Symposium
Volume 2 Issue 2 - 2018
Management and Outcomes of Esophageal Perforation: A Single Center 13-Year Experience
Department of Surgery, Tuen Mun Hospital, Tuen Mun, New Territories, HKSAR, Hong Kong
*Corresponding Author: Kevin KF Wong, Department of Surgery, Tuen Mun Hospital, Tuen Mun, New Territories, HKSAR, Hong Kong.
Received: May 11, 2018; Published: May 22, 2018
Abstract
Esophageal perforation is a potential life-threatening condition; carries with it a high morbidity and mortality rate if not treat these
patients appropriately and aggressively. Many factors, e.g. patients’ condition, etiologies, any underlying esophageal disorders, site
and size of perforation, duration of onset, degree of contamination, availability of expertise, can influence the choice of management
and outcome. Therefore, it is stressed that the treatment plan should be tailor-made instead of dogmatic. It remains challenging to
have timely diagnosis making and offers appropriate treatment. Prompt multi-disciplinary teams’ input is required to improve treatment
outcome.
Introduction
Esophageal perforation is uncommon. Majority of surgical centers encounter only a few cases of esophageal perforation cases annually [1-3]. At the same time, the outcome may be devastating. The overall mortality rate is around 20-30% [1-6]. In this study, we aimed to review our own center’s esophageal perforation cases. The patients’ demographics, etiologies, onset of symptoms, site and size of perforation, treatment methods, and factors that predict the clinical outcomes of our patients were studied.
Methods
From 7/2004 to 6/2017, esophageal perforation cases were retrieved from electronic record and clinical notes were studied. Data, including patients’ demographics, esophageal perforating site, size, etiologies, presence of contamination, management options and clinical outcomes, were reviewed and analyzed. Categorical and continuous variables were analyzed by using Chi-square test (Fisher-exact test if appropriate) and Mann-Whitney test respectively (SPSS version 22). Statistical significance was accepted for P value < 0.05.
Results
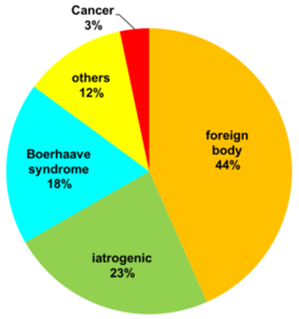
From 7/2004 to 6/2017(13 years), 60 esophageal perforation cases were managed. Many of the patients belonged to middle age or older age group. The median age was 65 (range: 25 to 93). 34 were male (56.7%) and 26 were female (43.3%) (Figure 1). Esophageal perforation size ranged from a tiny hole to a few centimeters. (Table 1). Concerning the perforating site, cervical esophagus is the commonest site for perforation (26 patients, 43.3%), followed by abdominal esophagus and esophageal-gastric junction (EGJ) (19 patients, 31.7%), and thoracic esophagus (15 patients, 25%). (Table 1) Foreign body ingestion is the most common cause for esophageal perforation (26 cases, 43.3%). This was followed by iatrogenic causes (14 cases, 23.3%), mainly due to endoscopy, such as EGD (esophago-gastro-duodenoscopy) (6 cases, 1 in 11208 EGD cases) and ERCP (endoscopic retrograde cholangiopancreatography) (2 cases, 1 in 3735 ERCP cases). There was one case caused by trans-esophageal echocardiogram (TEE) and one case related to spinal surgery. Boerhaave syndrome is the third commonest cause for esophageal perforation (11 cases, 18.3%). For the remaining cases, 2 (3.3%) were due to esophageal cancer and 7 (11.6%) due to other pathologies (Figure 2).
| Characteristics | Number (%) |
| Perforating site | |
| Cervical | 26 (43.3%) |
| Thoracic | 15 (25%) |
| Oesophago-gastric junction/ abdominal | 19 (31.7%) |
| Perforating size | |
| < 1cm | 42 (70%) |
| 1-2cm | 6 (10%) |
| > 2cm | 12 (20%) |
| Gross contamination | 28 (46.7%) |
| Treatment | |
| Operative | 25 (41.6%) |
| Endoscopic | 31 (51.7%) |
| Conservative | 4 (6.7%) |
| Multiple organisms infection (> = 3) | 13 (21.7%) |
| Need ICU admission | 28 (46.7%) |
| Need organ support | 26 (43.3%) |
| Leakage after initial treatment | 7 (11.7%) |
| 30-days mortality | 8 (13.3%) |
Table 1: Characteristics of oesophageal perforation cases.
| Number of leakage | Leakage rate (%) | P-value | Number of re-intervention | Re-intervention rate (%) | P-value | |
| Conservative treatment group (4 cases) | 1 | 25 | 0.399 | 0 | 0 | 0.81 |
| Endoscopic treatment group (31 cases) | 2 | 6.45 | 0.247 | 2 | 6.45 | 1.0 |
| Operative treatment group (25 cases) | 4 | 16 | 0.436 | 1 | 4 | 1.0 |
Table 2: Results among different treatment options.
Contamination (gross collection detected by X-ray or CT scan) occurred in almost half of the patients (Table 1), and contamination is the factor significantly related to 30-days mortality (p = 0.013). Other significant factors were: ICU admission (p < 0.001), the need for organ support (inotropic support, ventilator support or renal dialysis) (p < 0.001), multiple-organism infection (p < 0.001) and leakage after initial treatment (p = 0.043) (Table 3).
| Contamination | No contamination | P-value | |
| ICU admission | 20 | 8 | < 0.001* |
| Need organs support | 20 | 6 | < 0.001* |
| Multiple organisms infection | 13 | 0 | < 0.001* |
| Leakage after initial leakage | 6 | 1 | 0.043* |
| 30-days mortality | 7 | 1 | 0.013* |
Table 3: Association of contamination and different outcomes.
*p-value < 0.05
*p-value < 0.05
31 patients (51.7%) were treated by using endoscopic methods. These included application of clips to close the perforating site or deploying a covered stent to cover up the esophageal lesion. 25 patients (41.6%) were treated by using surgical intervention. 4 patients (6.7%) were treated conservatively. (Table 1) 2 cases in endoscopic treatment group had leakage (6.45%); one needed operation and the other case required further endoscopic treatment (stenting). Re-intervention rate in endoscopic treatment group was 6.45%. 4 cases in operative group (16%) had leakage. Only one of these cases needed another operation while the others were able to be managed conservatively. Re-intervention rate in operative group was 4%. One case in conservative management group (25%) leaked but was treated conservatively (re-intervention rate 0%) (Table 2).
Overall leakage rate was 11.7% (7 cases). Contamination (p = 0.043) and multiple-organism infection (p = 0.034) were the two risk factors significantly associated with leakage after initial treatment (Table 4).
| Risk factor | Leakage after initial treatment (total 7 cases) | P-value |
| Age | Median 65 (25-93) | 0.597 |
| Sex (M:F) | 6:1 | 0.126 |
| Etiology | ||
| Boerhavve syndrome | 2 | 0.456 |
| Foreign body | 1 | 0.402 |
| Iatrogenic | 3 | 0.546 |
| Perforating site | ||
| Cervical | 1 | 0.099 |
| Thoracic | 4 | 0.058 |
| OGJ/ abdominal | 2 | 0.851 |
| Perforating size | ||
| < 1cm | 3 | 0.93 |
| 1-2cm | 1 | 0.688 |
| > 2cm | 3 | 0.688 |
| > 24 hours onset of symptoms | 2 | 0.193 |
| Contamination | 6 | 0.043* |
| Multiple organisms infection | 4 | 0.034* |
| Treatment | ||
| Conservative | 1 | 0.399 |
| Endoscopic | 2 | 0.247 |
| Operative | 4 | 0.436 |
Table 4: Risk factors analysis for leakage after initial management.
*p-value < 0.05
*p-value < 0.05
The overall 30-day mortality was 13.3% (8 cases). The cause of perforation (swallowed foreign body, iatrogenic, Boerhaave syndrome), onset of symptoms > 24 hours and leakage after initial treatment were not statistically associated with 30-day mortality. (Table 5) The median hospital stay was 16 days (3 to 115 days). 28 patients (46.7%) required intensive care unit (ICU) admission (Table 1).
| Risk factor | Mortality cases (total 8 cases) | P-value |
| Age | Median 65 (25-93) | 0.34 |
| Sex (M:F) | 7:1 | 0.59 |
| Etiology | ||
| Boerhavve syndrome | 2 | 0.60 |
| Foreign body | 2 | 0.26 |
| Iatrogenic | 2 | 0.70 |
| Perforating site | ||
| Cervical | 2 | 0.26 |
| Thoracic | 4 | 0.08 |
| OGJ/abdominal | 2 | 0.66 |
| Perforating size | ||
| < 1cm | 4 | 0.19 |
| 1-2cm | 2 | 0.13 |
| >2cm | 2 | 0.70 |
| >24 hours onset of symptoms | 5 | 0.51 |
| Contamination | 7 | 0.013* |
| Multiple organisms infection | 3 | 0.243 |
| Treatment | ||
| Conservative | 1 | 0.445 |
| Endoscopic | 2 | 0.14 |
| Operative | 5 | 0.259 |
| Leakage after initial treatment | 2 | 0.207 |
Table 5: Risk factors analysis for 30-days mortality.
*p-value < 0.05
*p-value < 0.05
Discussion
Esophageal perforation is a rare but life-threatening condition. The mortality rate can be as high as 20 to 30%. The rarity of this disease makes clinician difficulty to get enough experience in treating and managing this disease. Treating esophageal perforation in a high volume center is one of the ways to improve the outcome and survival [1].
Another factor that makes esophageal perforation a life-threatening condition is the anatomy of the esophagus. Esophagus travels a long way, from the neck down to the abdomen. The clinical presentation of esophageal perforation can vary a lot, depending on the size, site and cause of perforation. Once the esophagus is perforated, the gastro-intestinal content can spread along the soft tissue planes in the head & neck and mediastinal region, resulting in extensive contamination and severe sepsis. Moreover, esophagus lacks a serosal layer, thus rendering it more vulnerable to rupture or perforation [4].
In real life, the situation may be more chaotic and complicated because other conditions, such as spontaneous pneumo-mediastinum and necrotizing mediastinitis, may mimic esophageal perforation. A high index of suspicion is required to make a timely diagnosis. After making the diagnosis, the management plan for esophageal perforation should be tailor-made. The patients’ general condition, etiology, onset of symptoms3, site and size of perforation, degree of contamination and presence of expertise will influence the treatment options [3,10]. Because of the limited information in the literature, no clear consensus exists regarding the management of esophageal perforation.
The following are the management principles:
Resuscitation
Many of these patients are suffering from severe sepsis. They may be in shock and require intubation. Aggressive resuscitation and stabilizing the patients are the keys for further investigations and management.
Resuscitation
Many of these patients are suffering from severe sepsis. They may be in shock and require intubation. Aggressive resuscitation and stabilizing the patients are the keys for further investigations and management.
Making the diagnosis
X-ray may reveal pleural effusion, pneumo-mediastinum, pneumothorax or surgical emphysema, but these are non-specific. CT scan with intravenous contrast is almost a must and easily accessible nowadays. If possible, adding oral contrast will give additional information like the location of perforation site and the severity of leakage. If the patient is intubated and not fit for taking oral contrast, OGD can be used [3]. Although some people may avoid the use of OGD in esophageal perforation due to the fear of increasing mediastinal contamination, study showed endoscopy is a safe and important component in treating esophageal perforation [9]. From our experience, the use of OGD has both diagnostic and therapeutic value. During the procedure, air insufflation should be minimized.
X-ray may reveal pleural effusion, pneumo-mediastinum, pneumothorax or surgical emphysema, but these are non-specific. CT scan with intravenous contrast is almost a must and easily accessible nowadays. If possible, adding oral contrast will give additional information like the location of perforation site and the severity of leakage. If the patient is intubated and not fit for taking oral contrast, OGD can be used [3]. Although some people may avoid the use of OGD in esophageal perforation due to the fear of increasing mediastinal contamination, study showed endoscopy is a safe and important component in treating esophageal perforation [9]. From our experience, the use of OGD has both diagnostic and therapeutic value. During the procedure, air insufflation should be minimized.
Managing the perforation site
This can be done by various means, such as applying metal clips endoscopically, deploying a covered metallic stent under fluoroscopic guidance, or by surgery. After treatment the patients need to keep nil-by-mouth. Enteric feeding should be considered first. This can be achieved by placing feeding tube (under endoscopic guidance) to duodenum or jejunum. A feeding jejunostomy tube can also be used [7]. Oral contrast study is done later to assess the healing of the perforating site. Selected cases may be treated conservatively if the perforating site is minute with localized contrast extravasation, especially those close to cricopharyngeus and in the absence of systemic infection [8].
This can be done by various means, such as applying metal clips endoscopically, deploying a covered metallic stent under fluoroscopic guidance, or by surgery. After treatment the patients need to keep nil-by-mouth. Enteric feeding should be considered first. This can be achieved by placing feeding tube (under endoscopic guidance) to duodenum or jejunum. A feeding jejunostomy tube can also be used [7]. Oral contrast study is done later to assess the healing of the perforating site. Selected cases may be treated conservatively if the perforating site is minute with localized contrast extravasation, especially those close to cricopharyngeus and in the absence of systemic infection [8].
Endoscopic therapy may have its own limitations. Clipping or stenting may not be feasible if the perforating site is too close to the cricopharynegeus. This is due to the difficulty in visualizing that region and the presence of foreign body sensation after endoscopic treatment. Stent migration is also a problem, particularly for covered stents as there is nothing to hold the stents in position. Around 3 to 4 weeks after successful stenting, we should remove the stent and assess the healing of the perforating site.
Surgery should be considered if the perforating site is large, or when patients deteriorate despite conservative or endoscopic treatment. Whether to do primary repair (+/- reinforcement) or esophageal exclusion surgery really depends on multiple factors. These include the location and size of perforation, degree of contamination, the cause of perforation, patient’s general condition and the presence of expertise [3]. Contamination and multiple organism infection are associated with leakage after initial treatment; overall re-intervention rate is around 5% in this study and many of them can be treated conservatively. (Table 2)
Protection of Vital Organs, Such as the Heart, Lung and Kidneys.
Many of these patients need organ support. ICU care and management will be beneficial. (Table 1)
Many of these patients need organ support. ICU care and management will be beneficial. (Table 1)
Drainage
If possible, all the collections over neck, mediastinum, thoracic cavity and abdominal region should be drained as the contaminant may be the potential sources for abscess collection and sepsis. These can be effected by percutaneous means if the perforation is treated by endoscopic method. If the treatment is by surgery, putting drains around the perforating site or performing tube esophagostomy should be considered if the repaired site’s healing is doubtful.
If possible, all the collections over neck, mediastinum, thoracic cavity and abdominal region should be drained as the contaminant may be the potential sources for abscess collection and sepsis. These can be effected by percutaneous means if the perforation is treated by endoscopic method. If the treatment is by surgery, putting drains around the perforating site or performing tube esophagostomy should be considered if the repaired site’s healing is doubtful.
Decontamination
Many of these patients have infection by multiple organisms. (Table 1) The administration of broad-spectrum antibiotics, sometimes multiple antibiotics, is needed to treat the sepsis. Some of the patients may need surgical debridement repeatedly to remove all the necrotic tissues or collections. In this study, contamination is the statistically significant risk factor for 30-days mortality, ICU admission, and the need for organ support, poly-microbial infection and leakage after initial treatment. (Table 3)
Many of these patients have infection by multiple organisms. (Table 1) The administration of broad-spectrum antibiotics, sometimes multiple antibiotics, is needed to treat the sepsis. Some of the patients may need surgical debridement repeatedly to remove all the necrotic tissues or collections. In this study, contamination is the statistically significant risk factor for 30-days mortality, ICU admission, and the need for organ support, poly-microbial infection and leakage after initial treatment. (Table 3)
If the esophagus is badly damaged and cannot be repaired primarily, emergency esophagectomy may be the treatment of choice. However, this should be the last resort and should only be considered with the availability of experienced upper GI surgeons. The mortality rate of emergency esophagectomy can be as high as 30 to 40%, which is much higher than that of elective oesophagectomy [6].
Conclusion
Up till now, the management of esophageal perforation is still challenging. This is an uncommon but a dire surgical emergency, with high morbidity and mortality. Making a diagnosis timely needs a high index of suspicion. Management plan should be tailor-made. Multi-disciplinary approach is needed to give the best chance of survival. Further accumulation of knowledge and advancement in endoscopic and minimal invasive techniques may generate the best management strategy in the future.
Declaration
All authors have disclosed no conflicts of interest.
All authors have disclosed no conflicts of interest.
Acknowledgement
The material in this paper was presented at the Annual Scientific Meeting of Hong Kong Society of Critical Care Medicine held in Hong Kong, November 2017.
The material in this paper was presented at the Annual Scientific Meeting of Hong Kong Society of Critical Care Medicine held in Hong Kong, November 2017.
References
- SR Markar., et al. “Management and outcomes of esophageal perforation: a national study of 2,564 patients in England”. American Journal of Gastroenterology 110.11 (2015): 1559-1566.
- TT Law., et al. “Outcomes after oesophageal perforation: a retrospective cohort study of patients with different aetiologies”. Hong Kong Medical Journal 23. 3 (2017): 231-238
- JA Soreide., et al. “Esophageal perforation: diagnostic work-up and clinical decision-making in the first 24 hours”. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine (2011): 19:66.
- Dale K Mueller., et al. “Esophageal rupture: background, anatomy, and pathophysiology”. Emedicine Medscape.
- Ronald V Romero., et al. “Esophageal perforation: continuing challenge to treatment”. Gastrointestinal Intervention 2.1 (2013): 1-6
- M Schweigert., et al. “Emergency oesophagectomy for oesophageal perforation after chemoradiotherapy for oesophageal cancer”. Annals of the Royal College of Surgeons of England 97.2 (2015): 140-145.
- Wu Gang., et al. “Treatment of spontaneous oesophageal rupture with transanal thoracic drainage and temporary oesophageal stent and jejunal feeding tube placement”. Journal of Trauma and Acute care surgery 82.1 (2017): 141-149.
- Perrone., et al. “Pharyngo-oesophageal perforation following anterior cervical discectomy and fusion”. European Journal of Cardio-thoracic surgery 51.1 (2017): 160-168.
- MK Kuppusamy., et al. “Impact of endoscopic assessment and treatment on operative and non-operative management of acute oesophageal perforation”. British Journal of Surgery 98.6 (2011): 818-824.
- Van der Zee., et al. “Management of oesophageal perforations; a tailored approach”. The Netherlands Journal of Surgery 38.2 (1986): 31-5.
Citation:
Kevin KF Wong., et al. “Management and Outcomes of Esophageal Perforation: A Single Center 13-Year Experience”. Chronicle
of Medicine and Surgery 2.2 (2018): 111-118.
Copyright: © 2018 Kevin KF Wong., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.