Research Article
Volume 4 Issue 1 - 2019
Efficacy of Native Trichoderma spp. in Controlling Fusarium wilt of Tomato Plants in Green House, Yemen
Biology Department, Faculty of Science, Sana’a University, Sana’a, Yemen
*Corresponding Author: Nesrain AbdulKarem Al-Mekhlafi. Biology Department, Faculty of Science, Sana’a University, Sana’a, Yemen.
Receieved: March 13, 2019; Published: March 23, 2019
Abstract
This study investigated the ability of Trichoderma spp. to control the Fusarium wilt of tomato, as well as the effect of these isolates on tomato plant growth in green house. Two native Trichoderma isolates [T. citrinoviride, (T33) and Trichoderma sp.anamorph of H. semiorbis, (T15)] significantly reduced tomato Fusarium wilt severity (19.77%) and increased all plant growth parameters (root length, 6.65 cm; shoot length,18.29; root fresh weight, 2.94g; shoot fresh weight, 28.66g; root dry weight, 0.46g; shoot dry weight,4.03g and no. of leaves, 24.33. The combination of both species of Trichoderma were more effective in controlling the Fusarium wilt disease compared to their application as alone.
Keywords: Tomato; Fusarium wilt; Trichoderma; biocontrol; green house
Introduction
Tomato (Lycopersicon esculentum Mill.) is one of the most important edible and nutritious vegetable crop grown all over the world [1]. Fusarium wilt of tomato caused by Fusarium oxysporum f. sp. Lycopersici that is one of the economically most important disease in major tomato growing regions worldwide [2]. It is a highly destructive pathogen, causing 10 to 50% yield loss in many tomato production areas [3]. However, the widespread use of chemicals fungicides has been a subject of public concern and security due to their potentially harmful effects on environment and human health and their undesirable effect on non-target organisms [4,5].
The control of Fusarium wilt of tomato is very difficult because pathogen progress within the vascular tissues which limits the effectiveness of fungicides. Biological control of plant pathogens through antagonistic offer environmentally safe and cost effective alternative to chemicals [2]. Trichoderma has gained immense importance since last few decades due to its biological control ability against several deadly plant pathogens [6] for their ability to increase root growth and development, crop productivity, resistance to abiotic stresses, uptake and use of nutrients [7]. Several studies have been published on ability of Trichoderma in biological control of Fusarium wilt of tomato [8-10]. Therefore, the objective of the present study was to assess the ability of two Trichoderma spp. in decreasing Fusarium wilt of tomato under in vivo conditions in Yemen.
Materials and Methods
Origin of Trichoderma isolates
Native isolates T. citrinoviride (T33) and Trichoderma sp.anamorph ofHypocrea semiorbis (T15)wereisolated from rhizosphere soil of corn in Yemen and selected for their high biocontrol activity against different soil-borne plant pathogens.
Native isolates T. citrinoviride (T33) and Trichoderma sp.anamorph ofHypocrea semiorbis (T15)wereisolated from rhizosphere soil of corn in Yemen and selected for their high biocontrol activity against different soil-borne plant pathogens.
Origin of Fusarium oxysporum
F. oxysporum (AUMC 208) was collected from Mycological Center, Faculty of Science, Assiut University, Assiut, Egypt.
F. oxysporum (AUMC 208) was collected from Mycological Center, Faculty of Science, Assiut University, Assiut, Egypt.
Sterilization of soil and seeds
Soil used through this experiment was clay soil, sand and peat moss at a ratio of 3:1:1 (w/w) then autoclaved for three successive days at 121°C for one hour/day. Pots of 15 cm ×10 cm diameter were surface sterilized with 1% sodium hypochlorite and were filled with autoclaved soil [11, 12]. Seeds of tomato were surface sterilized with 1% sodium hypochlorite for 2 min, rinsed three times in sterile distilled water and air dried [13].
Soil used through this experiment was clay soil, sand and peat moss at a ratio of 3:1:1 (w/w) then autoclaved for three successive days at 121°C for one hour/day. Pots of 15 cm ×10 cm diameter were surface sterilized with 1% sodium hypochlorite and were filled with autoclaved soil [11, 12]. Seeds of tomato were surface sterilized with 1% sodium hypochlorite for 2 min, rinsed three times in sterile distilled water and air dried [13].
Preparation of F. oxysporum inocula
Fusarium oxysporum inocula was prepared for incula by growing on PDA and incubated for 7 days at 28°C. The suspension for inoculation was prepared by pouring 30 ml of sterile distilled water into each of petri dishes containing F. oxysporum culture and the conidia were dislodged with a cell spreader, filtered through cheesecloth and counted with a haemacytometer. It was set to 1x106 conidia/ml [14].
Fusarium oxysporum inocula was prepared for incula by growing on PDA and incubated for 7 days at 28°C. The suspension for inoculation was prepared by pouring 30 ml of sterile distilled water into each of petri dishes containing F. oxysporum culture and the conidia were dislodged with a cell spreader, filtered through cheesecloth and counted with a haemacytometer. It was set to 1x106 conidia/ml [14].
Pathogenicity test
Pathogenicity test of F. oxysporum was carried out on tomato variety (Solanum lycopersicum L., cvs. ‘Rio grande, Faten) according to Jasnic ., et al. [15]. Pathogenicity test was tested by sowing of tomato seeds in artificially infected soil by F. oxysporum. Control plants were sown in soil with sterile distilled water. The severity of the Fusarium wilt was assessed from 2 weeks of inoculation up to 45 days. Disease incidence was determined as reported by Khanna., et al. [16]:
Pathogenicity test of F. oxysporum was carried out on tomato variety (Solanum lycopersicum L., cvs. ‘Rio grande, Faten) according to Jasnic ., et al. [15]. Pathogenicity test was tested by sowing of tomato seeds in artificially infected soil by F. oxysporum. Control plants were sown in soil with sterile distilled water. The severity of the Fusarium wilt was assessed from 2 weeks of inoculation up to 45 days. Disease incidence was determined as reported by Khanna., et al. [16]:
Percentage diseases incidence (DI %) = n × 100
N
Where:
n= number of plants showing wilts symptoms
N= Total number of plant sampled
N
Where:
n= number of plants showing wilts symptoms
N= Total number of plant sampled
Biological control of Fusarium wilt of tomato in greenhouse
The selected antagonists (T15 and T33) were tested for their ability to control the Fusarium wilt of tomato as well as their effect on tomato plant growth under greenhouse conditions. Each pot was taken with sterilized soil. Soil was infested with conidial suspension (30 ml) of F. oxysporum (1x106 conidia/ml). Simultaneously after 2 days of pathogen inoculation, soils were inoculated with (30 ml) of Trichoderma isolates (1x107 conidia/ml) and then pots were watered for 7 days before sowing. Ten tomato seeds were sown in each pot, three pot replicate for each treatment arranged in completed randomized design. Pots were kept under greenhouse condition till the end of the experiment [17]. The details of total 5 treatments as follow:
T (1): Control (without Fusarium and Trichoderma)
T (2): F. oxysporum
T (3): Trichoderma sp.anamorph of H. semiorbis+ F. oxysporum
T (4): T. citrinoviride + F. oxysporum
T (5): Trichoderma sp.anamorph of H. semiorbis +T. citrinoviride+ F. oxysporum
The selected antagonists (T15 and T33) were tested for their ability to control the Fusarium wilt of tomato as well as their effect on tomato plant growth under greenhouse conditions. Each pot was taken with sterilized soil. Soil was infested with conidial suspension (30 ml) of F. oxysporum (1x106 conidia/ml). Simultaneously after 2 days of pathogen inoculation, soils were inoculated with (30 ml) of Trichoderma isolates (1x107 conidia/ml) and then pots were watered for 7 days before sowing. Ten tomato seeds were sown in each pot, three pot replicate for each treatment arranged in completed randomized design. Pots were kept under greenhouse condition till the end of the experiment [17]. The details of total 5 treatments as follow:
T (1): Control (without Fusarium and Trichoderma)
T (2): F. oxysporum
T (3): Trichoderma sp.anamorph of H. semiorbis+ F. oxysporum
T (4): T. citrinoviride + F. oxysporum
T (5): Trichoderma sp.anamorph of H. semiorbis +T. citrinoviride+ F. oxysporum
At the end of experiment, the plants removed from the pots, shoot and root length, fresh weight (FW), dry weight (DW) and number of leaves per plant were determined [18]. The percentage of disease severity was determined using the formula given by Song et al., [19]:
Severity ratings for Fusarium oxysporum inoculated plants were done using a scale of 1-6 as described by Marley and Hillocks [20]. Where:
- no symptoms
- chlorosis and wilting of the first branches
- chlorosis and wilting of second and third branches
- chlorosis above third, second and third branches may be lost
- chlorosis and partial desiccation
- complete death of plant
Statistical analysis
Statistical analysis was performed in order to determine the effect of treatments on observed parameters. Significance of treatments was tested by One-way Analysis of Variance (ANOVA) and Tukey’s test (at P< 0.05) was applied for the differences in mean values. All the statistical analyses were completed using Graphpad 6.01 Statistics.
Statistical analysis was performed in order to determine the effect of treatments on observed parameters. Significance of treatments was tested by One-way Analysis of Variance (ANOVA) and Tukey’s test (at P< 0.05) was applied for the differences in mean values. All the statistical analyses were completed using Graphpad 6.01 Statistics.
Results and Discussion
The tomato plants were inoculated with F. oxysporum to test the capability of the fungusproducing disease symptoms in tomato in vivo. In the present study theinoculated plant showed wiltingsymptoms and percentage of diseases incidence was (62.64%). The result is supported by the findingsof Begum [21] who observed that F. oxysporum f. sp. lycopersici was able to produce wilting symptoms in tomato plants.
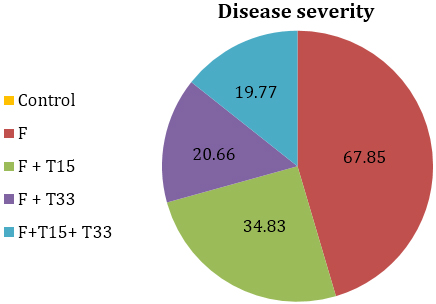
The results of severity of F. oxysporum on tomato gave the highest percentage of disease severity (67.85%) (Figure, 1) and reduced all plant growth parameters (Table, 1). The negative effects of F. oxysporum result from disruption to plant growth by the blocking of xylem vessels, causing leaf senescence and reduced photosynthesis [22].
It was revealed from the results, Trichoderma isolates varied in their effect on tomato plants and ability to reduce the effect of F. oxysporum when subsequently applied in the pot experiment (Table, 2). Reduction of Fusarium wilt disease was observed by T. citrinoviride and Trichoderma sp.anamorph of H. semiorbis in comparison to control. Best disease control was achieved in treatment (5)demonstrating only 19.77% of disease severity followed by treatment (4) and treatment (3). These results are in agreement with previous studies of several workers [3,8, 23,24] they reported that isolates of Trichoderma showed promise for controlling Fusarium wilt and improving the growth and yield of tomato.
The reduced Fusarium wilt on tomato may have been due to reduced population density of the pathogen [25], parasitism, lysis of pathogenic fungi, competition for limiting factors in the rhizosphere mainly iron and carbon [26,27].
The results of Trichoderma treatments showed statistically significant effects of Trichoderma treatments on plant growth parameters (Table, 1). Root and shoot length, fresh weight dry weight and number of leaves per plant found that the maximum was recorded in the treatment [5]. These results agree with Ozbay and Newman [28] who reported that seedling treated with T. harzianum increases seedling emergence, number of true leaves, fresh and dry weights of shoot and root of tomato plants.
| Treatments | Plant length (cm) | Fresh weight (g) | Dry weight (g) | No. of leaves | ||||
| root | shoot | root | shoot | root | shoot | |||
| T1 | Control | 5.53 | 15.03 | 1.67 | 17.5 | 0.34 | 2.34 | 19.55 |
| T2 | F | 4.28 | 13.45 | 0.9 | 8.50 | 0.20 | 1.31 | 18.58 |
| T3 | F + T15 | 5.60 | 15.76 | 2.85 | 18.66 | 0.38 | 2.62 | 20.68 |
| T4 | F + T33 | 5.75 | 16.64 | 2.90 | 21.12 | 0.44 | 3.01 | 22.62 |
| T5 | F+T15+ T33 | 6.65 | 18.29 | 2.94 | 28.66 | 0.46 | 4.03 | 24.33 |
| P value | 0.0906 | 0.0548 | 0.0001 | 0.0001 | 0.0021 | 0.0001 | 0.0870 | |
Table 1: Effects of Trichoderma treatments on tomato growth and disease incidence of
Fusarium wilt of tomato in green house.
Sundaramoorthy and Balaskar [29] also observed that tomato plants treated with T. harzianum (ANR-1) stimulate plant height by 73.62 cm and increased the dry weight by 288.38 g in comparison to untreated control.
Furthermore, Harman [30] established that Trichoderma spp., are opportunistic plant colonizers that affect plant growth by promoting abundant and healthy plant roots, possibly via the production or control of plant hormones. The increased growth response caused by Trichoderma isolates may be through modification of the rooting system as Yedida., et al. [31] reported for T. harzianum inoculation which improved uptake of nutrients by the plants at a very early growth stage.
Conclusion
Richoderma viride and Trichoderma sp. Decreased disease incidence of Fusarium sp., and increased plant yield in tomato. Moreover, the combination of both Trichoderma spp. proved better than their application alone. Thus, the finding of present investigation holds a good promise in tomato wilt management. However, further studies on the effect of these treatments in field conditions need to be undertaken so that Trichoderma could be recommended as a biocontrol agent richoderma viride and Trichoderma sp. Decreased disease incidence of Fusarium sp., and increased plant yield in tomato. Moreover, the combination of both Trichoderma spp. Proved better than their application alone. Thus, the finding of present investigation holds a good promise in tomato wilt management. However, further studies on the effect of these treatments in field conditions need to be undertaken so that Trichoderma could be recommended as a biocontrol agent
T. citrinoviride (T33) and Trichoderma sp.anamorph of H. semiorbis (T15), decreased disease severity of F. oxysporum and increased plant growth parameters in tomato. Moreover, the combination of both Trichoderma spp. proved better than their application alone in vivo in biological control of Fusarium wilt of tomato.
Acknowledgement
We thank Faculty of Science, University of Sana’a for their support during this investigation.
We thank Faculty of Science, University of Sana’a for their support during this investigation.
References
- Bauchet G and Causse M. “Genetic diversity in tomato (Solanum lycopersicum) and its wild relatives. In: Genetic diversity in plants” Caliskan, M. (Ed.). In Tech Publisher, Croatia (2010): 133-162.
- Abdallah RA., et al. “Biocontrol of Fusarium wilt and growth promotion of tomato plants using endophytic bacteria isolated from Solanum elaeagnifolium stems”. Journal of Phytopathology 164. 10(2016): 811-824.
- Ghazalibiglar H., et al. “Biological control of Fusarium wilt of tomato by Trichoderma isolates”. New Zealand Plant Protection 69 (2016): 57-63.
- Keswani C., et al. “Unraveling the efficient applications of secondary metabolites of various Trichoderma spp”. Applied Microbiology and Biotechnology 98. 2 (2014):533-544.
- Bisen K., et al. “ Unrealized potential of seed biopriming for versatile agriculture”. In nutrient use efficiency: from Basics to Advances (2015): 193-206.
- de Medeiros HA., et al. “Tomato progeny inherit resistance to the nematode Meloidogyne javanica linked to plant growth induced by the biocontrol fungus Trichoderma atroviride”. Scientific Reports 7 (2017): 40216.
- Ranasingh N., et al. “Use of Trichoderma in disease management”. Orissa Review (2006): 68-70.
- Cotxarrera L., et al. “Use of sewage sludge compost and Trichoderma asperellum isolates to suppress Fusarium wilt of tomato”. Soil Biology and Biochemistry 34.4 (2002): 467-476.
- Taghdi Y., et al. “Effectiveness of composts and Trichoderma strains for control of Fusarium wilt of tomato. Phytopathologia Mediterranea 54 (2015): 232–240.
- Debbi A., et al. “Distribution and genetic variability of Fusarium oxysporum associated with tomato diseases in algeria and a biocontrol strategy with indigenous Trichoderma spp.” Frontiers in Microbiology9 (2018): 1-9.
- Perveen K., et al. “Management of Sclerotinia sclerotiorum on Mentha arvensis cv. Gomti”. Journal of Mycology and Plant Pathology 37 (2007): 33-36.
- Mwangi MW., et al. “Inoculation of tomato seedling with Trichoderma harzianum and arbuscular mycorrhizal fungi and their effect on growth and control of wilt on tomato seedlings”.Brazilian Journal of Microbiology42.2 (2011): 508-513.
- Muthukumar A., et al. “Exploitation of Trichoderma species on the growth of Pythium aphanidermatum in chilli”. Brazilian Journal of Microbiology 42.4 (2011): 1598-1607.
- Nirmaladevi D and Srinivas C. “Cultural, morphological and pathogenicity variation in Fusarium oxysporum f. sp. lycopersici causing wilt of tomato”. Batman University. Journal of Life Sciences 2.1 (2012): 1-16.
- Jasnic S., et al. “Pathogenicity of Fusarium species in soybean. Matica Srpska Journal for Natural Sciences”109 (2005): 113-121.
- Khanna S., et al. “Identification of some high spectrum races of phytophtrra infestans in Khais hills”. Journal of the International Phonetic Association 4.1(1977): 18- 21.
- Kamala T and Indira S. “Evaluation of indigenous Trichoderma isolates from Manipur as biocontrol agent against Pythium aphanidermatum on common beans”. Biotechnology1.4 (2011):217–225.
- Clarke JM and McCaig TN. “Excised –leaf water retention capability as an indicator pf drought resistance of Triticum genotypes”. Canadian Journal of Plant Science62.3 (1982): 571-578.
- Song W., et al. “Tomato Fusarium wilt and its chemical control strategies in a hydroponic system”. Crop Protection 23. 3 (2004): 243-247.
- Marley PS and Hillocks RJ. “Effects of root-knot nematodes (Meloidogyne spp.) on Fusarium wilt in Pigeon pea (Cajanus cajan)”. Field crop Research. 46 (1996):15-20.
- Begum HA. “Studies on the integrated management for tomato wilt complex”. Ph.D. Thesis. Department of Plant Pathology. Agricultural University. Mymensingh, Bangladesh (2007): 324.
- Dimond A. “Pathogenesis in the wilt diseases”. Annual Review of Plant Physiology 6.1 (1955): 329-350.
- Srivastava R., et al. “Evaluation of arbuscular mycorrhizal fungus, fluorescent Pseudomonas and Trichoderma harzianum formulation against Fusarium oxysporum f. sp. lycopersici for the management of tomato wilt”. Biological Control 53.1 (2010): 24-31.
- Umashankar N., et al. “Biological control of Fusarium wilt in tomato”. Environment Ecology 28.2 ( 2010): 1111-1115.
- Otadoh JA., et al. “Assessment of Trichoderma isolates for virulence efficacy on Fusarium oxysporum f. sp. Phaseoli”. Tropical and Subtropical Agroecosystems 13 (2011): 99 – 107.
- Sivan A and Chet I. “Biological control of Fusarium spp. in cotton, wheat and muskmelon by Trichoderma harzianum”. Journal of Phytopathology 116. 1 (1986):39–47.
- Segarra G.,et al.“Trichoderma asperellum strain T34 controls Fusarium wilt disease in tomato plants in soilless culture through competition for iron”. Microbial Ecology 59.1 (2010): 141-149.
- Ozbay N and Newman SE. “Effect of Trichoderma harzianum strains to colonize tomato root and improve transplant growth”. Pakistan Journal of Biological Sciences 7.2 (2004): 253-257.
- Sundaramoorthy S and Balabaskar P. “Biocontrol efficacy of Trichoderma spp. against wilt of tomato caused by Fusarium oxysporum f. sp. Lycopersici”. Journal of Applied Biology and Biotechnology 1.03 (2013): 036-040.
- Harman GE. “Myths and dogmas of biocontrol: Changes in perceptions derived from research on Trichoderma harzianumT-22”. Plant Disease 84 (2000): 377-393.
- Yedida I., et al. “Induction of defense response in cucumber plants (Cucumis sativus L.) by the biocontrol agent Trichoderma harzianum”. Applied and Environmental Microbiology 65. 3 (1999): 1061-1070.
Citation:
Nesrain AbdulKarem Al-Mekhlafi., et al. “Efficacy of Native Trichoderma spp. in Controlling Fusarium wilt of Tomato Plants in Green House, Yemen”. Clinical Biotechnology and Microbiology 4.1 (2019): 1-6
Copyright: © 2019 Nesrain AbdulKarem Al-Mekhlafi., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.






























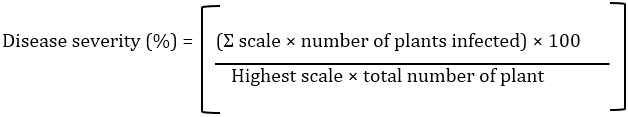
 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.