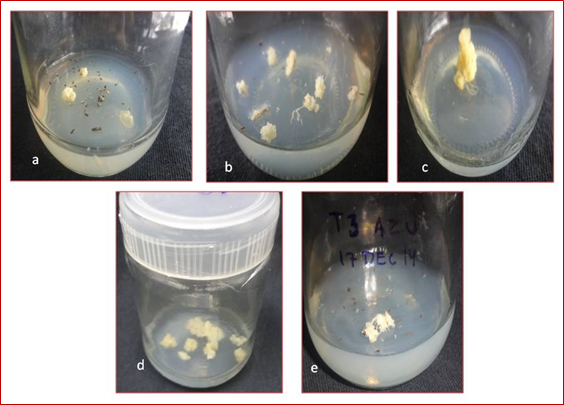
Figure 3: Callus morphology obtained from anthers of Azucena on modified N6 medium.
a) Callus produced in Azucena in callus induction treatment T1 (2, 4-D 1 mg L-1 + Kinetin 0.5 mg L-1) after 20 weeks of culture.
b) Callus produced in Azucena in callus induction treatment T10 (2, 4-D 1 mg L-1 + NAA 1 mg L-1 + Kinetin 1 mg L-1) after 8 weeks of culture.
c) Callus produced in Azucena in callus induction treatment T12 (2, 4-D 1 mg L-1 + NAA 2 mg L-1 + Kinetin 1 mg L-1) after 11 weeks of culture
d) Callus produced in Azucena in callus induction treatment T14 (2, 4-D 2 mg L-1 + NAA 1 mg L-1 + Kinetin 1 mg L-1) after 10 weeks of culture
e) Callus produced in Azucena in callus induction treatment T16 (2, 4-D 2 mg L-1 + NAA 2 mg L-1 + Kinetin 1 mg L-1) after 8 weeks of culture
a) Callus produced in Azucena in callus induction treatment T1 (2, 4-D 1 mg L-1 + Kinetin 0.5 mg L-1) after 20 weeks of culture.
b) Callus produced in Azucena in callus induction treatment T10 (2, 4-D 1 mg L-1 + NAA 1 mg L-1 + Kinetin 1 mg L-1) after 8 weeks of culture.
c) Callus produced in Azucena in callus induction treatment T12 (2, 4-D 1 mg L-1 + NAA 2 mg L-1 + Kinetin 1 mg L-1) after 11 weeks of culture
d) Callus produced in Azucena in callus induction treatment T14 (2, 4-D 2 mg L-1 + NAA 1 mg L-1 + Kinetin 1 mg L-1) after 10 weeks of culture
e) Callus produced in Azucena in callus induction treatment T16 (2, 4-D 2 mg L-1 + NAA 2 mg L-1 + Kinetin 1 mg L-1) after 8 weeks of culture
| Treatments | Type of callus | Colour of callus | Callus Growth | Time taken for callus induction (weeks) | Mean of treatment | ||||
| Azucena | Moroberekan | Azucena | Moroberekan | Azucena | Moroberekan | Azucena | Moroberekan | ||
| T0 (Control) | - | - | - | - | - | - | 0 | 0 | 0.0 |
| T1 | Compact | - | White | - | + | - | 20 | 0 | 10.0 |
| T2 | - | Compact | - | White | - | + | 0 | 13 | 6.5 |
| T3 | Compact | Compact | Yellow | White | + | + | 17 | 9 | 13.0 |
| T4 | Compact | Compact | Yellow | Yellow | ++ | + | 17 | 8 | 12.5 |
| T5 | Compact | Friable | White | Yellow | + | + | 13 | 8 | 10.5 |
| T6 | Compact | Compact | White | White | + | + | 10 | 9 | 9.5 |
| T7 | Compact | Compact | Yellow | Yellow | + | + | 10 | 11 | 10.5 |
| T8 | Compact | Compact | White | White | + | + | 10 | 8 | 9.0 |
| T9 | - | Compact | - | Yellow | - | + | 0 | 12 | 6.0 |
| T10 | Compact | Compact | White | White | + | + | 8 | 11 | 9.5 |
| T11 | Compact | Friable | White | Yellow | + | ++ | 16 | 9 | 12.5 |
| T12 | Compact | Compact | Yellow | White | + | + | 11 | 9 | 10.0 |
| T13 | Compact | Compact | Yellow | Yellow | + | ++ | 16 | 8 | 12.0 |
| T14 | Friable | Compact | Yellow | White | + | + | 10 | 10 | 10.0 |
| T15 | Compact | Compact | White | White | + | + | 13 | 11 | 12.0 |
| T16 | Compact | Compact | White | Yellow | + | + | 8 | 7 | 7.5 |
| Mean of variety | 10.53 | 8.41 | |||||||
Table 4: Morphological characteristics of the androgenic callus in Azucena and Moroberekan as influenced by growth regulators.
In japonica variety Moroberekan, among 16 growth regulator treatments, the treatment T0 (control) and T1 were not produced callus texture, callus colour or callus growth. All treatments were observed compact calli except treatment T5 and T11. The treatments T2, T3, T6, T8, T10, T12, T14 and T15 were showed white calli and remained treatments were showed yellow calli. The response of callus morphology was observed between 7-13 weeks. The treatment T16 (2, 4-D 2 mg L-1 + NAA 2 mg L-1+ Kinetin 1 mg L-1) was observed early callus morphology in 7 weeks whereas, the treatment T2 (2, 4-D 1 mg L-1 + Kinetin 1 mg L-1) was observed late callus morphology in 13 weeks (Figure 5) (Figure 4).
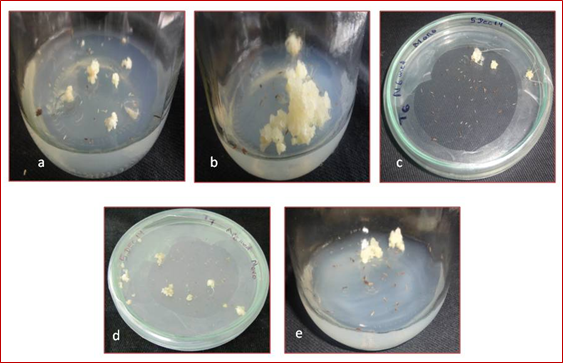
Figure 4: Callus morphology obtained from anthers of Moroberekan on modified N6 medium.
a) Callus produced in Moroberekan in treatment T2 (2, 4-D 1 mg L-1 + Kinetin 1 mg L-1) after 13 weeks of culture
b) Callus produced in Moroberekan in callus induction treatment T5 (NAA 1 mg L-1 + Kinetin 0.5 mg L-1) after 8 weeks of culture
c) Callus produced in Moroberekan in callus induction treatment T6 (NAA 1 mg L-1 + Kinetin 1 mg L-1) after 9 weeks of culture
d) Callus produced in Moroberekan in callus induction treatment T7 (NAA 2 mg L-1 + Kinetin 0.5 mg L-1) after 11 weeks of culture
e) Callus produced in Moroberekan in callus induction treatment T16 (2, 4-D 2 mg L-1 + NAA 2 mg L-1+ Kinetin 1 mg L-1) after 7
weeks of culture
a) Callus produced in Moroberekan in treatment T2 (2, 4-D 1 mg L-1 + Kinetin 1 mg L-1) after 13 weeks of culture
b) Callus produced in Moroberekan in callus induction treatment T5 (NAA 1 mg L-1 + Kinetin 0.5 mg L-1) after 8 weeks of culture
c) Callus produced in Moroberekan in callus induction treatment T6 (NAA 1 mg L-1 + Kinetin 1 mg L-1) after 9 weeks of culture
d) Callus produced in Moroberekan in callus induction treatment T7 (NAA 2 mg L-1 + Kinetin 0.5 mg L-1) after 11 weeks of culture
e) Callus produced in Moroberekan in callus induction treatment T16 (2, 4-D 2 mg L-1 + NAA 2 mg L-1+ Kinetin 1 mg L-1) after 7
weeks of culture
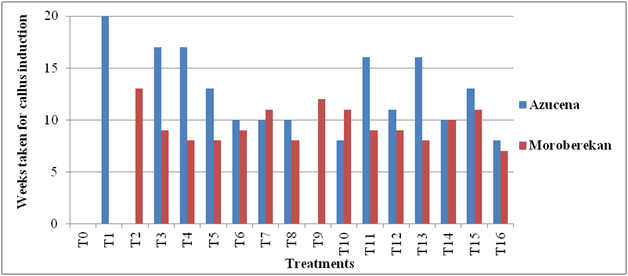
Figure 5: Time taken for callus initiation from anthers of Japonica rice varieties.
T0 = (Without growth regulators)
T1 = (2, 4-D 1 mg L-1 + Kinetin 0.5 mg L-1)
T2 = (2, 4-D 1 mg L-1 + Kinetin 1 mg L-1)
T3 = (2, 4-D 2 mg L-1 + Kinetin 0.5 mg L-1)
T4 = (2, 4-D 2 mg L-1 + Kinetin 1 mg L-1)
T5 = (NAA 1 mg L-1 + Kinetin 0.5 mg L-1)
T6 = (NAA 1 mg L-1 + Kinetin 1 mg L-1)
T7 = (NAA 2 mg L-1 + Kinetin 0.5 mg L-1)
T8 = (NAA 2 mg L-1 + Kinetin 1 mg L-1)
T9 = (2, 4-D 1 mg L-1 + NAA 1 mg L-1 + Kinetin 0.5 mg L-1)
T10 = (2, 4-D 1 mg L-1 + NAA 1 mg L-1 + Kinetin 1 mg L-1)
T11 = (2, 4-D 1 mg L-1 + NAA 2 mg L-1 + Kinetin 0.5 mg L-1)
T12 = (2, 4-D 1 mg L-1 + NAA 2 mg L-1 + Kinetin 1 mg L-1)
T13 = (2, 4-D 2 mg L-1 + NAA 1 mg L-1 + Kinetin 0.5 mg L-1)
T14 = (2, 4-D 2 mg L-1 + NAA 1 mg L-1 + Kinetin 1 mg L-1)
T15 = (2, 4-D 2 mg L-1 + NAA 2 mg L-1 + Kinetin 0.5 mg L-1)
T16 = (2, 4-D 2 mg L-1 + NAA 2 mg L-1 + Kinetin 1 mg L-1) weeks of culture
T0 = (Without growth regulators)
T1 = (2, 4-D 1 mg L-1 + Kinetin 0.5 mg L-1)
T2 = (2, 4-D 1 mg L-1 + Kinetin 1 mg L-1)
T3 = (2, 4-D 2 mg L-1 + Kinetin 0.5 mg L-1)
T4 = (2, 4-D 2 mg L-1 + Kinetin 1 mg L-1)
T5 = (NAA 1 mg L-1 + Kinetin 0.5 mg L-1)
T6 = (NAA 1 mg L-1 + Kinetin 1 mg L-1)
T7 = (NAA 2 mg L-1 + Kinetin 0.5 mg L-1)
T8 = (NAA 2 mg L-1 + Kinetin 1 mg L-1)
T9 = (2, 4-D 1 mg L-1 + NAA 1 mg L-1 + Kinetin 0.5 mg L-1)
T10 = (2, 4-D 1 mg L-1 + NAA 1 mg L-1 + Kinetin 1 mg L-1)
T11 = (2, 4-D 1 mg L-1 + NAA 2 mg L-1 + Kinetin 0.5 mg L-1)
T12 = (2, 4-D 1 mg L-1 + NAA 2 mg L-1 + Kinetin 1 mg L-1)
T13 = (2, 4-D 2 mg L-1 + NAA 1 mg L-1 + Kinetin 0.5 mg L-1)
T14 = (2, 4-D 2 mg L-1 + NAA 1 mg L-1 + Kinetin 1 mg L-1)
T15 = (2, 4-D 2 mg L-1 + NAA 2 mg L-1 + Kinetin 0.5 mg L-1)
T16 = (2, 4-D 2 mg L-1 + NAA 2 mg L-1 + Kinetin 1 mg L-1) weeks of culture
Discussion
Rice is one of the most important crops of Asia and it is the staple food of more than 90 per cent of the Asian population. The productivity of rice has to be improved upon continuously to meet the requirement of ever increasing population. This cannot be achieved through conventional techniques of crop improvement only and will require the involvement of plant biotechnology, including tissue culture.
Rice biotechnology has already contributed to the production of improved varieties; Wide hybridization, including use of embryo rescue technique has allowed the transfer of useful genes from wild Oryza species to elite cultivars. Many more potentially useful genes remain available in the wild species and new tools of biotechnology will speed up their utilization. Protoplast regeneration protocols have become more efficient and available for a broader range of rice cultivars; Protoplast fusion followed by regeneration should become another technique for wide crossing.
The potential of plant tissue culture techniques for the development of novel plant genotypes has been increasingly recognized. Progress has been made in developing techniques to culture and regenerate plants from somatic cells, pollen and protoplasts of a large number of plant species (Thorpe, 1990). Tissue culture techniques coupled with the recent developments in molecular biology have opened a new vista for broadening crop gene pools, increasing the efficiency of conventional plant breeding methods. Many new rice cultivars have been developed through biotechnological techniques like anther culture, embryo rescue and somaclonal variation (Zapata., et al. 2004).
Plant tissue culture techniques are recognized as one of the useful tools in crop improvement. Among these techniques, in vitro anther culture stands out and is an increasingly powerful tool when integrated into breeding programs (Hu and Zeng, 1984). This technique allows the acceleration of plant breeding by providing homozygous doubled haploids within a comparatively short time (Nurhidayah., et al. 1996). Anther culture has been known to produce a high proportion of haploids that could be doubled spontaneously or artificially to obtain doubled haploids carrying two sets of chromosomes from a single parent (homozygous) that can be further utilized in breeding process (Rukmini., et al. 2013). The major advantage of this technique is the recovery of homozygous diploids for breeding and establishment of haploid cell cultures for mutational studies and genetic manipulation experiments. An extensive study conducted on anther culture of rice has led to the identification of critical factors for successful development of haploids.
Various studies on rice anther culture have been conducted by several workers (Sengsai., et al. 2007; Sah, 2008 and Dalpat., et al. 2014). Thepresent investigation is an attempt to study Influence of growth regulators on callus morphology of japonica rice through anther culture. The results of the investigation arediscussed below.
Panicle harvest stage
During the present investigation panicles were harvested at the early flowering stage when young panicles were still enclosed within the sheath. Panicles with a distance of 12-13 cm between flag leaf and subtending leaf for Azucena and 14-15 cm for Moroberekan were selected because at this stage of panicle development microspores were in the mid uninucleate stage and it is considered to be the optimum stage of pollen development for callus induction. Mercy and Zapata (1986) studied the distance between flag leaf and subtending leaf as well as the late uninucleate and early binucleate pollen stage. Sengsai., et al. 2007; Niroula and Bimb, 2009 and Dalpat., et al. 2014 reported callus induction in late uninucleate and mid uninucleate microspore stage in japonica rice. Panicles with a distance of 11-13 cm (Afza., et al. 2000); 7-22 cm (Prabhu, 2013) and 8-11 cm (Dalpat., et al. 2014) between flag leaf and subtending leaf have been used successfully for callus induction in different japonica rice varieties.
During the present investigation panicles were harvested at the early flowering stage when young panicles were still enclosed within the sheath. Panicles with a distance of 12-13 cm between flag leaf and subtending leaf for Azucena and 14-15 cm for Moroberekan were selected because at this stage of panicle development microspores were in the mid uninucleate stage and it is considered to be the optimum stage of pollen development for callus induction. Mercy and Zapata (1986) studied the distance between flag leaf and subtending leaf as well as the late uninucleate and early binucleate pollen stage. Sengsai., et al. 2007; Niroula and Bimb, 2009 and Dalpat., et al. 2014 reported callus induction in late uninucleate and mid uninucleate microspore stage in japonica rice. Panicles with a distance of 11-13 cm (Afza., et al. 2000); 7-22 cm (Prabhu, 2013) and 8-11 cm (Dalpat., et al. 2014) between flag leaf and subtending leaf have been used successfully for callus induction in different japonica rice varieties.
Cold pre-treatment and dark incubation
In the present study, cold pre-treatment at 4°C for 8 days was given for the selected panicles. The cultures were incubated in dark for 10-20 weeks for enhancing callus induction (Cai and Chen, 1984; Trejo-Tapia., et al. 2002). Sunderland and Roberts (1979) reported that cold pre-treatment assures survival of a greater proportion of the embryogenic pollen grains. The total content of free amino acids is increased, which might be conductive for adaptation of microspores to the metabolic changes that results in embryogenesis induction (Claparols., et al. 1993 and Xie., et al. 1997). Kaushal., et al. (2014) reported that cold treatment is essential to improve anther culture response and manipulation of pre-treatment has ability to improve callus induction and subsequent plant regeneration. Cold treatments enhanced stoppage of the gametophytic development of microspores during cold stress and guides continuous division of the microspores to form callus (Tourev., et al. 1996 and Heberle-Bors, 1996). Pre-treatment longer than 9 days resulted in drastic decline in green plantlet regeneration capabilities of the calli of japonica rice (Chung, 1987 and Zhang, 1989). Pande (1997) stated that pre-treatment longer than 11 days resulted in albino production and Sen., et al. (2011) also reported that prolonged cold treatment of over 12 days induced albinos.
In the present study, cold pre-treatment at 4°C for 8 days was given for the selected panicles. The cultures were incubated in dark for 10-20 weeks for enhancing callus induction (Cai and Chen, 1984; Trejo-Tapia., et al. 2002). Sunderland and Roberts (1979) reported that cold pre-treatment assures survival of a greater proportion of the embryogenic pollen grains. The total content of free amino acids is increased, which might be conductive for adaptation of microspores to the metabolic changes that results in embryogenesis induction (Claparols., et al. 1993 and Xie., et al. 1997). Kaushal., et al. (2014) reported that cold treatment is essential to improve anther culture response and manipulation of pre-treatment has ability to improve callus induction and subsequent plant regeneration. Cold treatments enhanced stoppage of the gametophytic development of microspores during cold stress and guides continuous division of the microspores to form callus (Tourev., et al. 1996 and Heberle-Bors, 1996). Pre-treatment longer than 9 days resulted in drastic decline in green plantlet regeneration capabilities of the calli of japonica rice (Chung, 1987 and Zhang, 1989). Pande (1997) stated that pre-treatment longer than 11 days resulted in albino production and Sen., et al. (2011) also reported that prolonged cold treatment of over 12 days induced albinos.
Androgenic callus induction medium
During the present investigation selected panicles were rinsed in 70 per cent alcohol for 20 sec followed by surface sterilization with 0.1 per cent HgCl2 for 8-10 min. After that, sterilized anthers of Azucena (japonica var.) and Moroberekan (japonica var.) were cultured on N6 basal medium with growth regulators (2, 4-D, NAA, Kinetin), 3 per cent Maltose and pH was adjusted to 6. Usage of N6 medium for culture of anthers had been reported previously by Chu., et al. (1975); Herath., et al. (2007); Chen and Qin (2008) and Lapitan., et al. (2014). Bagheri., et al. (2009) had indicated that N6 medium was the best for callus induction from anthers of rice. Hence, N6 medium was selected in the present study. Maximum callus formation from anthers was achieved at pH 6 in Oryza sativa L. (Oono, 1976). In the present study, rice anthers showed positive response on N6 medium containing 30 gL-1 maltose. Similar results were reported by Ali., et al. (2004) and Prabhu (2013). The beneficial effect of maltose has been ascribed to its slow degradation which results in stabilization of medium osmolarity later on, promoting microspore division and callus formation in japonica rice varieties (Xie., et al. 1995 and Sengsai., et al. 2007).
During the present investigation selected panicles were rinsed in 70 per cent alcohol for 20 sec followed by surface sterilization with 0.1 per cent HgCl2 for 8-10 min. After that, sterilized anthers of Azucena (japonica var.) and Moroberekan (japonica var.) were cultured on N6 basal medium with growth regulators (2, 4-D, NAA, Kinetin), 3 per cent Maltose and pH was adjusted to 6. Usage of N6 medium for culture of anthers had been reported previously by Chu., et al. (1975); Herath., et al. (2007); Chen and Qin (2008) and Lapitan., et al. (2014). Bagheri., et al. (2009) had indicated that N6 medium was the best for callus induction from anthers of rice. Hence, N6 medium was selected in the present study. Maximum callus formation from anthers was achieved at pH 6 in Oryza sativa L. (Oono, 1976). In the present study, rice anthers showed positive response on N6 medium containing 30 gL-1 maltose. Similar results were reported by Ali., et al. (2004) and Prabhu (2013). The beneficial effect of maltose has been ascribed to its slow degradation which results in stabilization of medium osmolarity later on, promoting microspore division and callus formation in japonica rice varieties (Xie., et al. 1995 and Sengsai., et al. 2007).
Effect of growth regulators on callus morphology in japonica rice varieties
The anthers derived callus were produced embryogenic callus or non embryogenic callus with effect of growth regulators. The embryogenic callus is better than non embryogenic callus to conventional breeding. The compact type and white colour embryogenic callus are better than friable type or yellow colour embryogenic callus to homozygous line development. The development of callus morphology into finite time. In the present investigation, among 16 growth regulator treatments, the treatment T10 (2, 4-D 1 mg L-1 + NAA 1 mg L-1 + Kinetin 1 mg L-1) and T16 (2, 4-D 2 mg L-1 + NAA 2 mg L-1 + Kinetin 1 mg L-1) were observed early callus morphology in 8 weeks whereas, the treatment T1 (2, 4-D 1 mg L-1 + Kinetin 0.5 mg L-1) was observed late callus morphology in 13 weeks in japonica variety Azucena. Islam et al. (2004) reported callus morphology from BRRI commercial rice variety with 2, 4-D + NAA + Kinetin into 30 days and 45 days. Dalpat., et al. (2014) has also reported callus morphology from japonica rice variety Azucena with 2, 4 - D + NAA + Kinetin into 7-8 weeks. The japonica rice variety Azucena observed callus morphology with 2, 4 - D + Kinetin into limited time (Archana, 2015). Application of 2, 4-D and NAA in combination with Kinetin could lead to an increase of callus induction and plant regeneration (Rout and Sarma, 1991).Embryogenic calli with nodular structures appeared on the surface of the non-embryogenic callus. These calli tend to be light yellow to whitish in color which is slightly different from the non-embryogenic callus. Nonembryogenic calli with white, wet and friable characters were found predominantly under dark condition. It was documented that embryogenic callus displayed higher frequency of plant regeneration than the non-embryogenic one (Nabors., et al. 1983). Pandey., et al. (1994) reported that the success of invitro culture largely depends on the nutritional media, growth regulators, genotypes and the interaction of genotype with the medium. Production of embryogenic calli increased as the concentration of 2, 4 - D was increased from 0.5 mg L-1 to 1.5 mg L-1. Before now, it was reported that among the auxins, 2, 4 - D increase the rate of cell division and this attributes to increased amount of callus and to initiate and sustain embryogenic callus growth in rice (Wagiran., et al. 2008; Revathi and Pillai, 2011). However, the combination of 2, 4-D with kinetin was more effective in producing embryogenic and or organogenic calli (Wang., et al. 2004), when addition of NAA and or BAP could enhance the quality of the initiated callus (Turhan and Baser, 2004). While cytokinin may increase the growth rate of pre-embryogenic masses (Kommamine., et al. 1992). Results obtained by Rashid., et al. (2009) that increase in the concentration of 2, 4-D and kinetin reduced the embryogenic calli production.
The anthers derived callus were produced embryogenic callus or non embryogenic callus with effect of growth regulators. The embryogenic callus is better than non embryogenic callus to conventional breeding. The compact type and white colour embryogenic callus are better than friable type or yellow colour embryogenic callus to homozygous line development. The development of callus morphology into finite time. In the present investigation, among 16 growth regulator treatments, the treatment T10 (2, 4-D 1 mg L-1 + NAA 1 mg L-1 + Kinetin 1 mg L-1) and T16 (2, 4-D 2 mg L-1 + NAA 2 mg L-1 + Kinetin 1 mg L-1) were observed early callus morphology in 8 weeks whereas, the treatment T1 (2, 4-D 1 mg L-1 + Kinetin 0.5 mg L-1) was observed late callus morphology in 13 weeks in japonica variety Azucena. Islam et al. (2004) reported callus morphology from BRRI commercial rice variety with 2, 4-D + NAA + Kinetin into 30 days and 45 days. Dalpat., et al. (2014) has also reported callus morphology from japonica rice variety Azucena with 2, 4 - D + NAA + Kinetin into 7-8 weeks. The japonica rice variety Azucena observed callus morphology with 2, 4 - D + Kinetin into limited time (Archana, 2015). Application of 2, 4-D and NAA in combination with Kinetin could lead to an increase of callus induction and plant regeneration (Rout and Sarma, 1991).Embryogenic calli with nodular structures appeared on the surface of the non-embryogenic callus. These calli tend to be light yellow to whitish in color which is slightly different from the non-embryogenic callus. Nonembryogenic calli with white, wet and friable characters were found predominantly under dark condition. It was documented that embryogenic callus displayed higher frequency of plant regeneration than the non-embryogenic one (Nabors., et al. 1983). Pandey., et al. (1994) reported that the success of invitro culture largely depends on the nutritional media, growth regulators, genotypes and the interaction of genotype with the medium. Production of embryogenic calli increased as the concentration of 2, 4 - D was increased from 0.5 mg L-1 to 1.5 mg L-1. Before now, it was reported that among the auxins, 2, 4 - D increase the rate of cell division and this attributes to increased amount of callus and to initiate and sustain embryogenic callus growth in rice (Wagiran., et al. 2008; Revathi and Pillai, 2011). However, the combination of 2, 4-D with kinetin was more effective in producing embryogenic and or organogenic calli (Wang., et al. 2004), when addition of NAA and or BAP could enhance the quality of the initiated callus (Turhan and Baser, 2004). While cytokinin may increase the growth rate of pre-embryogenic masses (Kommamine., et al. 1992). Results obtained by Rashid., et al. (2009) that increase in the concentration of 2, 4-D and kinetin reduced the embryogenic calli production.
Among 16 growth regulator treatments, the treatment T16 (2, 4-D 2 mg L-1 + NAA 2 mg L-1+ Kinetin 1 mg L-1) was observed early callus morphology in 7 weeks whereas, the treatment T2 (2, 4 - D 1 mg L-1 + Kinetin 1 mg L-1) was observed late callus morphology in 13 weeks in japonica variety Moroberekan. Gueye and Nadir (2010) reported callus morphology from japonica genotype IKP with 2, 4 - D + NAA + Kinetin into 30-45 days. Zin and Irie (2017) observed callus morphology from F1 hybrid of japonica × indica with 2, 4-D + Kinetin into confined time. Combination auxin and cytokinin have been known for a long time to act either synergistically or antagonistically to control several significant developmental processes, such as the formation and maintenance of meristem (Ying-Hua., et al. 2011). A high auxin/cytokinin ratio is used for starting embryogenic callus formation compared to a low ratio for the regeneration of plantlets. The exact molecular function of plant growth regulators in tissue culture is unclear; however, it may probably be involved in the reprogramming of the expression of embryogenic genes (Shahsavari., et al. 2010). The callus induction and regeneration controlled through genetically factors which are in nucleus or cytoplasm and genotype is a determining factor in response to tissue culture even between varieties within species(Ghobeishavi., et al. 2014).
Summary
Rice (Oryza sativa L.) is one of the most important food crops of Southeast Asia, which feeds half of the world population. Anther culture is an efficient and convenient technique for rapid production of doubled haploids which are useful in crop breeding programs. The present investigation was designed to demonstrate effect of growth regulators on callus morphology of japonica rice varieties through anther culture.
Panicles with a distance of 12-13 cm between flag leaf and subtending leaf for Azucena and 14 -15 cm for Moroberekan were selected because at this stage of panicle development microspores were in the mid uninucleate stage and it is considered to be the optimum stage of pollen development for callus induction and regeneration.
The panicles were given a pre-culture cold treatment at 5°C for 8 days was given to the selected panicles in order to induce microspores in them to follow the sporophytic pathway instead of gametophytic pathway.
The anthers were placed on N6 medium containing different concentrations of growth regulators like 2, 4-D, NAA and Kinetin for callus induction. They were incubated in dark for 10-20 weeks in a culture room. The culture room was maintained at 23 ± 2°C, with a relative humidity of 50-60 per cent.
The present results were computed with Factorial Completely Randomized Design (FCRD) method for callus induction. In japonica variety Azucena, among 16 growth regulator treatments, the treatments T0 (control), T2 and T9 were not produced callus texture, callus colour or callus growth. All treatments were recorded compact calli except treatment T14. The treatments T1, T5, T6, T8, T10, T11, T15 and T16 were showed white calli and remained treatments were showed yellow calli. The response of callus morphology was recorded between 8-20 weeks. The treatment T10 (2, 4-D 1 mg L-1 + NAA 1 mg L-1 + Kinetin 1 mg L-1) and T16 (2, 4-D 2 mg L-1 + NAA 2 mg L-1+ Kinetin 1 mg L-1) were observed early callus induction in 8 weeks whereas, the treatment T1 (2, 4-D 1 mg L-1 + Kinetin 0.5 mg L-1) was observed late callus morphology in 13 weeks.
In japonica variety Moroberekan, among 16 growth regulator treatments, the treatment T0 (control) and T1 were not produced callus texture, callus colour or callus growth. All treatments were observed compact calli except treatment T5 and T11. The treatments T2, T3, T6, T8, T10, T12, T14 and T15 were showed white calli and remained treatments were showed yellow calli. The response of callus morphology was observed between 7-13 weeks. The treatment T16 (2, 4-D 2 mg L-1 + NAA 2 mg L-1+ Kinetin 1 mg L-1) was observed early callus morphology in 7 weeks whereas, the treatment T2 (2, 4-D 1 mg L-1 + Kinetin 1 mg L-1) was observed late callus morphology in 13 weeks.
The present investigation revealed that callus morphology is significant steps to invitro androgenesis. It forms step of indirect somatic embryogenesis that produces invitro plantlet. The effect of genotype, cold pre-treatment, pollen grain stages, culture media, growth regulator concentrations, culture media and culture conditions are formed type of callus morphology. Therefore, callus morphology is essential factor to the development of haploid plant through anther culture.
References
- ABE T and Futsuhara Y. “Genotypic variability for callus formation and plant regeneration in rice (Oryza sativa L.)”. Theoretical and Applied Genetics 72 (1986): 3-10.
- AFZA R., et al. “Effect of spikelet position on rice anther culture efficiency”. Plant Science 153 (2000): 155-159.
- ALI S., et al. “Assessment of various factors involved in the tissue culture system of rice”. Rice Science 11.5 (2004): 345-349.
- ALY., et al. “Somatic embryogenesis from Egyptian rice (Oryza sativa L.) Mature zygotic embryos as influenced by a function of genotype and growth regulators”. Arab Journal of Biotechnology 1.1 (1998): 107-116.
- AMBARWATI A. D., et al. “Rice anther culture to develop double haploid population and blast resistant lines”. Jurnal AgroBiogen 5.2 (2009): 71-77.
- Archana. “studies on varietal response to anther culture in rice (oryza sativa l.). m. sc. (agri.) thesis”. university agricultural sciences (2015).
- BAGHERI N., et al. “Evaluation of effective factors in another culture of Iranian rice (Oryza sativa L.) Cultivars”. North-Western Romanian Biology and Zoology Journals 3.2 (2009): 119-124.
- BENLIOGLU B., et al. “Effect of growth regulators on tissue culture parameters in rice (Oryza sativa L.)”. Ekin Journal of Crop Breeding and Genetics 2 (2015): 43-46.
- bidhan R and Mandal Ab. “Another culture response in indica rice and variations in major agronomic characters among the androclones of a scented cultivar, Karnal local”. African Journal of Biotechnology 4.3 (2005): 235-240.
- BIRCH RG., “Plant transformation: problems and strategies for practical application”. Annual Review of Plant Biology 48 (1997): 297-326.
- Biswas A and Mandal AB. “Plant regeneration in different genotypes of indica rice”. Indian Journal of Biotechnology 6 (200): 532-540.
- CAI XS AND CHEN LZ. “The effects of cold shock and liquid medium on callus formation in rice anther culture”. Agricultural Research Journal 33.1 (1984): 24-29.
- CHALEFF R AND STOLARZ A. “Factors influencing the frequency of callus formation among cultured rice (Oryza sativa L.) Anthers”. Plant Physiology 51 (1981): 201-206.
- CHEN CC., “In vitro development of plants from microspores of rice”. Plant Cell, Tissue and Organ Culture 86 (1977): 31-146.
- CHEN CC. “Effects of sucrose concentrations on plant production in another culture of rice”. Crop Science 18 (1978): 905-906.
- CHEN H AND QIN RZ. “Analysis of different effectors enhancing the anther culture ability of autotetraploid japonica rice”. Journal of Agricultural Science and Technology 10.3 (2008): 90-96.
- CHEN JJ., et al. “Effect of iron on rice anther culture Agricultural Sciences in China 35.3 (1986): 244-252.
- CHO MS AND ZAPATA FJ. “Callus formation and plant regeneration in isolated pollen culture in rice (Oryza sativa L. cv. Taipei 309)”. Plant Science 58 (1988): 239-244.
- CHU CC., et al. “Establishment of an efficient medium for another culture of rice through comparative experiment on the nitrogen source”. Scientia Sinica 18 (1975): 659-668.
- CHU C., “The N6 medium and its applications to anther culture of cereal crops. In: Proc. Symp. Plant tissue culture”. Science Press (1978): 43-50.
- CHUNG GS. “Application of anther culture technique for rice (Oryza sativa L.) Improvement. In: Proceeding of Korea-China Plant Tissue Culture Symposium”. Korea, Society of Plant Tissue Culture (1987): 36-56.
- CLAPAROLS I., et al. “Influence of some exogenous amino acids on the production of maize embryogenic callus and endogenous amino acid content”. Plant Cell, Tissue and Organ Culture 34 (1993): 1-11.
- DALPAT L., Anther culture studies in rice (Oryza sativa L.). University Agricultural Sciences (2013).
- DALPAT L., et al. “Callus induction and regeneration from in vitro anther culture of rice (Oryza sativa L.)”. International Journal of Agriculture, Environment and Biotechnology 7.2 (2014): 213-218.
- DARZ AE., et al. “Interaction of media for callus induction and for plant regeneration in rice anther culture”. IRRN 16.1 (1991): 7-8.
- DATTA SK AND WENZEL G. “Isolated microspore derived plant formation via embryogenesis in Triticum aestivum”. Plant Science 48 (1998): 49-54.
- DATTA SK., et al. “Culture of isolated pollen of wheat (Triticum aestivum L.)”. Biotechnology in Agriculture and Forestry 13 (1990): 435-447.
- DIN ARJ.M., et al. “Improvement of efficient in vitro regeneration potential of mature callus induced from Malaysian upland rice seed (Oryza sativa cv. Panderas)”. Saudi Journal of Biological Sciences 23 (2016): 69-77.
- DUNWELL JM AND THURLING N. “Role of sucrose in microspore embryo production in Brassica napus subsp oleifera”. Journal of Experimental Botany 36 (1985): 1478-1491.
- FAOSTAT. 2016, “Rice market monitor, statistical data base, food and agricultural organization (FAO) of the United Nations Rome”.
- FEHER, A., 2005. “Why somatic plant cells start to form embryos?”. Plant Cell Monograph 2: 85-101.
- GAMBORG QL., et al. “Nutrient requirement of suspension culture of soybean root cells”. Experimental Cell Research 50 (1968): 151-158.
- GHOBEISHAVI, H., et al. “The effect of plant growth regulators on embryogenic callus induction and regeneration from coleoptile in rice”. International Journal of Biosciences 5 (2014): 144-150.
- GUEYE T AND NDIR KN. “In vitro production of double haploid plants from two rice species (Oryza sativa L. and Oryza glaberrima Steudt.) For the rapid development of new breeding material”. Handbook of Oil Spill Science and Technology 5.7 (2010): 709-713.
- HEBERLE-BORS E., et al. “In vitro pollen cultures progress and perspectives. In: Pollen Biotechnology Gene Expression and Allergen Characterization”. International Thomson Publisher (1996): 85-109.
- HERATH HMI., et al. “Effect of culture media for anther culture of indica rice varieties and hybrids of indica and japonica”. Tropical Agricultural Research and Extension 10: 18-22.
- HU CH., et al. “On the inductive conditions of rice pollen plantlets in another culture. In: Proceedings of Symposium on Plant Tissue culture”. Science Press (1978): 87-95.
- IKEUCHI M., et al. “Plant callus: mechanisms of induction and repression”. Plant Cell 25 (2013): 3159-3173.
- ISLAM M., et al. “Effect of plant growth regulators on callus induction and plant regeneration in anther culture of rice”. Pakistan Journal of Biological Sciences 7.3 (2004): 331-334.
- Karim NH., et al. “Anther culture of rice”. Plant Biotechnology 6 (1986): 8.
- KAUSHAL L., et al. “Auxin to improve green plant regeneration of rice anther culture”. International journal of agriculture and crop sciences 8.1 (2016,): 15-26.
- KAUSHAL L., et al. “Effect of cold pretreatment on improving anther culture response of rice (Oryza sativa L.)”. Journal of Experimental Biology and Agriculture Sciences 2 (2014): 234-241.
- KHATUN R., et al. “Effect of cold pretreatment and different media in improving anther culture response in rice (Oryza sativa L.) in Bangladesh”. Indian Journal of Biotechnology 11 (2012): 458-463.
- KOMMAMINE A., et al. “Mechanisms of somatic embryogenesis in cell cultures: physiology, biochemistry and molecular biology”. In Vitro Cellular & Developmental Biology 28 (1992): 11-14.
- LAPITAN VC., et al. “Molecular characterization and agronomic performance of DH lines from the F1 of indica and japonica cultivars of rice (Oryza sativa L.)”. Field Crops Research 112 (2014): 222-228.
- LENKA N AND REDDY GM. “Role of media, plant growth regulators in callusing and plant regeneration from anthers of indica rice”. Proceedings of the Indian National Science Academy 860(1994,): 87-92.
- LENTINI Z., et al. “Androgenesis of highly recalcitrant rice genotypes with maltose and silver nitrate”. Plant Science 110 (1995):127-138.
- LIBIN A., et al. “Callus induction and plant regeneration of Sarawak rice (Oryza sativa L.) variety Biris”. African Journal of Agricultural Research 7.30 (2012,): 4260-4265.
- MERCY ST AND ZAPATA FJ. “Effect of pollen development stage on callus induction and its relation to auricle distance in two rice varieties”. International Rice Research Institute 11 (1986): 23-24.
- MURASHIGE T AND SKOOG F. “A revised medium for rapid growth and bioassays with tobacco tissue cultures”. Plant Physiology 15 (1962): 473-497.
- NABORS MW., et al. “Long duration, high frequency regeneration from cereal tissue cultures”. Planta 157 (1983): 385-391.
- NEELY D. “Tree wounds and wound closure”. Journal of Arboriculture Online 5 (1979): 135-140.
- NIIZEKI H AND OONO K. “Induction of haploid rice plant from anther culture”. Proceedings of the Japan Academy 44 (1968): 554-557.
- NIROULA RK AND BIMB HP. “Effect of genotype and callus induction medium on green plant regeneration from anther of Nepalese rice cultivars”. Asian Journal of Plant Sciences 8 (2009): 368-374.
- NITSCH JP AND NITSCH C. “Haploid plants from pollen grains”. Science 163 (1969): 85-87.
- NURHIDAYAH T., et al. “High regeneration rates in anther culture of inter specific sunflower hybrids”. Plant Cell Reports 16 (1996): 167-173.
- OGAWA T., et al. “Plant regeneration through direct culture of isolated pollen grains in rice”. Breeding Science 45.3 (1995): 301-307.
- OINAM GS and KOTHARI SL. “Totipotency of coleoptile tissue in Indica rice”. Plant Cell Reports 14 (1995):245-248.
- OONO K., “Production of haploid plants of rice (Oryza sativa L.) By anther culture and their use for breeding”. National Institute of Agricultural Sciences (1976): 139-222.
- PANDE H., Androgenesis in anther cultures of indica cultivar of Oryza sativa L Thesis. University of Delhi. (1997).
- PANDEY, S. K., et al. “Study on effect on genotype and culture medium on callus formation and plant regeneration in rice (Oryza sativa L.)”. Indian Journal of Genetics and Plant Breeding 54 (199): 293-299.
- PARK SGI., et al. “Effect of maltose concentration on plant regeneration of anther culture with different genotypes in rice (Oryza sativa L.)”. American Journal of Plant Sciences 201 (2013): 2265-2270.
- PAWAR BD., et al. “Development of in vitro plant regeneration protocol in rice (Oryza sativa L.) Using shoot tip explant”. International Journal of Environmental Research 9.1 (2015): 231-233.
- POWELL W., “Environmental and genetic aspects of pollen embryogenesis. In: Biotechnology in agriculture and forestry, part I. Haploids in crop improvement”. Springer 12 (1990): 44-65.
- PRABHU K N. “Anther culture studies in rice (Oryza sativa L.). Thesis, University Agricultural Sciences (2013).
- QUIROZ-FIGUEROA FR., et al. “Embryo production through somatic embryogenesis can be used to study cell differentiation in plants”. Plant Cell, Tissue and Organ Culture 86 (2006): 285-301.
- RAINA SK AND HADI S., “Simple device for mass extraction of rice anthers”. International Rice Research Newsletter (1987).
- RAMACHANDRA R., et al. “Scutelum derived high frequency invitro regeneration in two rice cultivars Rasi and Tarori basmati”. International Research Journal of Pharmacy 1.3 (2013): 68-74.
- RASHID A., et al. “Improving the yield of mungbean (Vigna radiata) in the North West frontier province of Pakistan using on-farm seed priming”. Australian Journal of Experimental Agriculture 40 (2004): 233-244.
- REVATHI S AND PILLAI MA. “In vitro callus induction in rice (Oryza sativa L.)”. International Journal of Current Research in Biosciences and Plant 1 (2011):13-15.
- ROUT JR AND SARMA NP., “Anther callus induction and green plant regeneration at high frequencies from an interspecific rice hybrid Oryza sativa”. 54.2 (1991): 155-159.
- ROUT P., et al. “Doubled Haploids generated through anther culture from an elite long duration rice hybrid, CRHR32: Method optimization and molecular characterization”. Plant Biotechnology 33 (2016): 177-186.
- RUEB S., et al. “Efficient plant regeneration through somatic embryogenesis from callus induced on mature rice embryos (Oryza sativa L.)”. Plant Cell, Tissue and Organ Culture 36 (1994): 259-264.
- RUKMINI M AND GUNDIMEDA JNR., “In-vitro androgenesis in rice: advantages, constraints and future prospects”. RICE SCIENCE 23.2 (2016): 57-68.
- RUKMINI M., et al. “Effect of cold pretreatment and phytohormones on anther culture efficiency of two indica rice (Oryza sativa L.) hybrids- Ajay and Rajalaxmi. Journal of Experimental Biology and Agriculture Sciences 1.2 (2013): 69-76.
- SAH BP., “Response of genotypes to culture media for callus induction and regeneration of plants from rice anthers”. Scientific World 6 (2008): 37-43.
- SEN C., et al. “Effect of cold pretreatment on anther culture of boro rice hybrids”. The International Journal of Plant Reproductive Biology 3 (2011): 69-73.
- SENGSAI S., et al. “Anther culture of BC1F1 (KDML105//IRBB5/KDMl105) hybrid to produce bacterial blight resistance doubled haploid rice”. Kasetsart Journal: Natural Science 41 (2007): 251-261.
- SHAHNEWAZ S., et al. “Induction of haploid rice plants through in vitro anther culture”. Pakistan Journal of Biological Sciences 6.14 (2003): 1250-1252.
- SHAHSAVARI E., et al. “The effect of plant growth regulators on optimization of tissue culture system in Malaysian upland rice”. African Journal of Biotechnology 9.14 (2010): 2089-2094.
- SHUKLA R., et al. “Production of high quality embryogenic callus of rice”. Publication of the Journal - The Bioscan 9.3 (2014): 1077-1080.
- SILVA TD AND RATNAYAKE WJ. “Anther Culture Potential of Indica Rice varieties, Kurulu Thuda And Bg 250”. Tropical Agricultural Research & Extension 12 (2009): 2-8.
- TALEBI R., et al. “In vitro plant regeneration through anther culture of some Iranian local rice (Oryza sativa L.) Cultivars”. Pakistan Journal of Biological Sciences 10.12 (2007): 2056-2060.
- TANG KX., et al. “A simple and rapid procedure to establish embryogenic cell suspension as a source of protoplasts for efficient plant regeneration from two Chinese commercial rice cultivars”. Plant Cell, Tissue and Organ Culture 66.2 (2001): 149-153.
- THADAVONG S., et al. “Callus induction and plant regeneration from mature embryos of glutinous rice (Oryza sativa L.) Cultivar TDK1”. Kasetsart Journal (Natural Science) 36 (2002): 334-344.
- THORPE TA. “The current status of plant tissue culture, in Plant Tissue Culture: Applications and Limitations”.Scientific publishing service (1990): 1-33.
- TOURAEV A., et al. “Efficient microspore embryogenesis in wheat (Triticum aestivum L.) Induced by starvation at high temperature””. Sexual Reproduction in Flowering Plants 9 (1996): 209-215.
- TRAN TTX AND NGUYEN TL. “Rice breeding for high grain quality through anther culture”. Omonrice 18 (2011): 68-72.
- TREJO-TAPIA G., et al. “The effects of cold pretreatment, auxins and carbon source on anther culture of rice”. Plant Cell, Tissue and Organ Culture 71 (2002): 41-46.
- TSAI SC AND LIN MH. “Production of rice plantlets by anther cultures”. Agricultural Sciences in China 26 (1977): 110-11.
- TURHAN H AND BASER I. “Callus induction from mature embryo of winter wheat (Triticum aestivum L.)”. Asian Journal of Plant Sciences 3 (2004): 17-19.
- VLADISLAVOVNA IM., “Тhe effect of auxin on plant regeneration on rice from anther culture in vitro”. Science Time 10.10 (2014): 160-167.
- WAGIRAN A., et al. “Improvement of plant regeneration from embryogenic suspension cell culture of japonica rice”. International Journal of Biological Sciences 1727.3048 (2008): 570-576.
- WANG CC., et al. “On the conditions for the induction of rice pollen plantlets and certain factors affecting the frequency of induction”. Acta Botanica Sinica 16 (1974): 43-53.
- WANG YQ., et al. “Efficient regeneration from in vitro culture of young panicles of rice (Oryza sativa L.). China”. 21 (2004): 52-60.
- XIE JH., et al. “The effect of cold pretreatment on the isolated microspore culture and the free amino acid change of anthers in japonica rice (Oryza sativa L.)”. Journal of Plant Physiology 151 (1997): 79-82.
- XIE J., et al. “Improved isolated microspore culture efficiency in medium with maltose and optimized growth regulator combination in japonica rice (Oryza sativa)”. Plant Cell, Tissue and Organ Culture 42 (1995): 245-250.
- YING-HUA S., et al. “Auxin-cytokinin interaction regulates meristem development”. Molecular Plant 4.4 (2011,): 616-625.
- ZAPATA AFJ., et al. “Current developments in plant biotechnology for genetic improvement: the case of rice (Oryza sativa L.)”. World Journal of Microbiology and Biotechnology 11 (2004): 393-399.
- ZHANG ZH AND CHU QR. “Advances in rice anther culture for varietal improvement in china”. Journal of Agricultural Science 2 (1986): 10-16.
- ZIN WM and IRIE K. “Evaluation of callus induction and plant regeneration on different media in rice F1 hybrids using anther culture”. International Journal of Scientific and Engineering Research 8.10 (2017): 363-369.
Citation:
Avinash Sharma. “Morphology Response of Growth Regulators on Callus of Japonica Rice Through Anther Culture”. Clinical
Biotechnology and Microbiology 2.4 (2018): 424-442.
Copyright: © 2018 Avinash Sharma. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.






























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.