Review Article
Volume 1 Issue 2 - 2017
What James Parkinson Really Thought was Behind Parkinson’s Disease
New York Institute of Medical Research, USA
*Corresponding Author: Dr. Lawrence Broxmeyer, MD, New York Institute of Medical Research, New York, USA.
Received: August 29, 2017; Published: September 05, 2017
Abstract
In May, 2017, a Norwegian study done by Berstad and Berstad threw its support behind revitalizing of the hypothesis that with
regard to Parkinson’s disease (PD), the preponderance of convincing evidence points to a chronic infectious cause – not viral and
most likely of the family Actinomycetales of which tuberculosis and the mycobacteria are premier members. The Berstads concluded
that in accordance with such thoughts and despite the diagnostic challenges ahead, that further studies in human brain tissue from
PD patients to detect such an underlying infectious cause were warranted. Such thoughts are really nothing new, and have been in
circulation since James Parkinson mentioned this same infectious cause in An Essay on the Shaking Palsy in which he was the first to
describe “paralysis agitans”, a condition that would later be renamed Parkinson’s disease by Jean-Martin Charcot. Here we review
further evidence in support for this particular infectious cause of Parkinson’s.
Keywords: Parkinson’s disease; James Parkinson; History of Parkinson’s disease; etiology of Parkinson’s disease; Infectious cause of
Parkinson’s disease; Anti-tubercular drugs used in Parkinson’s disease; the Actinomycetales; the Mycobacteria; scrofula; Mycobacterium
tuberculosis; substantia nigra; An Essay on the Shaking Palsy
Introduction
Recently, in the Introduction to their July, 2017 Norwegian study Parkinson’s disease: the hibernating spore hypothesis [1], Berstad and Berstad said this:
“In 2002, Broxmeyer concluded in his paper ‘Parkinson’s: another look’ [2]:. . .the preponderance of convincing evidence,. . . points to a chronic infectious cause, not viral and most likely of the family Actinomycetales …’. The aim of the present report is to support and revitalize Broxmeyer’s hypothesis.”
But was this hypothesis new? Historical reference reports differently.
As James Parkinson himself pondered the cause of the disease that would eventually be named after him in his Essay on the Shaking Palsy, he said this: “But taking all circumstances into due consideration, particularly the very gradual manner in which the disease commences, and proceeds in its attacks; as well as the inability to ascribe its origin to any more obvious cause, we are led to seek for it in some slow morbid change in the structure of the medulla, or its investing membranes, or theca, occasioned by simple inflammation, or rheumatic or scrophulous [tubercular] affection.” [3]
But in doing so, since both inflammation and rheumatic conditions had already been attributed to tubercular scrofula [4], Parkinson was really broaching the possibility that Parkinson’s disease happened as the result of slow morbid tubercular changes to the central nervous system. [4] After all, tuberculosis was already a known cause of palsy. [5]
James Parkinson continued, admitting how little was known about a disease whose tubercle bacilli had yet to be discovered by Robert Koch. Parkinson: “…although satisfied with ascribing those cases to scrofulous [tubercular] action, we are in fact……little informed
respecting the nature of the affection…”
Scrofula is the term generally used for lymphadenopathy of the neck, usually as a result of an infection in the lymph nodes, known as lymphadenitis –the terms “scrofulous,” and “tuberculous” being nearly interchangeable in the past. The particular characteristics associated with scrofula have varied at different periods, but essentially what was meant was tuberculosis of the bones and lymphatic glands. It is in this sense that the word survives. The old English popular name was “king’s evil,” so called from the belief that the sovereign’s touch could effect a cure. When Parkinson, in 1784, was approved by the City of London Corporation as a surgeon, the UK’s John Morley’s Essay on the Nature and Cure of Scrophulous Disorders, Commonly Called the King's Evil was already a widely read publication in its twentieth edition, on its way to a forty-second edition which would be in print by 1824. [4]
James Parkinson (1755–1824) worked as a general practitioner in the semi-urban hamlet of Hoxton, Northeast of the City of London, where he had been born, and where he lived all his life. The historian Roy Porter considered Parkinson a man “with impeccably enlightened credentials,” [6] a doctor with a highly developed empiricist bent, committed to that long British scientific tradition of observation. Therefore, although none of the six cases Parkinson described in his Essay on the Shaking Palsy came to necropsy, yet by careful steps of reasoning he finally conjectured that the illness depended on a diseased state of the high cervical cord, extending to the medulla. Considering the limited scope of neurological knowledge in his day it is astonishing that his deductions were so close to the target zone of the substantia nigra defined by present neuropathological data.
Today, there is mounting evidence that James Parkinson’s hypothesis that Parkinson’s disease was from systemic tubercular infection was correct to begin with.
The very drugs that have been used for Parkinson’s often show anti-tubercular activity. Tolcapone and Entacapone, once widely used to treat Parkinson’s are promising lead compounds against drug-resistant strains of M. tuberculosis. Both Tolcapone and Entacapone have liver toxicity, though entacapone, has much less hepatotoxicity. Entacapone and tolcapone both have inhibitory activity against M. tuberculosis. [7] And interestingly, studies such as Fung’s showed that when patients suffering from Parkinson's disease were treated with rifampin and isoniazid, two first line TB drugs, their condition was observed to improve [8]. Another group of medicines used against Parkinson’s from time to time are the MAO inhibitors. Deprenyl (Eldopril) a Parkinson’s mainstay, comes from a class, the MAO inhibitors originally designed to cure tuberculosis [9]. It was the important Parkinson's DATATOP study (1996) that unwittingly settled upon an agent originally designed to cure tuberculosis, the MAO inhibitor called Deprenyl. [10]
Even by the mid-1930s Burn of Yale University identified a gram-positive bacillus from the bloodstream of three infants that had died from Parkinson's causing von Economo's encephalitis [11], which bore an eerie resemblance to an atypical tubercular mycobacteria, the acid-fast forms of which Jackson [12] at Cornell found in a variety of degenerative diseases including Wilson’s disease, a primary cause of Parkinson's in the young. Independently, Fuente-Aguado and Bordon [13], Kurasawa Ikeda and Inoue [14], Otaki [15] and earlier, Mital, Sarkari and Singh [16] and Solanki and Kothari, [17] all cured symptomatic Parkinson's using anti-tubercular medications with TB drugs that have ever since been the subject of NIH and independent study against Parkinson’s.
Parkinson's and tuberculosis share many common threads; both came into force and were linked with the great Industrial Revolution. The substantia nigra was established as important to Parkinson's through a tuberculous attack on an autopsied patient [21]. Both diseases share a common preferential site of attack, the convexity of the brain. Parkinson's last great epidemic, von Economo's encephalitis, was, according to Hall [18], almost indistinguishable from tuberculosis. Even in AIDS, often associated with Parkinson's, mycobacteria such as tuberculosis, have been reported as the most common central nervous system pathogens in HIV-infected people [62]. Furthermore, many of the patients with either Parkinson's or tuberculosis share the common denominator of a chronic wasting disease, a cachexic eating away of the flesh; and both are primarily diseases of older people with a slight male preference.
Indirect evidence exists in that tuberculosis and Parkinson’s disease are linked by a unique protein. That protein, named “Parkin”, already is the focus of intense investigation in Parkinson’s disease, in which its malfunction is associated with a loss of nerve cells. But Parkin also acts on tuberculosis, triggering destruction of the bacteria by immune cells known as macrophages. This lends itself to the possibility that malfunctioning Parkin can lead to a loss of nerve cells through the inability to destroy tubercular mycobacteria in the macrophage. [19]
We live in an age where science and medicine are encouraged to apply new technology towards older afflictions and thus the rush to surgical and stem-cell correction of Parkinson’s. However, here as elsewhere the roots of the present often lie deep and securely buried in the past. Let us now take a moment to uncover that past.
The Setting
The Industrial Revolution swept through James Parkinson’s (1755-1824) England, and life was different. Crowding and the growth of cities had taken their toll. England as well as Europe was in the grip of a seething caldron of tuberculous (TB) epidemics. Labeled "The captain of all these men of death" by John Bunyon, tuberculosis was rampant. Measured in centuries, wave upon wave of the disease, punctuated by a low decline in one area, only to rise in another, brought England and the Continent to its knees. Although an ancient disease, TB had never really showed its truly devastating side until the Industrial Revolution. England’s wave of the disease began in the 16th century, probably peaking around 1780, while Parkinson was still a pharmacist with medical ambitions. At that point TB was killing one quarter of all adults in England. By the end of the 19th century, there was fear that the disease would destroy the entire civilization of Europe. Seven years before he died, James Parkinson published An Essay on the Shaking Palsy, which became a classic for the disease named after him. This essay, known more for its descriptive qualities than anything else, first used the term "paralysis agitans" to describe tremors which occurred at rest and improved with action.
The Industrial Revolution swept through James Parkinson’s (1755-1824) England, and life was different. Crowding and the growth of cities had taken their toll. England as well as Europe was in the grip of a seething caldron of tuberculous (TB) epidemics. Labeled "The captain of all these men of death" by John Bunyon, tuberculosis was rampant. Measured in centuries, wave upon wave of the disease, punctuated by a low decline in one area, only to rise in another, brought England and the Continent to its knees. Although an ancient disease, TB had never really showed its truly devastating side until the Industrial Revolution. England’s wave of the disease began in the 16th century, probably peaking around 1780, while Parkinson was still a pharmacist with medical ambitions. At that point TB was killing one quarter of all adults in England. By the end of the 19th century, there was fear that the disease would destroy the entire civilization of Europe. Seven years before he died, James Parkinson published An Essay on the Shaking Palsy, which became a classic for the disease named after him. This essay, known more for its descriptive qualities than anything else, first used the term "paralysis agitans" to describe tremors which occurred at rest and improved with action.
As Jankovic and Tolosa [20] point out, modern conceptualization of Parkinson's only really began with Blocq and Marinesco's historical 1893 watershed, in which their "Temblement Parkinsomen Hemiplegique" spoke of a tubercular mass (tuberculoma) lodged in a 38-year-old parkinsonian man's midbrain [21]. It was this study that led to the finding that two small linear arrangements on the bottom of the brain, the substantia nigra, once damaged, could lead to Parkinson's. The size and shape of a filbert nut, the tuberculoma, lodged in the patient's right midbrain had destroyed his substantia, effectively leaving him with left-sided Parkinson's from the crossover that nerves take on their way from the brain to the spine. As Blocq and Marinesco autopsied, Charcot, the patient’s legendary physician, filled them in on clinical details. The man had come to Charcot with left-sided muscle rigidity and dull lower back pain. As the rigidity slowly worsened, tremors began and progressed. Soon the fingers of his left hand became half-flexed and glued together. He walked with small irregular steps, his left arm close to his body. His left lower leg experienced intermittent pain and trembled, especially with fatigue. Bent forward and rigid, he was the picture of Parkinsonism. Weak, he lost noticeable weight over a few months, then suddenly, died – from pulmonary tuberculosis. Upon autopsy, and before uncovering the tubercular brain mass, Blocq opened the deceased’s chest.
The vertebral portions of his 1st and 2nd ribs were completely eaten away as a result of spread from his tubercular lung. Next, this man's cranial cavity at first appeared normal. But Marinesco noticed an increase in the right peduncle, a bundle of nearby neurons, accompanied by gross attack on the substantia nigra. Inside the peduncle lay the tuberculous mass. Was this mass a coincidence or had it caused the Parkinson’s? The pathologists doubted that it was a coincidence. Not only had Charcot related a second case, with a similar tubercular brain mass (Blocq and Marinesco, 1893) but Mendel [22] of Germany spoke of a 4½ -year-old Parkinsonian child with right arm tremors, right leg weakness and moderate facial paralysis involving two cranial nerves. Again, this child died of tuberculosis and at autopsy, a tuberculoma, this time the size of an almond, was lodged in the left peduncle of her tiny midbrain. Blocq had also read Kraft-Edding's (Blocq, 1893) study, in which a tuberculous mass of the midbrain also led to Parkinson's. And so, for his student thesis, Bechet [23] described Blocq and Marinesco's work and at the same time could not help thinking of the long and tortuous struggle to prove that TB was a contagious disease and not just contracted from some environmental toxin. It was a road similar to the one Parkinson's would have to travel.
White Plague
British researcher William Stark (1765), carefully couching his words "consumption" or "phthisis"(Greek for a consumptive), described how, after a flu-like lung involvement, TB could and was carried by the bloodstream to various parts of the body, including the brain. It could then reactivate, even decades later. These were the “little lumps” he uncovered on autopsy after autopsy [24]. Stark thus anticipated the famed Laennec, contemporary of Parkinson. But Stark's words fell on deaf ears, only to become a curious controversy among students of various countries throughout Europe. Among these students was Bechet (1771-1882), a French pathologist, who had autopsied countless tuberculosis patients before he died of the disease at the age of 31, uttering: "Not much is really known about the pathology of phthisis (tuberculosis), for few autopsies have been done because of the foolish fear among physicians that the disease is catching." (Gordon, 1944). His early death proved otherwise. His mentor and teacher, Corvisart (1755-1821), wrote to Napoleon: "Bechet has just fallen on a battlefield which numbers more than one victim: no one has done so much and as well in so short a time" [24].
British researcher William Stark (1765), carefully couching his words "consumption" or "phthisis"(Greek for a consumptive), described how, after a flu-like lung involvement, TB could and was carried by the bloodstream to various parts of the body, including the brain. It could then reactivate, even decades later. These were the “little lumps” he uncovered on autopsy after autopsy [24]. Stark thus anticipated the famed Laennec, contemporary of Parkinson. But Stark's words fell on deaf ears, only to become a curious controversy among students of various countries throughout Europe. Among these students was Bechet (1771-1882), a French pathologist, who had autopsied countless tuberculosis patients before he died of the disease at the age of 31, uttering: "Not much is really known about the pathology of phthisis (tuberculosis), for few autopsies have been done because of the foolish fear among physicians that the disease is catching." (Gordon, 1944). His early death proved otherwise. His mentor and teacher, Corvisart (1755-1821), wrote to Napoleon: "Bechet has just fallen on a battlefield which numbers more than one victim: no one has done so much and as well in so short a time" [24].
The Emperor later ordered a bust of Bechet placed in the Hotel Dieu. Corvisart's utterance might have well been the understatement of the millennium, for by the first half of the 20th century, tuberculosis had already killed five million in its upward spiral. In the 100 years from 1850-1950, it was estimated that 1 billion persons died from tuberculosis [25]. Today, the World Health Organization estimates that one third of the world's population, or 1.86 billion people are infected [26]. At about the same time as Bechet, Frenchman Gaspard Laurent Bayle (1810) wrote in “Recherches sur la phithisis pulmonale,” describing "millet seed" bodies in phthisis as “tubercles." Bayle, a seasoned pathologist, also said phthisis was an infectious disease. Bayle himself died of tuberculosis in 1816 at the age of 42. But it was his junior collaborator, Rene Laennec (1781-1826), whom Parkinson knew, who would indelibly stamp on the Western mind that Stark was right: Not only was tuberculosis infectious, but its tubercles could be found in the lungs, skin, intestines, bones, brain, eyes, mucous membranes and literally every tissue of the body. Indeed, the basis of all modern knowledge regarding tuberculosis came from Laennec (Gordon, 1944), except for identifying the tuberculosis bacillus. Three years after James Parkinson died, tuberculosis recognition was greatly advanced by Villemin (1827-1892). Villemin demonstrated the infectious nature of the disease by injecting tuberculous tissue into rabbits. He, too, however, was unable to identify its causative factor. Flick (1937) reported that von Baumgarten observed migratory cells congregating around tuberculosis inoculated into the anterior chambers of the eyes of rabbits. But it was Koch (1843-1910) who stirred and stunned the scientific world of his day by announcing his discovery of a living a microorganism, a tubercle bacillus, in sections of tuberculosis taken from the body of a young worker [27]. Although Koch proved his accuracy by inoculating guinea pigs, as an unknown, he would have a difficult task in convincing his colleagues of his momentous discovery. In the end, though, Robert Koch prevailed, one of the great scientists of his or any age.
In an attempt to correlate Blocq and Marinesco’s work on Charcot’s Paris patient, Bechet found many reasons to suspect Parkinson’s developing in a TB patient. But occupying more of his attention was that tuberculosis of the spine (Pott’s disease) explained the patient’s lower back and leg pain. Blocq and Marinesco held firm –although Pott's disease might have accounted for the lower back pain, it was the brain mass destroying the substantia that led to the bulk of the patient's symptoms, which were mainly Parkinson's. History would bear out Blocq, but not for some years.
The Fibrinous Veil
Parkinson's disease results from an attack on certain structures at the bottom or base of the brain. If the brain were lifted from the top of the head like a helmet and placed on a table directly in front of us with its inside facing up, we would clearly notice the graded indentation in its center, marked by two diagonally linear lines of dark pigmentation, the substantia nigra; just millimeters below this lie structures of the basal ganglia. Parkinsonism, with its rigidity, resting tremor, mask-like facies and shuffling gait is associated with degeneration in the substantia, the basal ganglia, and the nearby cranial nerves. Significantly, the area of preference that tuberculosis shows in attacking the brain is the same basal or bottom portion of the brain, also known as the convexity of the brain. No other known disease has this exact basal area of predilection, which appears, when infected, like a cheesy, fibrinous veil.
Parkinson's disease results from an attack on certain structures at the bottom or base of the brain. If the brain were lifted from the top of the head like a helmet and placed on a table directly in front of us with its inside facing up, we would clearly notice the graded indentation in its center, marked by two diagonally linear lines of dark pigmentation, the substantia nigra; just millimeters below this lie structures of the basal ganglia. Parkinsonism, with its rigidity, resting tremor, mask-like facies and shuffling gait is associated with degeneration in the substantia, the basal ganglia, and the nearby cranial nerves. Significantly, the area of preference that tuberculosis shows in attacking the brain is the same basal or bottom portion of the brain, also known as the convexity of the brain. No other known disease has this exact basal area of predilection, which appears, when infected, like a cheesy, fibrinous veil.
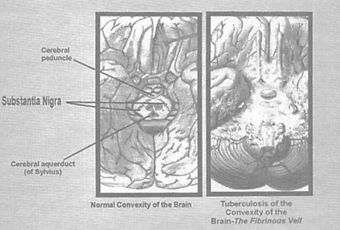
Figure 2: The picture on the right shows the cheesy fibrinous veil sitting on top of the normal
convexity of the brain. Note how the substantia nigra is covered by this infectious residue.
In Pathologic Basis of Disease, Robbins [28] used the term "diffuse chronic meningoencephalitis" as the most common pattern of tubercular involvement of the brain. Robbins acknowledged that its symptoms could often take the form of encephalitis. Indeed, symptoms here varied greatly, ranging from simply a chronic or intermittent headache with subtle mental changes to full-blown meningitis. In most cases the original infection that leads to central nervous system tuberculosis is assumed to have been present since the time it was seeded through the blood into the brain during a flu-like illness, perhaps in childhood, called primary pulmonary tuberculosis. Such a condition is commonly misdiagnosed as the flu. The intense inflammatory reaction at the base of the brain, sometimes reactivating years later, can also lead to stroke through occlusion of blood vessels, as well as movement disorders, sleepiness or lethargy. Also, through direct compression of the cranial nerves, including not only the facial nerve, which controls expression, but cranial nerves 3, 4 and 6, – which control ocular movement, other Parkinson’s manifestations can result. All of these are well documented in central nervous system (CNS) tuberculosis as are the parkinsonian-like movement disorders which can result as the disease attacks the substantia and nearby basal ganglia. In fact, as we shall soon see, every symptom related to Parkinson's or Parkinsonism finds its counterpart in TB of the CNS.
It had been many years since Blocq and Marinesco's (1893) landmark study when Dr. Levy-Valensi admitted a 22-year-old glassblower for pulmonary tuberculosis to the French hospital Brevannes on January 26th, 1927 [29]. After several months of pregnancy, the young woman experienced considerable weight loss. TB of both lungs was clinically confirmed on December1926, accompanied by high temperature and followed by severe motor problems to the point where she could hardly leave her bed. She complained that it seemed that her weakness stemmed from a rigidity of her lower extremities. The patient had no signs of meningitis. [29] Nevertheless, when he first admitted her, he saw a very sick woman with pallor, cyanosis of the lips and nose and with extreme difficulty breathing. With his stethoscope, Levy-Valensi identified the soundless cavities of tuberculosis in both lungs. Her neurologic exam showed decreased muscle strength, mostly left sided. She had no Babinski or Kemig's signs which would have indicated possible meningitis. Yet something was wrong, as typified by the way her eyes stared straight ahead without expression and her arms hung close against her body when standing, both characteristic of Parkinson's disease. In addition, she demonstrated the extreme rigidity typical of advanced Parkinson's. On the other hand the patient had no trouble with coordination or equilibrium and suffered no tremors of the hand. All the same Levy-Valensi felt there was central nervous system involvement and asked for a spinal tap, which the patient refused. A certain period of improvement followed, but by February 12, eighteen days after entering the hospital, she became worse and died of pulmonary tuberculosis at a time when her motor problems from Parkinson's were actually temporarily regressing. Autopsy showed extensive tuberculosis in both lungs. In the brain, a fibrinous veil lay over almost the entire bottom or convexity of her brain.
Tuberculosis Encephalitis
In 1936, I. Scheinker [30] of Vienna wrote of a case of tuberculosis encephalitis, an inflammation of the brain, presenting as Parkinsonism. In that case a 48-year-old woman appeared lethargic, with a general lessening of affect and lack of spontaneity. In another era this woman would have been quickly diagnosed as having von Economo's disease; a deadly epidemic that swept Europe in the early 1900's, thought to be caused by a virus and which in many cases caused Parkinsonism. Working out of his Vienna office, Scheinker continued to describe his patient's Parkinson's-like symptoms, which included a rigidity affecting total body movement, a fixed facial expression, small steps, a slow walk and a stiff posture. In addition, the woman had retropulsion, an involuntary walking backwards, occurring in some patients with Parkinsonism. Yet in the end she proved to have tuberculosis encephalitis without pus, a little-discussed possibility for the epidemic encephalitis which just 10 years previously had swept the world. And just as surely as von Economo's disease, also known as encephalitis lethargica, killed up to 35% of those infected, this woman died four months after admission to Scheinker's hospital. Lethargic and sleepy, she became apathetic before death to the point where she could not even speak. A mask-like face, and resting tremors in both arms all were present. Scheinker (1936) did a spinal tap, but her spinal fluid was completely normal. Waxing and waning continued.
In 1936, I. Scheinker [30] of Vienna wrote of a case of tuberculosis encephalitis, an inflammation of the brain, presenting as Parkinsonism. In that case a 48-year-old woman appeared lethargic, with a general lessening of affect and lack of spontaneity. In another era this woman would have been quickly diagnosed as having von Economo's disease; a deadly epidemic that swept Europe in the early 1900's, thought to be caused by a virus and which in many cases caused Parkinsonism. Working out of his Vienna office, Scheinker continued to describe his patient's Parkinson's-like symptoms, which included a rigidity affecting total body movement, a fixed facial expression, small steps, a slow walk and a stiff posture. In addition, the woman had retropulsion, an involuntary walking backwards, occurring in some patients with Parkinsonism. Yet in the end she proved to have tuberculosis encephalitis without pus, a little-discussed possibility for the epidemic encephalitis which just 10 years previously had swept the world. And just as surely as von Economo's disease, also known as encephalitis lethargica, killed up to 35% of those infected, this woman died four months after admission to Scheinker's hospital. Lethargic and sleepy, she became apathetic before death to the point where she could not even speak. A mask-like face, and resting tremors in both arms all were present. Scheinker (1936) did a spinal tap, but her spinal fluid was completely normal. Waxing and waning continued.
For a brief time her fever lessened, and she was tremor-free. But her temperature kept spiking and suddenly, on July 24th, she died. An autopsy was ordered. If this Parkinson's patient had tuberculosis, as Scheinker suspected, it was not to be found in her lungs or the other organs outside her nervous system. Then, in an area of damaged basal ganglia inside the brain, he found what he was looking for: a conglomerate of small knots appeared with a walnut-sized tubercular mass. The lesion reminded him of others he had seen in scarred, healed tuberculosis of the lung, and he began to think of what Simmonds had earlier reported in England. Simmonds had found a similar tubercular mass in the brain of a 71-year-old female with hyaline fibers and a granulomatous mass, also interpreted as scarred tuberculosis. Simmonds spoke of the brain's neurons, called glial cells, being attacked until their central parts were destroyed. (Scheinker, 1936) Again and again Scheinker now probed the substantia nigra, but found few noticeable changes other than a slight widening of the vascular spaces leaving the pigmented substantia. Yet this, along with the more extensive damage to the woman's basal ganglia, had been enough to cause her Parkinsonism. In his review of the literature, Scheinker (1936) cited Nonne, who in 1900 first showed that a tuberculous growth could be responsible for hemorrhaging encephalitis. [31]. Wohwill (1918) further elaborated on several cases, which seemed quite similar to Encephalitis Lethargica, at that time perched to sweep Europe. And Sittig [32] concurrently pointed to the fact that even the smallest glia and ganglions of the brain showed a falling apart under tubercular attack. (Sittig, 1914). Wilder [33] observed Parkinson's in the course of severe lung tuberculosis. After his patient’s death, Wilder's autopsy showed diffuse degenerative changes of the brain's parenchyma with particular preference for the substantia nigra, all caused by a tuberculosis –the gross lesions of which were outside the CNS. (Wilder, 1926) As he finished his autopsy, Scheinker thought about the great epidemic of Encephalitis Lethargica or von Economo's disease, which had first swept Europe, then the world and was now history. Was it really a virus, as some maintained, or was it just a new strain of tuberculosis-like mycobacteria such as the ones, which for centuries had been wreaking havoc on the very same populations?
The Last Great Encephalitis
Around 1917, the last great epidemic of encephalitis, named "Encephalitis Lethargica," struck terror into the hearts of first Europe and then the rest of the world. The disease attacked the brain, leaving some victims in a statue-like condition, speechless and motionless. Between 1915 and 1926, nearly five million people were affected, a third of whom died in the acute stages. And many of those who survived never returned to their pre-existing "aliveness". If it did not immediately kill its victim, Lethargica caused a Parkinsonism characterized by unusual behavior. These odd behaviors included bouts of marching in place followed by running and an oculogyric crisis characterized by wild rotation of the eyeballs. As scientists and doctors pondered its cause and cure, Lethargica, of necessity, sparked a sharp renewal of interest in Parkinson's disease. After all, it was once broadly held that mid-20th century Parkinsonism was almost entirely attributable to this Encephalitis Lethargica epidemic immediately after the First World War. And prior to that event, many authorities described Parkinson's as a rare disease.
Around 1917, the last great epidemic of encephalitis, named "Encephalitis Lethargica," struck terror into the hearts of first Europe and then the rest of the world. The disease attacked the brain, leaving some victims in a statue-like condition, speechless and motionless. Between 1915 and 1926, nearly five million people were affected, a third of whom died in the acute stages. And many of those who survived never returned to their pre-existing "aliveness". If it did not immediately kill its victim, Lethargica caused a Parkinsonism characterized by unusual behavior. These odd behaviors included bouts of marching in place followed by running and an oculogyric crisis characterized by wild rotation of the eyeballs. As scientists and doctors pondered its cause and cure, Lethargica, of necessity, sparked a sharp renewal of interest in Parkinson's disease. After all, it was once broadly held that mid-20th century Parkinsonism was almost entirely attributable to this Encephalitis Lethargica epidemic immediately after the First World War. And prior to that event, many authorities described Parkinson's as a rare disease.
Although the world had never seen anything quite like this type of Encephalitis, also called von Economo's, the disease becomes more understandable when taken into context. The far more serious TB pandemic peaked first in Europe and then in the U.S. by the early 1920's. It is into this arena that the purportedly new epidemic entered. Acting in truly tuberculesque fashion, von Economo's typified the path that a new strain of the mycobacterial killer often takes: striking preferentially among infants and young adults. Doctors sat by helplessly as their youthful populations reeled from the disease. Juvenile Parkinson's, to that date practically unheard of, became commonplace [34]. Lethargica was even said to cause Parkinson’s to some degree in all who survived [35] and at one time was more common than Parkinson's itself [36].
Were it not for von Economo's penchant for causing Parkinson's, it would have quietly slipped into being just another footnote in the history of unknown epidemics. The exact cause of the Encephalitis was never sorted out, though occasional European and Russian pockets still persist [37]. Years ago in an attempt to round up the usual suspects, some unknown toxin or perhaps virus was singled out. The fact that von Economo's was contagious could not be denied. Yahr [38] pointed out that an almost concomitant influenza epidemic was suspected, but most serious studies found little basis for the link, which was at best speculative. Von Economo himself pointed to the fact that he had isolated cases of Lethargica eighteen months before Europe's influenza epidemic even began. Never seeming to affect two patients the same way, von Economo's could happen suddenly or gradually, with flu-like symptoms before or after Parkinsonism. Classically, the disease began as fever, sleepiness or stupor with ocular disturbances –but many other symptoms were possible.
In the U.S., then in the midst of its own von Economo's plight, investigators at the Association for Research in Nervous and Mental Diseases (ARNMD) met urgently in New York on December 28, 1920. [39] Concluded was that although the cause of Lethargica was beyond medical grasp, with very few exceptions, it could be differentiated from other diseases. But first among these few exceptions was tuberculosis of the CNS (Central Nervous System). Though the ARNMD pointed out that the spinal fluid picture in von Economo's and tuberculosis were identical except for their sugar content, it was later realized that this was indeed not always the case. Subsequent publications, such as Kennedy’s [40] large study, showed that greater than 83% of cases of TB meningitis could present with cerebrospinal sugars of greater than 45 mg/100. Normal sugar values were also found in similar cases by Smith [41], Hinman [42], Sumaya and Simek [43], Lincoln [44] and Virmani and Geeta [45].
Furthermore, as Yahr [38] pointed out, although the sugar content of the cerebrospinal fluid (CSF) was reported elevated in a fair number of von Economo's cases, these were not done in conjunction with blood sugars, so that such elevation was all but meaningless. The first spinal tap itself was only performed in 1918, at a time when the technology for a long, thin, hollow bore steel needle was in its infancy. Fuente-Aguado and Bordon [13] made it clear that the wide clinical spectrum of CNS TB includes simply a cerebritis or inflammation of the brain associated with chronic headaches, with or without meningitis. And only a relatively small number of cases break through to involve the spinal fluid, a precondition for low spinal sugar. In fact, during the pandemic Encephalitis Lethargica was frequently diagnosed by a mere history and physical, with either the immediate or stalled appearance of Parkinson's up to 15 years later [46].
Blunt and Lane's more recent sporadic cases not only showed normal spinal sugar but further put a viral theory for von Economo's to rest by reporting negative serology in both blood and spinal fluid for adenovirus, influenza A and B, mycoplasma, Q fever, cytomegalovirus, mumps, measles, herpes simplex, varicella, coxsackie and even aviral PCR viral polymerase chain reaction (PCR) [46]. Furthermore, no improvement was noted in his cases when put on the antiviral acyclovir. Jancovic [20] cited other viruses in mentioning Japanese B, Western Equine and Coxsackie as being those complicated by Parkinsonism. Lessell [47] though, who did work in Guam, where a Parkinson-dementia complex was prevalent among its indigenous native population called the Chamorros, relates that an epidemic of Japanese encephalitis, which struck the Island in 1948, failed to show any unusual number of Parkinson’s cases in any one year thereafter. (Lessell, 1961) At the same time Andrews noted that tuberculosis meningitis is anything but rare on Guam [48].
Duvoisin and Yahr, after an exhaustive review of the various viruses periodically implicated in Parkinson’s causing von Economo's, stated bluntly: "Except for rare instances of transient Parkinson-like states in the course of various types of viral encephalitis, we found no case of parkinsonism following any type of (viral) encephalitis other than Encephalitis Lethargica (von Economo's disease)" [49]. But the question still lingered as to whether Lethargica itself was caused by a virus. Hall [18] in his authoritative Epidemic Encephalitis, written at a time when the disease had already done extensive damage, insisted that one of the most common difficulties with von Economo's was distinguishing it from CNS (central nervous system) tuberculosis. Hall maintained that the spinal fluid differences in the two conditions are often only slight or inconclusive. As well, Hall remarked that since the site of entry appeared to be through the nasopharynx in epidemic Encephalitis Lethargica – that insect-borne viral diseases, like Japanese B and Eastern equine encephalitis were highly unlikely causes (Hall, 1924). Meanwhile, no single symptom or pathologic finding has emerged on what von Economo's caused that is not found in the literature of tuberculous involvement of the CNS.
Probably the most well-known individual with post-encephalitic von Economo's Parkinsonism was Adolph Hitler. Although Hitler was afraid of cancer, which took his mother's life, in general the Nazi's fear of cancer paled to that of his fear about tuberculosis, the second highest cause of death until the 1920's. Lieberman [50] stated that not only did Hitler have Parkinson's as a result of Encephalitis Lethargica, but also that he became cognitively impaired by it in 1941, changing the entire course of World War II. Deciding to invade the Soviet Union before first defeating England and without the assurance of Japan concomitantly attacking Russia, Hitler, by June of 1941, effectively placed himself in the double jeopardy of a two-front war. Then, indecisive and confused, the Nazi dictator stopped von Bock's victorious Panzer thrust dead in its tracks, just as this general’s divisions were about to advance upon and capture Moscow, an offensive which conceivably could have dealt Russia a fatal blow (Lieberman,1999). But a closer look at Hitler's medical records reveals more than mere von Economo's. At 16 he suffered a severe respiratory illness involving both lungs, followed by a prolonged convalescence in which he remained weak for a month or two, only slowly recovering. As Heston, Leonard and Heston [51] remarked, this illness had the main features of tuberculosis, and since it was contracted in 1905, this would have given the disease 13 years to fester and reactivate, this time involving the CNS. Thus, the many pictures capturing the Nazi dictator covering one hand with the other in an effort to momentarily stop or lesson the tremors in his arms and hands could quite easily have come from tubercular involvement of the CNS.
Behind The Frozen Addicts
Parkinsonism is distinct from Parkinson's disease in that with our present state of knowledge, a variety of chemical, biologic and degenerative conditions are thought to cause Parkinsonism. Whether in the future these various agents prove to be only contributory to an underlying degenerative disease remains to be seen. An example of Parkinsonism can be seen in patients taking certain drugs that act on the nervous system, such as chlorpromazine, a major tranquilizer that causes drug-induced Parkinsonism. In fact, even manganese has been noted to cause "miner's parkinsonism," first described in South America and later in certain U.S. locales. (Caine and Chu, 1994) Yet even this type of Parkinsonism was effectively treated by the anti-tubercular drug PAS or p-aminosalicylic acid. (Jiang, 2006). Manganese supposedly acts by destroying the dopamine producing cells of the substantia in much the same way as the "designer drug" 1-methyl-4-phenyl-1,2,3,6-tetrahydro-pyridine (MPTP). But as we shall see, manganese does much more. Fortunately rare, MPTP Parkinsonism was dramatized as part of the public television series NOVA in "The Frozen Addicts." [52]
Parkinsonism is distinct from Parkinson's disease in that with our present state of knowledge, a variety of chemical, biologic and degenerative conditions are thought to cause Parkinsonism. Whether in the future these various agents prove to be only contributory to an underlying degenerative disease remains to be seen. An example of Parkinsonism can be seen in patients taking certain drugs that act on the nervous system, such as chlorpromazine, a major tranquilizer that causes drug-induced Parkinsonism. In fact, even manganese has been noted to cause "miner's parkinsonism," first described in South America and later in certain U.S. locales. (Caine and Chu, 1994) Yet even this type of Parkinsonism was effectively treated by the anti-tubercular drug PAS or p-aminosalicylic acid. (Jiang, 2006). Manganese supposedly acts by destroying the dopamine producing cells of the substantia in much the same way as the "designer drug" 1-methyl-4-phenyl-1,2,3,6-tetrahydro-pyridine (MPTP). But as we shall see, manganese does much more. Fortunately rare, MPTP Parkinsonism was dramatized as part of the public television series NOVA in "The Frozen Addicts." [52]
The positive function of MPTP seemed to be as method to induce Parkinsonism in test animals, then given various anti-Parkinson's agents. Thoughts that environmental toxins might be responsible for Parkinsonism, centuries old, are still very much with us and led to the 1996 use of Deprenyl and tocopherol as a treatment for Parkinson's disease (DATATOP) study [10]. Here, patients either received combination Deprenyl and tocopherol (vitamin E) or a placebo tablet with nothing in it. Researchers reasoned that the combined use of a monoamine oxidase (MAO) inhibitor, which is what Deprenyl (also called Eldepryl) is, and a free-scavenger like vitamin E might prevent some unknown toxin similar to MPTP from destroying dopamine-producing cells. But the patients receiving the MAO inhibitor (Deprenyl) and the vitamin did so much better that the study had to be called to an early halt. For a while, it seemed that the combination was not only increasing serum dopamine and reducing symptoms, but might even be halting Parkinson’s progression altogether. Apparently overlooked, however, was that MAO inhibitors were first introduced as anti-tuberculous medicines in the 1950's. Then, when it became evident that these inhibitors improved the moods of TB patients, their use was expanded into psychiatry. By 1951, a tuberculosis drug milestone called isoniazid was discovered along with its isopropyl twin iproniazid [9]. Soon, it was evident that iproniazid was mood enhancing and Zeller [53] found that iproniazid acted by inhibiting the enzyme MAO in humans. Subsequent studies [54], put iproniazid on the map, but within psychiatry's borders. DATATOP might have expanded MAO use into Parkinson’s when it became obvious that it had some activity against the disease, but it failed to notice the direct link between its main ingredient, an MAO inhibitor, and the specific infectious disease with which MAO inhibitors were originally designed to deal with.
Independent Support
By the 1990’s, the most convincing animal models to that date for a bacterial cause of Parkinson's were done, on murine models. Kohbata and Beaman [55], seemingly cemented a relationship between what appeared to be nocardia and Parkinson’s by detecting antibodies to tuberculosis' first-cousin in 20 of 20 patients with Parkinson’s. But so close are tuberculosis and nocardia that attempts to devise a blood test for nocardia were repeatedly stifled because the test also proved positive for mycobacteria such as TB and leprosy [56]. And a well-known medical school text on microbiology points towards other problems in the differentiation of the nocardia from tubercular mycobacteria [57]:
By the 1990’s, the most convincing animal models to that date for a bacterial cause of Parkinson's were done, on murine models. Kohbata and Beaman [55], seemingly cemented a relationship between what appeared to be nocardia and Parkinson’s by detecting antibodies to tuberculosis' first-cousin in 20 of 20 patients with Parkinson’s. But so close are tuberculosis and nocardia that attempts to devise a blood test for nocardia were repeatedly stifled because the test also proved positive for mycobacteria such as TB and leprosy [56]. And a well-known medical school text on microbiology points towards other problems in the differentiation of the nocardia from tubercular mycobacteria [57]:
The mycobacteria and nocardioforms have traditionally been considered as related to the coryneforms and/or filamentous actinomycetes, and the separation between mycobacteria, corynebacteria and the various pleomorphic (many-formed) bacteria is not easy. Different observers may classify the same strain as belonging to the genera corynebacterium, arthobacter, nocardia or mycobacterium (Atlas, 1988)
So when Kohbata revisited his initial study, this time with Shimokawa, they purposely used the term "nocardia related organisms," to include mycobacteria, acknowledging that their positive antibody blood titers could also signify previous infection with tuberculosis [58]. Meanwhile, Gao and Raine [59] proved through the use of mycobacterial heat shock proteins against the blood of two Parkinson’s patients that it was nocardia's close relative, mycobacteria like TB, that were somehow linked to Parkinson’s.
A Linkage Through Cure
Mital, Sarkari and Singh [16] of the GSVM. Medical College in Kanpur, India published two cases of T.B. meningitis with Parkinsonism in which Parkinson's symptoms almost completely disappeared during anti-TB therapy. In their first case, a 19-year-old farmer, seen six weeks after hospitalization, went from full-blown Parkinson’s towards a tremendous improvement in both his gait and tremors when put on antibiotics designed to combat tuberculosis. He was then lost to further follow-up. A second case, involving a 25-year-old female, completely lost her tremors, parkinsonian facies and rigidity, again on anti-TB drugs. Mital, Sarkari and Singh's conclusion: Not only did these cases illustrate that Parkinson's could come from CNS tuberculosis, but this conclusion was reinforced by the fact that Parkinsonism almost completely disappeared through the use of anti-TB drugs.
Mital, Sarkari and Singh [16] of the GSVM. Medical College in Kanpur, India published two cases of T.B. meningitis with Parkinsonism in which Parkinson's symptoms almost completely disappeared during anti-TB therapy. In their first case, a 19-year-old farmer, seen six weeks after hospitalization, went from full-blown Parkinson’s towards a tremendous improvement in both his gait and tremors when put on antibiotics designed to combat tuberculosis. He was then lost to further follow-up. A second case, involving a 25-year-old female, completely lost her tremors, parkinsonian facies and rigidity, again on anti-TB drugs. Mital, Sarkari and Singh's conclusion: Not only did these cases illustrate that Parkinson's could come from CNS tuberculosis, but this conclusion was reinforced by the fact that Parkinsonism almost completely disappeared through the use of anti-TB drugs.
A study by Otaki, at Ichikawa and Oizumi [15] at the Kirume University in Japan showed similar marked improvement in a 79-year-old man placed on anti-tuberculous treatment after his upper right lung collapsed from the disease. Fuente-Aguado and Bordon [13], working out of the Infectious Disease Unit of Hospital Xeral in Bigo, Spain, treated a case of parkinsonism associated with tuberculous meningoencephalitis with TB medications. Seven days after starting tuberculous therapy, signs of Parkinsonism disappeared entirely, accompanied by overall improvement in the patient's clinical picture. Fuente-Aguado and Bordon's conclusion: The extinction of Parkinsonism after tuberculosis therapy supported tuberculosis as a cause of Parkinsonism. Solanki and Kothari [17] reported two cases of full-blown Parkinsonism developing in a 30-year-old Indian housewife and a 32-year-old male laborer, both of whom were being treated for CNS tuberculosis and both of whom lost their Parkinson's with continued anti-tubercular medicines. Concurrently, Kurasawa, Ikeda, and Inoue [14], puzzled by a lung mass in a 71-year-old Parkinsonian man's chest, thought it to be malignant. But when it proved mycobacterial instead and the patient was put on two first-line drugs for tuberculosis, the patient lost his Parkinson's –and two years later remained without it.
AIDS and Parkinson's
Fully one-third of the world's populations, 2 billion people, are now infected with tuberculosis [60]. Jancovic and Tolosa [20] pointed out that today possibly the most common cause of infectious Parkinsonism is HIV itself or an AIDS-related opportunistic infection. But it has become increasingly difficult altogether to untie CNS AIDS from tuberculosis and its related Actinomycetales. Harrison [61] pointed out that CNS tuberculosis develops especially in adults infected with HIV. As of that publication, an estimated 8.5 million persons were infected with both HIV and TB at the same time. In New York City alone the rate of HIV-positive blood tests in TB patients is 50% and clearly, the upswing in TB from 1985 to the present has been linked to the AIDS epidemic. Not only is TB now considered an AIDS-reportable disease, but also Berenguer and Moreno [62] reported that there is reason to believe that Mycobacteria tuberculosis is the commonest CNS pathogen in HIV-infected people. And although other AIDS-related opportunistic infections can cause CNS involvement, in Bishburg., et al.'s [63] study, TB preceded these infections by from one to 10 months. Nor is tuberculosis the only mycobacteria capable of attacking HIV patients.
Fully one-third of the world's populations, 2 billion people, are now infected with tuberculosis [60]. Jancovic and Tolosa [20] pointed out that today possibly the most common cause of infectious Parkinsonism is HIV itself or an AIDS-related opportunistic infection. But it has become increasingly difficult altogether to untie CNS AIDS from tuberculosis and its related Actinomycetales. Harrison [61] pointed out that CNS tuberculosis develops especially in adults infected with HIV. As of that publication, an estimated 8.5 million persons were infected with both HIV and TB at the same time. In New York City alone the rate of HIV-positive blood tests in TB patients is 50% and clearly, the upswing in TB from 1985 to the present has been linked to the AIDS epidemic. Not only is TB now considered an AIDS-reportable disease, but also Berenguer and Moreno [62] reported that there is reason to believe that Mycobacteria tuberculosis is the commonest CNS pathogen in HIV-infected people. And although other AIDS-related opportunistic infections can cause CNS involvement, in Bishburg., et al.'s [63] study, TB preceded these infections by from one to 10 months. Nor is tuberculosis the only mycobacteria capable of attacking HIV patients.
A University of Texas study went so far as to conclude that most, if not all, HIV patients who did not die from another HIV-related event would eventually become infected with Mycobacterium avium-intercellulare (MAI), formerly called avian or fowl tuberculosis [64]. Jacob and Henein [65], writing about CNS involvement with nontuberculous mycobacteria such as MAI, concluded that MAI should be strongly considered as a serious CNS pathogen in any patients with AIDS. LeBlang, Whitman and Post [66], broadened the discussion by seeing another Actinomycetales, tuberculosis-like nocardia in CNS AIDS. Under-reported and often unrecognized, these researchers noted that since nocardia, unlike TB, is presently not an AIDS-defining illness; its true incidence is not really known. However, a study cited in Javaly's [67] review, in which 42% of nocardia-infected HIV patients had nocardia as their initial CNS opportunistic infection, brings to mind Bishburg., et al.'s [63] similar findings with tuberculosis which seemed to precede other infections from one to 10 months –again prompting the possibility that in certain cases, investigators could have actually been looking at evolutionary nocardial/tuberculosis crosses or simply typical or atypical tubercular organisms that they were erroneously interpreting as nocardia.
Fuente-Aguado and Bordon [13] pointed out that CNS tuberculosis is relatively frequent in HIV patients in Spain, as in much of the rest of the world. One of his patients, a 49-year-old, had flexed posture, a shuffling gait, muscular rigidity, and slow movements. In short, he was the picture of Parkinsonism, complete with a Meyerson's sign (inability to inhibit blinking by tapping the muscle on the forehead, the glabella, between the eyebrows). Although this patient denied any previous HIV exposure, blood tests proved HIV-positive. Fuente-Aguado and Bordon requested a spinal tap, and tuberculosis was found. By the tenth day after admission, just seven days after anti-tuberculous antibiotics were started, his signs of Parkinsonism disappeared.
Smoke and Mirrors
Stephenson's [68] article connected home pesticide use to Parkinson's based on a Stanford study fueled by previous research finding an increased incidence of Parkinson's in city dwellers who gardened as a hobby. This Stanford study seemed on target, as many pesticides are indeed neurotoxic. However, as Miami Parkinson's expert, Koller cautioned: "The whole concept is tantalizing, but there's never been a single agent or even a class of agents that have been identified as a risk factor" [68]. In the meantime, researchers' interest gelled on finding some inherited predisposition towards certain environmental toxins, such as pesticides, that lay at the root of the problem. Indeed, since the 200 hundred years since James Parkinson's "Essay on the Shaking Palsy," a mind-boggling number of infectious, pharmacologic, industrial and degenerative causes have, on the surface, been implicated in the disease he first documented. And in few instances was the warning of researcher James Young more applicable. Young said this [69]:
Stephenson's [68] article connected home pesticide use to Parkinson's based on a Stanford study fueled by previous research finding an increased incidence of Parkinson's in city dwellers who gardened as a hobby. This Stanford study seemed on target, as many pesticides are indeed neurotoxic. However, as Miami Parkinson's expert, Koller cautioned: "The whole concept is tantalizing, but there's never been a single agent or even a class of agents that have been identified as a risk factor" [68]. In the meantime, researchers' interest gelled on finding some inherited predisposition towards certain environmental toxins, such as pesticides, that lay at the root of the problem. Indeed, since the 200 hundred years since James Parkinson's "Essay on the Shaking Palsy," a mind-boggling number of infectious, pharmacologic, industrial and degenerative causes have, on the surface, been implicated in the disease he first documented. And in few instances was the warning of researcher James Young more applicable. Young said this [69]:
“Sixty years ago, to claim that the specific disease, tuberculosis of the lungs had a multiplicity of direct causes was in keeping with the knowledge of the period. Thus it was believed that the inflammation of a bronchitis, the irritation of the inhaled fragments of stone with which this mason worked, etc., were each one a direct 'cause' of pulmonary tuberculosis, and that there were many other causes as well –for example heredity, unhealthy environment, etc. Time has shown that the greatly different direct irritants and the other indirect agencies operate in one common way –by increasing cell and tissue susceptibility to the one common factor, the tubercle bacillus. In many other cases the history of medical progress has shown that, where several causes have been advanced to account for a disease, these have proved to be nothing more than factors preceding, and predisposing to, the single common agent.” (Young, 1924)
As Jancovic and Tolosa [20] relate, if Parkinson’s was from a genetic cause, it would cluster primarily within at-risk gene lineage, but this in general has not been the case. Studies of twins, in which twins from a single egg show no higher a rate of Parkinson's than those from two eggs, argue against a genetic basis for Parkinson’s. Lux and Kurtske [70] saw evidence from a wide variety of sources that Parkinson’s is an acquired disorder, as by infection and that twin studies only strengthen this notion. Moreover, Betemps and Buncher [71] saw twin studies in Parkinson's as altogether ruling out a hereditary process. Deadly copper-hoarding Wilson's disease is thought to be a hereditary autosomal recessive condition. A major player in Parkinson's before 40-years-of-age, almost half of Wilson's victims present with Parkinson's [72]. But Alexander-Jackson [12], isolated acid-fast forms from Wilson's and felt that mycobacterial infection, not heredity, was behind the "genetic changes" on Wilson's 13Q chromosome.
Furthermore, most of the single-gene mutations found more commonly in Parkinson's code for metabolic enzymes. Merril, Geier and Petricciani [73] induced human cells to initiate enzyme synthesis through infectious bacterial gene change. Validating Morse and Lederberg's [74] earlier work, which showed that the genetic code is truly universal and that genetic changes in human cells could come about by bacteria and their phage viruses within, the work of Merril et al. stood as a landmark in the age-old argument of environment or infection versus heredity.
Studies globally identified rural living, gardening, pesticide use and well water as Parkinson's risk factors. But Koller and Veters-Overfelt's Kansas study, having found rural living and well water as significant, found the largest differences in the first decade of life as becoming increasingly smaller, until no differences in these risk factors were found by the fifth decade [75]. Hubble and Cao [76] thought that they found nocardia blood seropositivity especially high in older subjects from Kansas, which might suggest that nocardia exposure increases with age and rural residency; however, Mycobacteria kansii is also prevalent in the soil of these same areas. And rural living affords closer exposure to these soils. Insofar as those who would link rural living to increased chemical exposure as a cause, Jancovic and Tolosa [20] noted this proximity could easily reflect exposure to an infectious agent rather then chemicals.
Gorell and Johnson [77] documented that prolonged occupational exposure to copper, manganese and iron are all associated with Parkinson’s. But David [78], an expert on mycobacteria, pointed out that mycobacterial cell mass can be greatly increased in the lab by adding any of these agents to existing mycobacterial culture medium.
Martyn and Osmond [79] found Parkinson’s linked more to early diphtheria than with control studies. But Corynebacterium diphtheria, long associated with Parkinson's, is closely related to the mycobacteria, sharing many properties and even exchanging genetic material through common phage transfer [80]. On a biochemical level, oxidative stress has been mentioned as contributing to Parkinson's. The substantia nigra is under triple jeopardy from oxidant stress injury. In the body, tyrosine is first converted to dopamine, itself highly toxic to catecholamine cells [81]. Dopamine, in turn, is metabolized to hydrogen peroxide and free radical byproducts, which again can damage cell components of the substantia [82]. In addition, neuromelanin, found in the substantia's pigmented neurons, contains large amounts of iron [83], which as we have just seen, is a significant growth factor for mycobacteria. Dopamine-derived hydrogen peroxide reacts with this iron generating extremely neurotoxic free hydroxyl radicals. Ben-Shacher and Youdim, [84] by intra-nigral iron injections, induced Parkinson's in rats. But again, just how much of what has been documented in Parkinson’s is secondary to mycobacterial metabolism is open to question. Yet the mycobacteria are strict aerobes, and oxygen must be available as the final electron acceptor. Three distinct respiratory chains have been described, one of which leads to the formation of hydrogen peroxide. The disposal of accumulated hydrogen peroxide is then accomplished by two iron-porphyrin enzymes, catalase and peroxidase, which occur in all mycobacteria, and the products of which release their own free oxygen radicals [78]. In addition, mycobacteria synthesize their own tyrosine, precursor to dopamine in humans [78], and a possible source of "oxidant stress overload" as individual generations of the microbes are replaced during ensuing infection.
The question of how much of the dead mycobacteria, whose lipid content can amount to 40% of their dry weight [85] accounts for the higher levels of lipid peroxidation [86] seen in the Parkinson's substantia – is also open to interpretation, as is whether the increased level of iron found in Parkinson’s substantia [87] could not at least be in part due to mycobacterial iron porphyrin release. Pathologically, in Parkinson's there is an accumulation of melanin containing nerve cells in the brain stem, chiefly in the substantia nigra and the locus coeruleus [88]. Yet again, melanin may be formed biologically in vitro by oxidation of tyrosine or tryptophan, both of which are aromatic amino acids manufactured by the mycobacteria [78].
Fire Surfaces
It might have been coincidental that both Mycobacteria tuberculosis and Parkinson's came into force and were linked with the great Industrial Revolution. And it might be thought unusual that in his An Essay on the Shaking Palsy James Parkinson mentioned scrofular tuberculosis not once, but twice, as a possible causative agent for Parkinson’s disease [3]. It might seem merely fortuitous that Blocq and Marinesco [21] established through tuberculoma attack that the substantia nigra was important to Parkinson's. Similarly, that Mendel [22] established tuberculosis in a parkinsonian child. It could be considered incidental that the preferred site of attack for tuberculous meningoencephalitis is the convexity of the brain, where it covers the substantia, the basal ganglia and has ready access to the cranial nerves. Perhaps even coincidental that it has a propensity to cause the endothelial inflammation and disease that often leads to stroke as well as the ability to choke off the aqueduct of Sylvius, leading to a blockage of cerebrospinal fluid known as hydrocephalus –both stroke and hydrocephalus being well established causes of Parkinson's. It might have been chance that like Blocq and Marinesco, Levy-Valensi [29] and Scheinker [30] found tuberculosis in patients with Parkinson's following in the footsteps of Simmonds (Scheinker, 1936), Nonne [31], Sittig [32], and Wilder [33]. It might have also been coincidental that von Economo's Encephalitis, which caused Parkinson’s regularly, was, according to Hall and the ARNMD [18,39] almost indistinguishable from CNS TB, even to its spinal fluid findings. It also may have been coincidental that Hitler, who had von Economo's, also, had a lengthy illness in adolescence, which, according to Heston, Leonard and Heston [51] had the main features of tuberculosis. It might be considered a fluke that Deprenyl, also known as Eldepryl, a Parkinson's mainstay, comes from a class of drugs, the MAO inhibitors, originally designed to cure tuberculosis [9] – just as the fact that the two well-known Parkinson’s drugs Tolcapone and Entacapone also have strong anti-tubercular activity [7].
It might have been coincidental that both Mycobacteria tuberculosis and Parkinson's came into force and were linked with the great Industrial Revolution. And it might be thought unusual that in his An Essay on the Shaking Palsy James Parkinson mentioned scrofular tuberculosis not once, but twice, as a possible causative agent for Parkinson’s disease [3]. It might seem merely fortuitous that Blocq and Marinesco [21] established through tuberculoma attack that the substantia nigra was important to Parkinson's. Similarly, that Mendel [22] established tuberculosis in a parkinsonian child. It could be considered incidental that the preferred site of attack for tuberculous meningoencephalitis is the convexity of the brain, where it covers the substantia, the basal ganglia and has ready access to the cranial nerves. Perhaps even coincidental that it has a propensity to cause the endothelial inflammation and disease that often leads to stroke as well as the ability to choke off the aqueduct of Sylvius, leading to a blockage of cerebrospinal fluid known as hydrocephalus –both stroke and hydrocephalus being well established causes of Parkinson's. It might have been chance that like Blocq and Marinesco, Levy-Valensi [29] and Scheinker [30] found tuberculosis in patients with Parkinson's following in the footsteps of Simmonds (Scheinker, 1936), Nonne [31], Sittig [32], and Wilder [33]. It might have also been coincidental that von Economo's Encephalitis, which caused Parkinson’s regularly, was, according to Hall and the ARNMD [18,39] almost indistinguishable from CNS TB, even to its spinal fluid findings. It also may have been coincidental that Hitler, who had von Economo's, also, had a lengthy illness in adolescence, which, according to Heston, Leonard and Heston [51] had the main features of tuberculosis. It might be considered a fluke that Deprenyl, also known as Eldepryl, a Parkinson's mainstay, comes from a class of drugs, the MAO inhibitors, originally designed to cure tuberculosis [9] – just as the fact that the two well-known Parkinson’s drugs Tolcapone and Entacapone also have strong anti-tubercular activity [7].
As well, it might seem circumstantial that clinical and epidemiologic studies have peppered Parkinson's literature for decades linking it to tuberculosis-like germs of the order Actinomycetales –including nocardia, Corynebacterium (diphtheria) and mycobacteria: microorganisms not only easily confused during initial identification [57], but which also cross-react when a blood test is tried to identify one of them [56]. It might be considered accidental that Burn [11] isolated a germ with an eerie resemblance to Alexander-Jackson's [12] atypical mycobacteria in three von Economo's infants at autopsy, or that Alexander-Jackson found in that study acid-fast forms in Burn's bacillus that she implicated as causing Wilson's disease, dominant cause of Parkinson's in the young. It might be considered insignificant that tuberculosis-like nocardia has a specific affinity for substantia nigral neurons [55] or that in a follow-up study Kohbata and Shimokawa [58] cemented a relationship by blood serology between nocardia or mycobacteria in 20 of 20 Parkinson's patients or that Gao and Raine [59] linked Parkinson's to mycobacteria in blood through diagnostic heat shock proteins. It could be considered anecdotal that Mital, Sarkari and Singh [16], Otaki, Ichikawa and Oizumi [15], Fuente-Aguado and Bordon [13], Solanki and Kothari [17] and Kurasawa, Ikeda and Innoue [14], all cured Parkinson's with anti-tuberculous therapy –or that recently Cox found that tuberculosis and Parkinson’s disease are linked by the unique protein called “Parkin” [19].
It could be called inconsequential that in AIDS-related Parkinson's, claimed by some as the most common cause of infectious Parkinson's, that Berenguer and Moreno [62] reported mycobacteria as the most common CNS pathogen in HIV-infected people. And it could also be inconsequential that even by the year 2000, 8.5 million people were co-infected with both HIV and TB concurrently [61]. It could be considered coincidental that Guam, where TB meningitis at one time ran rampant, once hosted an epidemic proportion of neurologic disorders including Parkinson's-Dementia [89]. It might seem that there is lack of essential connection between the post-traumatic Parkinson's, say, of an ex-boxer and the fact that such trauma could also cause long-standing tubercles in the brain [90], planted even decades before, to discharge bacilli into the meningeal spaces, reactivating a long quiescent disease from one punch too many. It might seem unrelated that occupational exposure to copper, manganese and iron [77] are all not only associated with Parkinsonism but the very substances which have been used as growth factors to increase mycobacterial cell mass in the laboratory [78]. And it might seem chance that the triple jeopardy of oxidative stress under which the substantia nigra sits, namely catecholamine toxic dopamine derived from tyrosine, hydrogen peroxide and free radical by-products, all of which can damage the substantia [82] are also manufactured by mycobacterial intermediary metabolism and cell-respiration [78]. It might be characterized as random that many Parkinson's victims show the same cachexic eating away of the flesh that has long characterized tuberculosis or consumption –or that the dopaminergic neuron loss McGeer and Ragaki [91] found as an active process, even after Parkinson's death, is just the sort of chronic process that so characterizes a disease like TB, which often begins in youth. It could be considered incidental that tuberculosis and Parkinson’s are presently considered diseases of the older population with more men than women affected. It could be considered happenstance that first line TB drugs such as Isoniazid or Rifampin took away Parkinson’s symptoms in clinical trials [8,92] such as that of Anthony Fink’s at UC Santa Cruz which showed that Rifampin actually inhibited and disaggregated α-synuclein fibrils; or that the older anti-tubercular PAS eradicated symptoms in manganese-induced Parkinsonism in Jiang’s university study. [93] But in the end, and taken together, there are just too many coincidences in an ever-growing list to casually dismiss. In conclusion, the preponderance of convincing evidence, as we pass the bicentennial of James Parkinson's original Essay points, to a chronic infectious cause, not viral, and most likely of the family Actinomycetales –either mycobacterial or an atypical mycobacteria, fortunately still susceptible to known and already approved antimicrobials. Further clinical trials await.
References
- Berstad K and Berstad JER. “Parkinson's disease; the hibernating spore hypothesis”. Medical Hypotheses 104 (2017): 48-53.
- Broxmeyer L. “Parkinson's: another look”. Medical Hypotheses59:4 (2002): 373-377.
- Parkinson J. “An essay on the shaking palsy. 1817”. Journal of Neuropsychiatry Clinical Neuroscience 14:2 (2002): 223-236.
- Morley J. “Essay on the Nature and Cure of Scrophulous Disorders, Commonly Called the King's Evil”. 20th Edition. James Buckland Publisher 1781.96pp
- Copland J. Of the Causes, Nature and Treatment of Palsy and Apoplexy: of the forms, seats, complications, and morbid relations of paralytic and apoplectic diseases”. Longman, Brown, Green & Longmans 1850.414: 211.
- Porter R. Enlightenment. London: Penguin, 2000. 752 pages.
- Kinnings SL., et al. “Drug Discovery Using Chemical Systems Biology: Repositioning the Safe Medicine Comtan to Treat Multi-Drug and Extensively Drug Resistant Tuberculosis”. PLoS Computation Biology 5:7 (2009): e1000423.
- Fung VS and Thompson PD. “Rigidity and spasticity". In Tolosa E, Jankovic. Parkinson's disease and movement disorders”. Hagerstown, MD: Lippincott Williams & Wilkins (2007): 504–513.
- Hardman IG and Gilman AG. Goodman & Gillman’s The Pharmacologic Basis of Therapeutics, 9th Ed. New York: McGraw Hill, (1996).
- DATATOP Parkinson's Study Group. Ann Neurology. 39 (1996): 29-45.
- Burn CG. “Unidentified Gram-Positive Bacillus Associated with Meningoencephalitis”. Proceedings of the Society for Experimental Biology and Medicine 31 (1934): 109.
- Alexander-Jackson EA. “A Specific Type of Microorganism Isolated from Animal and Human Cancer: Bacteriology of the Organism”. Growth 18:1 (1954): 37-51.
- Fuente-Aguado J de la and Bordon J. “Parkinson in an HIV infected patient with hypodense cerebral lesion”. Tubercle and Lung Disease77:2 (1996): 191-192.
- Kurasawa T., et al. “Pulmonary infection with Mycobacterium kansasii presenting as solitary nodule shadow in the left anterior basal segment”. Japanese Journal of Thoracic Diseases 35:2(1997): 215-219.
- Otaki M., et al.“A case of endobronchial tuberculosis complicated with atelectasis of right upper lobe”. 69:7 (1994): 491-495.
- Mital OP., et al. “Parkinsonian symptoms in TBM. (a case report)”. Journal of the Association of Physicians of India 22:8 (1974): 629-631.
- Solanki SV and Kothari VR. “Parkinsonian Syndrome”. Journal of the Indian Medical Association 68:12 (1977): 255-257.
- Hall AJ: Epidemic Encephalitis (Encephalitis Lethargica) John Wright & Sons LTD. Bristol. 1924.
- Manzanillo PS., et al. “The ubiquitin ligase Parkin mediates resistance to intracellular pathogens”. Nature 501:7468 (2013): 512–516.
- Jancovic J and Tolosa E. “Parkinson's Disease And Movement Disorders”. Williams and Wilkins. Baltimore. 1998
- Blocq P. Marinesco G. “Tremblement Parkinsonian Hemiplegique Symtomatique d'une Tumeur du Pedoncule Cerebral”. Comptes Pendus Hebo Madaires Des Seances Et Memoires De La Societe Paris (1893): 105-111.
- Mendel. Berliner Klinische Wochensrift. No. 29. 1885 in Blocq, P; Marinesco.G: Tremblement Parkinsonian Hemiplegique Symtomatique d'une Tumeur du Pedoncule Cerebral. Comptes Pendus Hebo Madaires Des Seances Et Memoires De La Societe Paris, (1893): 105-111.
- Bechet E. “Formes cliniques et diagnostic de la maladie Parkinson". These, Paris Obs. XIX: (1892) p. 135.
- Gordon BL. “Ocular Tuberculosis”. Archives of Ophthalmology 31.5 (1944): 541-556.
- Iseman, MD. “Evolution of drug resistant tuberculosis: A tale of two species”. Proceedings of the National Academy of Sciences 91:7 (1994): 28-29.
- Dye C., et al. “Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 282:7 (1999): 677-686.
- Koch R: Die Aetiologie der Tuberculosis. Berliner Klinische Wochenscrift. XIX 1882
- Robbins SL. Robbin’s Pathologic Basis of Disease. WB Saunders Company, Philadelphia 5th Edition. 1994
- Levy-Valensi J. Meningite tuberculeuse ayam un syndrome parkinsonien. Semaine des Hopitaux de Paris. 20 (1931) 653-654.
- Scheinker I. Encephalitis tuberculosa im Striatom-unter dem Bilde eines Parkinsonismus. Deutsche Zeitschrift fur Nervenheil Kunde. Veriag Bon FCW Vogel. Berlin. 1936
- Nonne. Dtach Z Novenhe. Vol 18. 1900
- Sittig. Z. Neur Vol 2,3.1914
- Wilder J. Mschr. Psychiatv. Vol 61.1926
- Wilson SAK. Neurology. The Williams and Wilkin’s Company. Baltimore 1 (1940): 115
- Borthwick GA. “Sequelae of Epidemic Encephalitis”. Clin J 60 (1931): 510-524.
- Naville F. “Les sequelles de I'epidemic d'encephalitis de”. Rev Med Suisse Romande 43 (1918-1921): 1923.
- Ulitovsky DA: Epidemic lethargic encephalitis. Ivkutsk State Medical Institute of Infectious and Toxic Diseases of the Nervous Sytem. 1961.
- Yahr MD; Encephalitis lethargica (Von Economo's disease epidemic encephalitis). Handbook of Clinical Neurology 34 (1983): 451-457.
- ARNMD (An Investigation by the Association for Research in Nervous and Mental Disorders). Acute Epidemic Encephalitis (Lethargic Encephalitis). New York. Paul B. Hoeber (1922).
- Kennedy DH. “Tuberculous Meningitis”. Journal of the American Medical Association 241:3 (1979): 264-268.
- Smith AL. “Tuberculosis meningitis in childhood”. Current Treatment Options in Neurology4:3 (2002): 57-60.
- Hinman AR. “Tuberculous meningitis at Cleveland Metropolitan Hospital1959 to 1963”. The American Review of Respiratory Disease Returns 95:4 (1967): 670-673.
- Sumaya CV and Simek M. “Tuberculosis meningitis in children during the isoniazid era”. Journal of. Pediatrics 87:1 (1975): 43-49.
- Lincoln EM and Sordillo SVR. “Tuberculosis meningitis in children”. Journal of Pediatrics 57 (1960): 807-823.
- Virmani V and Geeta R. “A study of the cerebrospinal fluid in atypical presentations of tuberculosis meningitis”. Journal of Neurosurgical Sciences26:4 (1987): 587-592.
- Blunt SB and Lane RJ. “Clinical Features and Management of Two Cases of Encephalitis Lethargica”. Moment disorders 12:3 (1997): 354-359.
- Lessel S. “Seizure in a Guamanian Village”. Archives of Neurology 7 (1962): 37-44.
- Andrews JM. “Neurological disease on Guam. A review of past and present investigations”. Bulletin of the Los Angeles Neurological Societies 32:1 (1967): 30-42.
- Duvoisin RC and Yahr MD. “Encephalitis and Parkinsonism”. Archives of Neurology 12 (1965): 227.
- Lieberman A. “Adolph Hitler's Cognitive Disorder and How It Affected His Conduct of World War II”. Advances in Neurology 80 (1999): 459-466.
- Heston MD., et al. “The Medical Casebook of Adolph Hitler”. Stein and Day Publishers. New York. 1980.
- Nova: The Frozen Addicts aired: July, 1982
- Zeller EA and Barsky J. “Influence of isonicotinic acid hydrazide (INH) and 1-isonicotinyl-2-isopropyl hydrazide (IIH) on bacterial and mammalian enzymes”. Experimentia(1952): 349-350.
- Crane GE Clinical psychopharmacology in its 20th year. Science Wash 181 (1973): 124-128.
- Kohbata S and Beaman BL. “L-Dopa-responsive movement disorder caused by Nocardia Asteroides localized in the brains of mice”. Infection and Immunity59:1 (1991): 181-191.
- Beaman BL and Bolivon P. “Nocardia and Nocardiosis”. Journal of Medical and Veterinary Mycology 30. Suppl 1 (1992): 317-331.
- Atlas R. Microbiology: Fundamentals and Applications. 2nd Edition. New York. Macmillan. 1988
- Kohbata S and Shimokawa K. “Circulating Antibody to Nocardia in the Serum of Patient's with Parkinson's Disease”. Advances in Neurology 60 (1993): 355-357.
- Gao YL and Raine CS. “Humoral response to hsp 65 in multiple sclerosis and other neurologic conditions”. Neurology 44:5 (1994): 941-945.
- Roberts L. “The comeback plague”. U.S. News & World Report. March 27, 2000.
- Kasper D., et al. “Harrison's Principles of Internal Medicine. New York. McGraw-Hill. 1998.
- Berenguer J and Moreno S. “Tuberculous Meningitis in Patients Infected with the Human Immunodeficiency Virus”. New England Journal of Medicine 326:10 (1992): 668.
- Bishburg E., et al. “Central Nervous System Tuberculosis with the Acquired Immunodeficiency Syndrome and Its Related Complex”. Annals of Internal Medicine105:2 (1986): 210-213.
- Nightingale SD. Journal of Infectious Diseases. 165 (1992) 1082-1085.
- Jacob NJ and Henein SS. “Nontuberculous Mycobacterial Infection of the CNS in Patients with AIDS”. Southern Medical Journal 86:6 (1993): 638-640.
- LeBlang SD., et al. “CNS Nocardia in AIDS Patients: CT and MRI with Pathologic Correlation”. Journal of Computer Assisted Tomography l9:1 (1995): 15-22.
- Javaly K., et al. “Nocardiosis in patients with human immunodeficiency virus infection. Report of 2 cases and review of the literature”. Medicine71:3 (1992): 128-38.
- Stephensen. “Exposure to Home Pesticides Linked to Parkinson's disease”. The Journal of the American Medical Association 283:23 (2000): 3055-3056.
- Young J. “A New Outlook on Cancer. Irritation and Infection”. British Medical Journal 10 (1925): 60-61.
- Lux WE and Kurtzke JF. “Is Parkinson's disease acquired? Evidence from a geographic comparison with multiple sclerosis”. Neurology 37:3 (1987): 471-487.
- Betemps EJ and Buncher CR. “Birthplace as a Risk Factor in Motor Neurons Disease and Parkinson's disease”. International Journal of Epidemiology 22:5 (1993): 898-904.
- Walshe JM and Yealland M. “Wilson's disease: The problem of delayed diagnosis”. Journal of Neurology, Neurosurgery, and Psychiatry 55 (1992): 592-596.
- Merril CR., et al. “Bacterial Virus Gene Expression in Human Cells”. Nature 233:5319 (1971): 398-400.
- Morse Ml, Lederberg EM. Genetics 41: pp.142, 758. 1956
- Koller W and Veters-Overfelt B. “Environmental risk factors in Parkinson’s disease”. Neurology 40 (1990): 1218-1221.
- Hubble JP and Cao T. “Nocardia Species as an Etiologic Agent in Parkinson's Disease . Serologic Testing in a Case-Control Study”. Journal of Clinical Microbiology 33:10 (1995): 2768-2769.
- Gorell JM and Johnson CC. “Occupational exposures to metals as risk factors for Parkinson's disease”. Neurology 48:3 (1997): 650-658.
- David HL. “Bacteriology of the Mycobacteriosis”. U.S. Dept. of Health, Education and Welfare. Public Health Service. Center for Disease Control. Atlanta, March 1976.
- Martyn CN and Osmond C. “Parkinson's disease and the environment in early life”. Journal of the Neurologic Sciences 132 (1995): 201-206.
- Mankiewicz E and Jackson EA. “Bacteriophages that Lyse Mycobacteria and Corynebacteria show Cytopathogenic Effect on Tissue Cultures of Renal Cells of Cercopithecus Aethiops”. Canadian Medical Association Journal 92 (1965): 31-33.
- Hastings TG and Lewis DA. “Role of Oxidation in the NeurotoxicEffects of Intrastriatal Dopamine Injections”. Proceedings of the National Academy of Sciences 93:5 (1996): 1956.
- Cross CE. “Oxygen radicals and human disease”. Annals of Internal Medicine 107:4 (1987):.526-545.
- Zecca L and Pietra R. “Iron and other metals in neuromelanin, substantia nigra and putamen in human brain”. Journal of Neurochemistry 62 (1994): 1097-1101.
- Ben-Shacher D and Youdim MB. “Internigral iron injection induces behavioral and biochemical Parkinsonism in rats”. Journal of Neurochemistry57 (1999): 2133-2135.
- Anderson RJ. “The chemistry of the lipids of the tubercle bacillus”. Yale Journal of Biology and Medicine15:3 (1943): 311.
- Jenner P and Dexter DT. “Oxidative stress as a cause of nigral cell death in Parkinson's disease and incidental Lewy body disease”. Annals of Neurology32 S82-7 (1992): 82-87.
- Softic E., “Paulus W. Selective increase of iron in substantia nigra zona compacta of parkinsonian brains”. Journal of Neurochemistry56 (1991): 978-982.
- Jablonski S. Jablonski's Dictionary of Syndromes & Eponymic Diseases. Malabar, Florida. Krieger Publishing Company. 1991. 665pp.
- Elizan TS. “Amyotrophic lateral sclerosis and parkinsonism-dementia complex: a study in Non-Chamorros of the Mariana and Caroline Islands”. Archives of neurology14 (1996): 347-355.
- Rom WN, Garay SM. Tuberculosis. New York. Little Brown and Company. 1998
- McGeer PL and Ragaki S. “Rate of cell death in parkinsonism indicates active neuropathological process”. Annals of Neurology 24 (1988): 574-576.
- Li J., et al. “Rifampicin Inhibits α-Synuclein Fibrillation and Disaggregates Fibrils”. Chemistry & Biology 11:11 (2004): 1513–1521.
- Jiang YM., et al. “Effective treatment of manganese-induced occupational Parkinsonism with p-aminosalicylic acid: a case of 17- year follow-up study”. Journal of Occupational and Environmental Medicine 48:6 (2006): 644-649.
Citation:
Lawrence Broxmeyer. “What James Parkinson Really Thought was Behind Parkinson’s Disease”. Current Opinions in Neurological
Science 1.2 (2017): 103-119.
Copyright: © 2017 Lawrence Broxmeyer. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.






























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.