Case Report
Volume 1 Issue 1 - 2017
Potential Benefits of Cellular Transplantation in a Patient with Amyotrophic Lateral Sclerosis
1Department of regenerative medicine and medical Services, NeuroGen Brain and Spine Institute, India
2Department of Research & Development, NeuroGen Brain and Spine Institute, India
3Department of NeuroRehabilitation, NeuroGen Brain and Spine Institute, India
4Department of regenerative laboratory services, NeuroGen Brain and Spine Institute, India
2Department of Research & Development, NeuroGen Brain and Spine Institute, India
3Department of NeuroRehabilitation, NeuroGen Brain and Spine Institute, India
4Department of regenerative laboratory services, NeuroGen Brain and Spine Institute, India
*Corresponding Author: Amruta Paranjape, Department of Research & Development, NeuroGen Brain and Spine Institute, India.
Received: March 28, 2017; Published: April 24, 2017
Abstract
Background: Amyotrophic Lateral Sclerosis (ALS) is a rapidly progressive neurodegenerative disorder which has a fatal prognosis. The primary pathology is loss of motor neurons in the brain and spinal cord. Recently cellular therapy has been studied for ALS in various animal models and has emerged as potential therapeutic modality. Autologous bone marrow mononuclear cells (BMMNCSs) have been studied and found to be ethical, safe and feasible.
Case Report: We present a detailed case report of 41 years old female diagnosed with amyotrophic lateral sclerosis since 3 years. She was given intrathecal autologous bone marrow mononuclear cell therapy combined with riluzole, neurorehabilitation and 6 weeks of lithium. On the outcome measures, Amyotrophic Lateral Sclerosis Functional Rating (ALSFRS-r) score increased from 29 to 32 and Functional Independence Measure (FIM) score increased from 48 to 64.
Conclusion: The highlight of the case study is halting of disease progression with symptomatic improvements over a period of 12 months after intervention.
Keywords: Amyotrophic Lateral Sclerosis (ALS); Stem cells; Cellular therapy; Autologous Bone Marrow Mononuclear Cells (BMMNCSs) transplantation; ALSFRS-r; FIM
Abbreviations: NRRT: Neuro regenerative rehabilitative therapy; BMMNCSs: Bone marrow mononuclear cells; ALS: Amyotrophic lateral sclerosis; mMRC-MMT: modified medical research council’s manual muscle testing; mMRC-MMT (I): revised modified medical research council’s manual muscle testing; EMG: Electromyography; IC-SCRT: Institutional committee for stem cell research and therapy; G-CSF: Granulocyte colony stimulation factor; MNC: Mononuclear cell; FACS: Fluorescence activated cell sorting; ALS FRS r: Revised ALS Fucntional rating scale; FIM: Functional independence measure; ALSFRS: ALS functional rating scale
Introduction
Amyotrophic lateral sclerosis (ALS) a neurodegenerative disease affecting motor neurons in the cerebral cortex, brainstem, and spinal cord. This results in muscle denervation that leads to physical symptoms of muscle weakness, atrophy, and alteration in muscle tone, which progresses to the loss of voluntary movements [1] ALS disease usually progresses to death within 2 to 4 years [2]. Rapid progression and unknown etiology together contribute to inability to provide an effective treatment for ALS.
Disease-modifying options for patients are limited to treatment with medical management, noninvasive positive pressure ventilation, and nutritional support, each having only a modest effect on disease progression and survival which increases the life span of patient by 3-5 months. With recent advances in regenerative medicine, cellular therapy has become an option for the treatment of ALS [3]. Various animal studies [4,5,6] have shown beneficial effects of cell therapy in ALS. Initial trials and studies with autologous bone marrow mononuclear cells have demonstrated that, it has been safe and effective in patients with other neurological disorders [7,8].
Herein, we present a case report pertaining to potential use of autologous bone marrow mononuclear stem cells given as a therapeutic agent in ALS. The neuroregenerative rehabilitation therapy (NRRT) integrates regenerative medicine using intrathecal administration of autologous bone marrow mononuclear cells (BMMNCs) and various rehabilitation therapies such as physiotherapy, occupational therapy, psychological counseling and speech therapy. Cell therapy was given to this patient, since there was continuous deterioration despite the treatment with conventional medical therapies and treatments. And the patient still continued to experience major deficits in activities of daily living, ambulation and transfers.
Materials and Methods
Case report
We present 41 years old female diagnosed with ALS since 3 years. She presented with complaints of frequent falls while walking after the birth of her second daughter. She noted weakness in bilateral lower extremities, progressed from distal to proximal muscles. Six months later, symptoms progressed to weakness in the left upper extremity from distal to proximal muscles and then to right upper extremity. She had dysarthria and dysphagia. Gradually she developed difficulties in walking, stairs climbing, getting up from supine to sit and getting up from chair. She also experienced high fatigability while performing functional activities. She had no family history, no relevant medical or surgical history. On clinical evaluation, she was hypertonic and hyper-reflexic, with Babinski sign and ankle clonus positive. Cramps and fasciculations were present. Muscle strength was assessed using revised version of the medical research council’s manual muscle testing scale (mMRC-MMT (I)) as given in table 1. Her muscles strength was below functional grade in shoulder, trunk and hands muscles and at the level of functional grade in elbows, knee, hip and foot muscles. She had poor dynamic standing and walking balance. Her inspiratory and expiratory effort and capacity were poor. She was moderately dependent for ADL. She complained about urinary urgency.
We present 41 years old female diagnosed with ALS since 3 years. She presented with complaints of frequent falls while walking after the birth of her second daughter. She noted weakness in bilateral lower extremities, progressed from distal to proximal muscles. Six months later, symptoms progressed to weakness in the left upper extremity from distal to proximal muscles and then to right upper extremity. She had dysarthria and dysphagia. Gradually she developed difficulties in walking, stairs climbing, getting up from supine to sit and getting up from chair. She also experienced high fatigability while performing functional activities. She had no family history, no relevant medical or surgical history. On clinical evaluation, she was hypertonic and hyper-reflexic, with Babinski sign and ankle clonus positive. Cramps and fasciculations were present. Muscle strength was assessed using revised version of the medical research council’s manual muscle testing scale (mMRC-MMT (I)) as given in table 1. Her muscles strength was below functional grade in shoulder, trunk and hands muscles and at the level of functional grade in elbows, knee, hip and foot muscles. She had poor dynamic standing and walking balance. Her inspiratory and expiratory effort and capacity were poor. She was moderately dependent for ADL. She complained about urinary urgency.
| mMRC –MMT | Description | mMRC-MMT (I) | Description |
| 0 | No Movement | 0 | No movement |
| 1 | A flicker of movement is seen or felt in the muscle | 1 | A flicker of movement is seen or felt in the muscle |
| 2 | Muscle moves the joint when gravity is eliminated | 1+ | Muscle moves the joint through up to 1/3rd of the ROM when gravity is eliminated |
| 1++ | Muscle moves the joint more than 1/3rd less than 2/3rd of the ROM when gravity is eliminated | ||
| 2- | Muscle moves the joint more than 2/3rd but less than the full ROM | ||
| 2 | Muscle moves the joint through complete ROM when gravity is eliminated | ||
| 3- | Muscle moves the joint against gravity, but not through full mechanical range of motion | 2+ | Muscle moves the joint up to 1/3rd ROM against gravity |
| 2++ | Muscle moves the joint >1/3rd, < 2/3rd of ROM against gravity | ||
| 3- | Muscle moves the joint more than 2/3rd but less than complete ROM | ||
| 3 | Muscle cannot hold the joint against resistance but moved the joint fully against gravity | 3 | Muscle moves the joint through complete ROM against gravity |
| 3+ | Muscle moves the joint fully against gravity and is capable of transient resistance, but collapses abruptly | 3+ | Muscle moves the joint against gravity and moderate resistance up to 1/3rd of ROM |
| 3++ | Muscle moves the joint against gravity and moderate resistance from 1/3rd to 2/3rd of ROM | ||
| 4- | Same as grade 4, but muscle holds the joint only against minimal resistance | 4- | Muscle moves the joint more than 2/3rd but less than complete ROM against gravity and moderate resistance |
| 4 | Muscle holds the joint against a combination of gravity and moderate resistance | 4 | Muscle moves the joint against gravity and moderate resistance though complete ROM |
| 4+ | Same as grade 4 but muscle holds the joints against moderate to maximal resistance | 4+ | Muscle moves the joint against gravity and moderate to maximal resistance up to 1/3rd of ROM |
| 5- | Barely detectable weakness | 4++ | Muscle moves the joint against gravity and moderate to maximal resistance from 1/3rd to 2/3rd of ROM (Barely detectable weakness) |
| 5 | Normal strength | 5 | Muscle moves the joint against gravity and moderate to maximal resistance though complete ROM (Normal Strength) |
Table 1: Comparison of the grades of the scales mMRC-MMT and mMRC-MMT(I).
She was diagnosed based on Electromyographic (EMG) studies suggestive of evidence of acute denervation in all the muscles. Findings
showed acute and wide spread denervation involving tongue, cervical, thoracic and lumbo-sacral spine and all limbs. This was
suggestive of Anterior Horn Cell Disease. Her FIM score was 48/126 and ALSFRS r score was 29/48. Muscle strength before stem cell
therapy is given in Table 2.
| Date | mMRC-MMT (I) grade at assessment months (Before 1st transplantation) | mMRC-MMT (I) grade at 2 months (After 1st transplantation) | mMRC-MMT (I) grade at 4 months (After 1st transplantation) | mMRC-MMT (I) grade at 6 months (After 1st transplantation) | mMRC-MMT (I) grade at 9 months (After 1st transplantation) | mMRC-MMT (I) grade at 12 months (After 1st transplantation) | |
| Muscle groups | |||||||
| Hip | Flexor | 3++ | 3++ | 3++ | 3++ | 3++ | 3++ |
| Extensors | 3+ | 3++ | 3+ | 3++ | 3++ | 3++ | |
| Abductors | 3+ | 4 | 3++ | 3++ | 3++ | 3++ | |
| Adductors | 3+ | 3++ | 3++ | 3++ | 3++ | 3++ | |
| Knee | Flexors | 3++ | 4 | 4 | 4 | 4 | 4 |
| Extensors | 4 | 4 | 4 | 4 | 4 | 4 | |
| Ankle and Foot | Tibialis | 3++ | 4 | 4 | 4 | 4 | 4 |
| Anterior Tibialis |
3+ | 4 | 4 | 4 | 4 | 4 | |
| Posterior Peronius |
3++ | 3++ | 3++ | 3++ | 3++ | 3++ | |
| Longus Peronius |
3++ | 3++ | 3++ | 3++ | 3++ | 3++ | |
| Bravis Peronius |
2 | 2 | 2 | 2 | 2 | 1+ | |
| Tertius Plantar |
4 | 4 | 4 | 4 | 4 | 4 | |
| Flexor Ext. |
3++ | 4 | 3+ | 3+ | 3+ | 3+ | |
| Hallusis Longus Ext. Digitorum Longus |
3 | 4 | 4 | 4 | 4 | 3 | |
| Upper Abdominal | 2 | 2 | 2- | 1+ | 2 | 2+ | |
| Lower | 3+ | 3++ | 3++ | 3++ | 3++ | 3++ | |
| Abdominal Back Extensors |
2 | 1+ | 1+ | 1++ | 2 | 2 | |
| Trunk | Flexors | 3- | 3- | 3- | 3- | 3- | 2 |
| Extensors | 3+ | 3+ | 3+ | 3+ | 3+ | 2++ | |
| Trapezius | 3++ | 3++ | 3++ | 3++ | 3++ | 3++ | |
| Neck | Rhomboids | 2 | 2 | 2 | 2 | 2 | 2 |
| Serratus Anterior | 1++ | 1++ | 3+ | 3+ | 3+ | 3+ | |
| Flexor | 2++ | 2++ | 3- | 3- | 3- | 3- | |
| Extensors Abductors Adductors |
3+ 2+ 3++ |
3+ 2++ 3++ |
3++ 3- 3++ |
3++ 2+ 3++ |
3++ 2+ 3++ |
3++ 2+ 3++ |
|
| Biceps | 3++ | 3++ | 3++ | 3++ | 3++ | 3++ | |
| Shoulder | Brachialis | 3++ | 3++ | 3++ | 3++ | 3++ | 3++ |
| Triceps | 3+ | 3+ | 3++ | 3++ | 3++ | 3++ | |
| Brachioradialis | 3++ | 3++ | 3++ | 3++ | 3++ | 3++ | |
| Elbow | Supinators | 2++ | 3 | 3++ | 3++ | 3++ | 2+ |
| Pronators | 2+ | 3 | 3++ | 3++ | 3++ | 3++ | |
| Extensors | 3++ | 3++ | 3++ | 3++ | 3++ | 3++ | |
| Flexors | 3- | 3+ | 3++ | 3+ | 3+ | 3++ | |
| Forearm, Wrist and Hand |
Flexor | 3+ | 3++ | 3++ | 3++ | 3++ | 3+ |
| Pollicis Longus Flexor |
1+ | 1++ | 1++ | 1+ | 1++ | 1+ | |
| Policis Brevis Extensor |
3- | 3++ | 3++ | 3+ | 3+ | 3+ | |
| Pollicis Longus Extensor |
3+ | 3++ | 3++ | 3+ | 3+ | 3+ | |
| Policis Brevis Adductor |
1+ | 1+ | 1+ | 1+ | 1+ | 3++ | |
| Policis Abductor |
1+ | 1+ | 1+ | 1+ | 1+ | 1+ | |
| Pollicis Longus and Brevis Opponens |
1++ | 1++ | 1++ | 1 | 1++ | 0 | |
| Pollicis | |||||||
Table 2: Changes in the muscle strength over 12 months as measured by mMRC-MMT (I) scale.
The patient selection was in compliance with the World Medical Associations Helsinki declaration criteria [9]. The protocol of autologous BMMNCs intrathecal administration has been reviewed and approved by the Institutional Committee for Stem Cell Research and Therapy (IC-SCRT). The patient was informed about the procedure and a duly filled informed consent was obtained.
Bone marrow aspiration
300 mcg of Granulocyte colony-stimulating factor (G-CSF) injections were administrated 48 hours and 24 hours prior to BMMNCs transplantation, to stimulate CD34+ cells and increase their survival and multiplication. 100 ml bone marrow was aspirated from the anterior superior iliac spine, using bone marrow aspiration needle and collected in heparinized tubes.
300 mcg of Granulocyte colony-stimulating factor (G-CSF) injections were administrated 48 hours and 24 hours prior to BMMNCs transplantation, to stimulate CD34+ cells and increase their survival and multiplication. 100 ml bone marrow was aspirated from the anterior superior iliac spine, using bone marrow aspiration needle and collected in heparinized tubes.
Separation of BMMNCSs: The bone marrow is further subjected to mononuclear cell (MNC) separation using differential centrifugation. Bone marrow is diluted in the ratio of 1:1 with normal saline. The diluted bone marrow is subjected to density gradient separation using Ficoll-Paque media by centrifuging it at 440 xg rpm for 35 minutes in a swinging bucket rotar without brake at 20°C. MNCs are obtained as a buffy coat. The MNCs are washed thrice with normal saline by centrifuging at 300 xg for 15 minutes in a swinging bucket rotar without brake at 20°C and finally resuspended in 1 ml of normal saline. Viable count of the isolated MNCs is done using trypan blue vital dye which is mixed in 1:1 proportion and loaded on to the haemocytometer for the total cell count and viable count. So the viable count was found to be 99% and the MNC were 2.68 x 108. This is further confirmed by TALI cell counter. CD34+ analysis was done using Fluorescence activated cell sorting (FACS) using CD34 PE antibody.
Administration of BMMNCs: Isolated BMMNCs were administered intrathecally immediately post separation, in L4-L5 using a lumbar puncture needle. 1 gm methyl prednisolone in 500 ml Ringer’s Lactate was simultaneously injected intravenously to improve stem cell multiplication and transplantation.
The patient was given bilateral upper limbs and lower limbs active strengthening exercises, trunk strengthening exercises, Proprioceptive Neuromuscular Facilitation pattern to reduce synergic pattern, sitting and standing balance training and gait training. She was also given oromotor and facial exercises. To improve the effectiveness of the stem cell therapy patient was given exercise home program. To enhance potency of cells, 300 mg lithium once in a day was prescribed and serum lithium levels were maintained between 0.5 to 0.8 mEq/L for six weeks. In view of significant improvements, the patient was given the cell therapy for second time after 6 months.
Revised ALS Fucntional rating scale (ALS FRS r) and Functional independence measure (FIM) scores were marked at 2, 4, 6, 9 and 12 months period. A graph was plotted with ALS FRS-r score along with Y- axis and duration in months along X-axis to compare the disease progression of this patient with natural disease progression in ALS. (Figure-1).
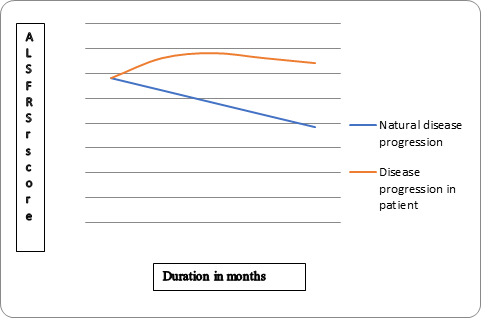
Figure 1: ALS FRS r and FIM scores were marked at 2, 4, 6, 9 and 12 months period for this patient. A graph
was plotted with ALS FRS-r score along with Y- axis and duration in months along X-axis to compare the disease
progression of this patient with natural disease progression in ALS. In the natural disease progression
there is 17% decline in ALSFRS score in every 6 months. (29 score on ALS FRSr should become 19 in the natural
progression). However, in this patient ALS FRS r score was 32 at 12 months.
The patient was followed up at regular intervals for one year.
Results
Post one week stem cell therapy
Oromotor Functions: Speech became louder and clear.
Ambulation and transfer: There was reduction in leg muscle spasticity and leg scissoring so sit to stand from chair became easier.
Oromotor Functions: Speech became louder and clear.
Ambulation and transfer: There was reduction in leg muscle spasticity and leg scissoring so sit to stand from chair became easier.
Post two months stem cell therapy
Oromotor Functions: Improved clarity and duration of speech. Swallowing became more faster and she didn’t require sipper. Night drooling, tongue fasciculations were stopped completely.
Oromotor Functions: Improved clarity and duration of speech. Swallowing became more faster and she didn’t require sipper. Night drooling, tongue fasciculations were stopped completely.
Hand Functions:Improvement was seen in fine, gross motor activities and hand coordination. Also handwriting (showed in appendix) and writing speed has improved.
Bed mobility:Rolling, supine to sit and shifting in the bed became more faster than before. Posture: Neck drop reduced. Kyphotic posture was reduced.
Balance: Improvement was seen in sitting static and dynamic balance. He was able to stand from chair independently.
Ambulation: Ambulation became easier, less tiring, walks longer than before with assistance. Stamina to perform exercises had improved.
Functional Improvements: Functionally was able to eat independently without assistance, brush teeth with less effort, could apply soap while bathing and perform perineal hygiene with some assistance, earlier completely dependent for these activities. Social interaction has improved secondary to improvement in speech.
Post four months stem cell therapy
Hand Functions:Handwriting became more legible. Pen and spoon holding was better. Improvement was seen in fine and gross motor activities as she could use wash basin, open and close tap, switch on and off the light and fans and could pick up and eat small things like dry fruits.
Hand Functions:Handwriting became more legible. Pen and spoon holding was better. Improvement was seen in fine and gross motor activities as she could use wash basin, open and close tap, switch on and off the light and fans and could pick up and eat small things like dry fruits.
Ambulation: She could walk with walker for around 4-5 minutes. Ground clearance was better with increased knee flexion and she had started doing stepping.
Functional Improvements: Could do bed to chair transfer, toilet transfer.
Post six month stem cell therapy
Ambulation: Leg movements were easier so she could walk with help of walker independently.
Post six month stem cell therapy
Ambulation: Leg movements were easier so she could walk with help of walker independently.
Bladder and bowl: As urinary urgency had reduced, she could hold urine for one hour.
In view of the improvements a second cell transplantation procedure was done at 6 months. The procedure was same as the first transplantation, which involved intrathecal administration of autologous BMMNCSs followed by 6 weeks of lithium. Riluzole and neurorehabilitation was continued.
Post 9 months of first stem cell therapy
3 months after second transplantation and 9 months since the first transplantation; she could climb flight of stairs with the help of side railing. Neck drop reduced significantly. Improvements were also noted in muscle strength (Table 1 and 2) and in handwriting (Appendix 1).
3 months after second transplantation and 9 months since the first transplantation; she could climb flight of stairs with the help of side railing. Neck drop reduced significantly. Improvements were also noted in muscle strength (Table 1 and 2) and in handwriting (Appendix 1).
Appendix 1
Changes in the handwriting over the period of 9 months
1A. Handwriting sample at assessment
1A. Handwriting sample at assessment
1B. Handwriting sample at 2 month follow up
1C. Handwriting sample at 4 month follow up
1D. Handwriting sample at assessment before second shot at 6 month
Post 12 months of first stem cell therapy
Patient’s condition was maintained. Her frequency of falls reduced i. e. 1 in 6 months.
Patient’s condition was maintained. Her frequency of falls reduced i. e. 1 in 6 months.
Objective evaluation
Over a period of 12 months, there were significant changes in ALS FRS-r and FIM score. (Table 3 and 4). 6 months post intervention ALS FRS-r score increased from 29 to 34 with improvement noted in speech, swallowing, handwriting, cutting food and handling utensils, turning in bed and adjusting cloths components of scale. On follow up after 12 months ALS FRS-r score was 32 with change in handwriting and stair climbing components. Post 12 months of intervention, FIM score improved from 48 to 64, with functional improvements noted in self-care, bowel management, transfer and locomotion.
Over a period of 12 months, there were significant changes in ALS FRS-r and FIM score. (Table 3 and 4). 6 months post intervention ALS FRS-r score increased from 29 to 34 with improvement noted in speech, swallowing, handwriting, cutting food and handling utensils, turning in bed and adjusting cloths components of scale. On follow up after 12 months ALS FRS-r score was 32 with change in handwriting and stair climbing components. Post 12 months of intervention, FIM score improved from 48 to 64, with functional improvements noted in self-care, bowel management, transfer and locomotion.
| At assessment | 2 months after transplantation | 4 months after transplantation | 6 months after transplantation | 9 months after transplantation | 12 months after transplantation | |
| Speech 4 – Normal Speech processes 3 – Detectable speech with disturbances 2 – Intelligible with repeating 1 – Speech combined with nonvocal communication 0 – Loss of useful speech |
2 | 2 | 3 | 2 | 2 | 2 |
| Salivation 4 – Normal 3 – Slight but definite excess of saliva in mouth; may have nighttime drooling 2 – Moderately excessive saliva; may have minimal drooling 1 – Marked excess of saliva with some drooling 0 – Marked drooling; requires constant tissue or handkerchief |
3 | 4 | 3 | 3 | 3 | 3 |
| Swallowing 4 – Normal eating habits 3 – Early eating problems – occasional choking 2 – Dietary consistency changes 1 – Needs supplemental tube feeding 0 – NPO (exclusively parenteral or enteral feeding) |
3 | 3 | 4 | 4 | 4 | 4 |
| Handwriting 4 – Normal 3 – Slow or sloppy; all words are legible 2 – Not all words are legible 1 – Able to grip pen but unable to write 0 – Unable to grip pen |
2 | 3 | 2 | 3 | 3 | 2 |
| Does subject have gastrostomy? No – Answer 5a Yes – Answer 5b
3 – Somewhat slow and clumsy, but no help needed 2 – Can cut most foods, although clumsy and slow; some help needed 1 – Food must be cut by someone, but can still feed slowly 0 – Needs to be fed |
No 2 | No 3 | No 2 | No 2 | No 1 | No 2 |
| b Cutting Food and Handling Utensils (alternate scale for patients with gastrostomy) 4 – Normal 3 – Clumsy but able to perform all manipulations independently 2 – Some help needed with closures and fasteners 1 – Provides minimal assistance to caregivers 0 – Unable to perform any aspect of task |
||||||
| Dressing and Hygiene 4 – Normal function 3 – Independent and complete self-care with effort or decreased efficiency 2 – Intermittent assistance or substitute methods 1 – Needs attendant for self-care 0 – Total dependence |
1 | 1 | 1 | 1 | 1 | 1 |
| Turning in bed and adjusting bed clothes 4 – Normal 3 – Somewhat slow and clumsy, but no help needed 2 – Can turn alone or adjust sheets, but with great difficulty 1 – Can initiate, but not turn or adjust sheets alone 0 – Helpless |
1 | 2 | 2 | 2 | 2 | 2 |
| Walking 4 – Normal 3 – Early ambulation difficulties 2 – Walks with assistance 1 – Nonambulatory functional movement only 0 – No purposeful leg movement |
2 | 2 | 3 | 2 | 2 | 2 |
| Climbing Stairs 4 – Normal 3 – Slow 2 – Mild unsteadiness or fatigue 1 – Needs assistance 0 – Cannot do |
1 | 1 | 3 | 3 | 3 | 2 |
| R-1- Dyspnea 4 – None 3 – Occurs when walking 2 – Occurs with one or more of the following: eating, bathing, dressing 1 – Occurs at rest, difficulty breathing when either sitting or lying 0 – Significant difficulty, considering using mechanical respiratory support |
4 | 4 | 3 | 4 | 4 | 4 |
| R-2 Orthopnea 4 – None 3 – Some difficulty sleeping at night due to shortness of breath, does not routinely use more than two pillows 2 – Needs extra pillow in order to sleep (more than two) 1 – Can only sleep sitting up 0 – Unable to sleep |
4 | 4 | 4 | 4 | 4 | 4 |
| R-3 Respiratory Insufficiency 4 – None 3 – Intermittent use of NIPPV 2 – Continuous use of NIPPV during the night 1 – Continuous use of NIPPV during the night and day 0 – Invasive mechanical ventilation by intubation or tracheostomy |
4 | 4 | 4 | 4 | 4 | 4 |
| Total | 29 | 33 | 34 | 34 | 33 | 32 |
Table 3: Changes in ALS-FRS Revised scale score.
| Outcome measures | At assessment Before 1st transplantation | At 2 months (After 1st transplantation) | At 6 months (After 1st transplantation) | At 9 months (1st transplantation) | At 12 months (1st transplantation) |
| ALSFRS-r | 29 | 33 | 34 | 33 | 32 |
| FIM | 48 | 72 | 73 | 71 | 64 |
Table 4: Changes in measures over the period of 12 months.
In the natural disease progression there is 17% decline in ALS functional rating scale (ALSFRS) score in every 6 months [10] however as shown in graph 1, in this patient we observed that ALS FRS r score increased from 29 to 32 over 12 months.
Discussion
ALS is a rapidly progressing neurodegenerative disease which leads to severe motor disability due to axonal degeneration of motor neurons in brain and spinal cord with sparing of sensory system [11-13]. Defect in the astrocytic glutamate metabolism leads to exotoxicity and autoimmunity causes a widespread neuroinflammatory response. Recent studies demonstrated that various genes are also involved in this process [14]
Evidences have shown that, stem cells help to replace lost cells, provide neurotrophic support, and improve the diseased microenvironment. [15] Due to its self-renewal and differentiation ability, into different types of mature cells, stem cells may aid in modifying or arresting the deterioration of the disease process [14,16,17]. Autologous BMMNCSs are safe and reduces the risk of immunological rejection and of tumor formation [18]. Intrathecal delivery was aimed at increasing the number of neuromuscular connections, decrease pro-inflammatory cytokines in the brain and spinal cord, decrease microglia and astroglia density and promote migration and differentiation of endogenous precursors [19,20].
On follow up after cellular therapy and neurorehabilitation, no significant decline in function or acceleration of disease progression was evident, in fact the patient showed consistent positive functional changes. De Carvahelo., et al. 2005 [10] reported a 17% decline of ALS-FRS scores every 6 months. However, we observed that in this patient ALS-FRSr increased from 29 to 32 over 12 months (Figure 1). FIM score increased from 48 to 62. The functional changes noted in ambulation, oromotor function, hand function. The BMMNCSs protect the existing motor neurons and replace degenerated motor neurons in ALS. Also the neuroprotective mechanisms are believe to be more effective in bringing about the changes seen in ALS post transplantation [14]. These cells are capable of migrating to the injured sites, as guided by various chemo attractant pathways [21].
The foremost importance of neurorehabilitation is to assist in movement restoration, to increase muscle strength, endurance, co-ordination and balance to reduce spasticity and increase joint range of motion and prevent contractures. Exercise enhances the effect of local stem cell by mobilization and angiogenesis [22]. Hence combination of neuroregeneration and rehabilitation was given to augment outcome of cell therapy. A systematic review of literature including a total of 1100 patients treated with Lithium showed no statistically significant differences in the rates of functional decline, deterioration of respiratory function or survival time since onset of the disease as compared to patients treated with Riluzole or placebo [23]. The rationale for prescribing Lithium to our patient was to enhance the survival and potency of transplanted cells [24].
Sharma., et al. 2014 in retrospective controlled study [14] treated 37 patients with autologous BMMNCSs intrathecally. These cases showed increased survival duration by 30.38 months as compared to the control population. Prabhakar., et al. [25] transplanted the autologous BMMNCSs intrathecally in 10 ALS patients. These patients were followed up for one year, 4 points drop in the ALSFRS-r was calculated. Blanquer., et al. 2012 [26] suggests that intraspinal administration of BMMNCSs leads to neurotrophic effect and preserves more number of motor neurons in the treated spinal segments as compared to the non-treated spinal segments. Therefore our results are consistent with the previous findings of the safe use, beneficial effects of cell transplantations and halting of disease progression in ALS patients.
To establish the effectiveness of treatment further randomized control studies are required. We have studies the combination of cellular transplantation and rehabilitation; individual effects of them can be studied in future.
Limitations
The limitation of this case report is absence of advanced neuroimaging techniques, which should be incorporated in future research. It is a solitary example of benefits of the intervention in absence of the control group and unblinded assessment. Therefore larger randomized controlled studies with blinded assessments are required to explore the efficacy of the intervention. However, the improvement noticed in this case contrary to the natural progression of the disease post cellular therapy, medicines and neurorehabilitation, which highlights the potential of this novel approach to stabilize disease progression.
The limitation of this case report is absence of advanced neuroimaging techniques, which should be incorporated in future research. It is a solitary example of benefits of the intervention in absence of the control group and unblinded assessment. Therefore larger randomized controlled studies with blinded assessments are required to explore the efficacy of the intervention. However, the improvement noticed in this case contrary to the natural progression of the disease post cellular therapy, medicines and neurorehabilitation, which highlights the potential of this novel approach to stabilize disease progression.
Conclusion
Though a solitary case, the significant improvements on objective scales of ALSFRS-r and FIM demonstrate that autologous BMMNCS transplantation has slowed the progression of disease and improved her functions. The positive benefits observed could be attributed to regenerative properties of cell transplantation as well as the paracrine effects resulting in neuroprotection. Therefore cellular transplantation when used in conjunction with lithium, riluzole and rehabilitation treatment may improve the quality of life and halt the disease progression of ALS patients. Further studies are needed.
Conflict of interest statement
The author declares that there is no conflict of interest regarding the publication of this article.
The author declares that there is no conflict of interest regarding the publication of this article.
References
- Witiuk Kelsey., et al. "Cognitive deterioration and functional compensation in ALS measured with fMRI using an inhibitory task". Journal of Neuroscience 34.43 (2014): 14260-14271.
- Karussis Dimitrios., et al. "Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis." Archives of neurology 67.10 (2010): 1187-1194.
- Glass Jonathan D., et al. "Lumbar intraspinal injection of neural stem cells in patients with amyotrophic lateral sclerosis: results of a phase I trial in 12 patients" Stem cells 30.6 (2012): 1144-1151.
- Rizvanov Albert A., et al. "Genetically modified human umbilical cord blood cells expressing vascular endothelial growth factor and fibroblast growth factor 2 differentiate into glial cells after transplantation into amyotrophic lateral sclerosis transgenic mice". Experimental biology and medicine 236.1 (2011): 91-98.
- Garbuzova Davis Svitlana., et al. "Multiple intravenous administrations of human umbilical cord blood cells benefit in a mouse model of ALS." PLoS One 7.2 (2012): e31254.
- Garbuzova Davis Svitlana., et al. "Intravenous administration of human umbilical cord blood cells in a mouse model of amyotrophic lateral sclerosis: distribution, migration, and differentiation". Journal of hematotherapy & stem cell research 12.3 (2003): 255-270.
- Sharma Alok., et al. "cellular transplantation may modulate disease progression in spino-cerebellar ataxia–a case report". Indian Journal of Medical Research and Pharmaceutical Sciences 1.3 (2014).
- Sharma Alok., et al. "Autologous bone marrow mononuclear cells intrathecal transplantation in chronic stroke". Stroke research and treatment (2014).
- Carlson Robert V., et al. "The revision of the Declaration of Helsinki: past, present and future". British journal of clinical pharmacology 57.6 (2004): 695-713.
- de Carvalho., et al. "Clinical trials in ALS: a review of the role of clinical and neurophysiological measurements". Amyotrophic Lateral Sclerosis 6.4 (2005): 202-212.
- Meininger., et al. "Safety, pharmacokinetic, and functional effects of the nogo-a monoclonal antibody in amyotrophic lateral sclerosis: a randomized, first-in-human clinical trial". PLoS One 9.5 (2014): e97803.
- Abe., et al. "Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients". Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration 15.7-8 (2014): 610-617.
- Sharma., et al. "The effect of autologous bone marrow mononuclear cell transplantation on the survival duration in Amyotrophic Lateral Sclerosis-a retrospective controlled study". American journal of stem cells 4.1 (2015): 50.
- Al-Chalabi., et al. "The genetics and neuropathology of amyotrophic lateral sclerosis". Acta neuropathologica 124.3 (2012): 339-352.
- Kim Seung U and Jean De Vellis. "Stem cell‐based cell therapy in neurological diseases: a review". Journal of neuroscience research 87.10 (2009): 2183-2200.
- Miller., et al. "Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND)". The Cochrane Library (2007):
- Sharma., et al. "Role of autologous bone marrow mononuclear cells in chronic cervical spinal cord injury-a longterm follow up study". Journal of Neurological Disorders (2013):
- Feldman., et al. "Intraspinal neural stem cell transplantation in amyotrophic lateral sclerosis: phase 1 trial outcomes". Annals of neurology 75.3 (2014): 363-373.
- Thonhoff., et al. "Stem cell-derived motor neurons: applications and challenges in amyotrophic lateral sclerosis". Current stem cell research & therapy 4.3 (2009): 178-199.
- Mazzini Letizia., et al. "Stem cells in amyotrophic lateral sclerosis: state of the art". Expert opinion on biological therapy 9.10 (2009): 1245-1258.
- Borlongan Cesar V., et al. "The great migration of bone marrow-derived stem cells toward the ischemic brain: therapeutic implications for stroke and other neurological disorders". Progress in neurobiology 95.2 (2011): 213-228.
- Drory Vivian E., et al. "The value of muscle exercise in patients with amyotrophic lateral sclerosis". Journal of the neurological sciences 191.1 (2001): 133-137.
- Gamez J., et al. "Lithium for treatment of amyotrophic lateral sclerosis: much ado about nothing". Neurología (English Edition) 31.8 (2016): 550-561.
- Richman CM., et al. "Granulopoietic effects of lithium on human bone marrow in vitro". Experimental hematology 9.4 (1981): 449-455.
- Prabhakar Sudesh., et al. "Autologous bone marrow-derived stem cells in amyotrophic lateral sclerosis: a pilot study". Neurology India 60.5 (2012): 465.
- Blanquer Miguel., et al. "Neurotrophic bone marrow cellular nests prevent spinal motoneuron degeneration in amyotrophic lateral sclerosis patients: a pilot safety study". Stem Cells 30.6 (2012): 1277-1285.
Citation:
Amruta Paranjape., et al. “Potential Benefits of Cellular Transplantation in a Patient with Amyotrophic Lateral Sclerosis”. Current
Opinions in Neurological Science 1.1 (2017): 31-43.
Copyright: © 2017 Amruta Paranjape., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.