Case Report
Volume 2 Issue 2 - 2018
West Nile Neuroinvasive Disease: A Case Report
Director of Neuro-Immunology Program, Division of Multiple Sclerosis and other Demyelinating Disorders, Department of Neurology, Palmetto Health-University of South Carolina, Columbia, SC, USA
*Corresponding Author: Renu Pokharna, MD, FAANEM, MSCS, CRND, CNP, NM, Director of Neuro-Immunology Program, Division of Multiple Sclerosis and other Demyelinating Disorders, Department of Neurology, Palmetto Health-University of South Carolina, Columbia, SC, USA.
Received: January 01, 2018; Published: March 27, 2018
Abstract
West Nile virus (WNV) may cause a neuroinvasive disease via inoculation of the virus through a Culex mosquito bite. WNV may present with meningitis, encephalitis and/or acute flaccid paralysis, which is referred to as West Nile neuroinvasive disease (WNND). We report a case of a 28 year old African American female who presented with WNND, which manifested as a rapidly progressive, bilaterally symmetrical, proximal and distal limb weakness with atrophy of muscles. Increased awareness of this uncommon presentation of a progressive, disabling, and potentially fatal disease may facilitate earlier diagnosis and lead to preventative measures to eradicate mosquitos in infested areas.
Keywords: West Nile virus; West Nile neuroinvasive disease; Poliomyelitis; Arthropod borne infection; Encephalitis; Meningitis; Upper motor neuron paralysis; Lower motor neuron paralysis
Introduction
West Nile virus (WNV) is flavivirus that is transmitted via the Culex mosquito bite. WNV is most commonly asymptomatic, but can also present as West Nile fever or West Nile neuroinvasive disease (WNND) [1-3]. Most patients with WNV manifest with fever, headache, malaise, myalgia, fatigue, skin rash, lymphadenopathy, vomiting, and diarrhea. Approximately 1% of individuals with WNV will go on to develop WNND, which is characterized by meningitis, encephalitis and/or acute flaccid paralysis [4]. We present a case of a serologically proven WNV infection in a 28 year old African American female, which progressed to WNND with an unusual presentation of rapidly progressive, bilaterally symmetrical, proximal and distal limb weakness.
Case Description
A twenty-eight year old, right-handed, African-American female with obesity presented to an outside hospital for a 5-day history of fever and bi-frontal headache without neck stiffness, photophobia, nausea, or vomiting. Over the next few days she had intermittent headaches that resolved with ibuprofen, high-grade fever and vomiting for which she received IV fluids and antipyretics at an urgent care facility.
She later presented to an outside Emergency Department with persistent headache and fever. She denied history of recent travel, trauma, tick bite, rash, pets in the house, or sick contacts. She complained of generalized weakness, but denied focal weakness or numbness. There were no meningeal signs on exam. Admission labs were remarkable for hypokalemia, hypomagnesaemia, and severe hypophosphatemia. She had a leukocytosis with a white blood cell count of 16 k/UL and elevated lactic acid of 2.5 mmol/L.
A computerized tomography (CT) head without intravenous (IV) contrast did not show acute intracranial abnormality. Cerebrospinal fluid (CSF) study showed 1534 nucleated cells (33% polymorphic neutrophils, 55% lymphocytes) with 152 red blood cells, mildly elevated protein of 80 mg/dl and normal glucose. She was started on empiric vancomycin and ceftriaxone along with Decadron for presumed meningitis/encephalitis. CSF Polymerase Chain Reaction (PCR) encephalitis panel and Venereal Disease Research Laboratory test (VDRL) were both negative. Serum studies including Human Immunodeficiency Virus (HIV), Cryptococcal antigen, anti-nuclear antigen antibody, and blood cultures were all negative. CSF WNV IgM and IgG antibodies were sent out. Antibiotics were continued. Decadron was discontinued after two doses. Magnetic resonance imaging (MRI) of the brain with and without contrast showed mild cerebellar and brain stem meningeal enhancement, as seen in Figure 1.
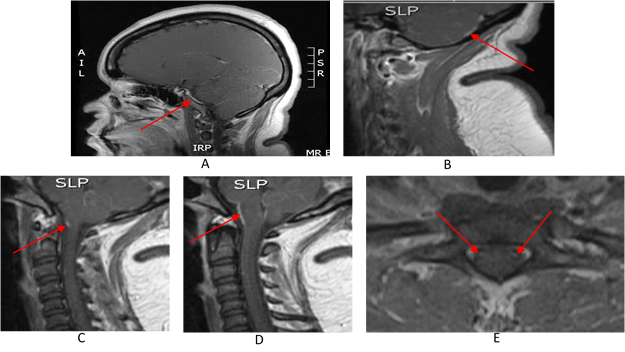
Figure 1A: MRI brain T1 with contrast – sagittal view with meningeal enhancement of the temporal lobe (arrow).
B) MRI cervical spine T1 with contrast – sagittal view of cerebellar enhancement (arrow).
C) MRI cervical spine T1 with contrast – sagittal view showing intramedullary enhancing lesion in the spine (arrow).
D) MRI cervical spine T1 with contrast – sagittal view showing meningeal enhancement of cerebellum, pons and medulla oblongata (arrow).
E) MRI cervical spine T1 with contrast – axial view showing enhancing lesions within the anterior horn cells of the spinal cord (arrows).
B) MRI cervical spine T1 with contrast – sagittal view of cerebellar enhancement (arrow).
C) MRI cervical spine T1 with contrast – sagittal view showing intramedullary enhancing lesion in the spine (arrow).
D) MRI cervical spine T1 with contrast – sagittal view showing meningeal enhancement of cerebellum, pons and medulla oblongata (arrow).
E) MRI cervical spine T1 with contrast – axial view showing enhancing lesions within the anterior horn cells of the spinal cord (arrows).
The patient’s weakness continued to progress over the course of her hospitalization. Initially, the weakness was bilateral, proximal, symmetric, and mostly present in the upper extremities. The next day she developed distal bilateral weakness as well. She denied loss of bowel or bladder function. Creatinine phosphokinase (CPK) was elevated at 800 U/L. MRI of the cervical and thoracic spine without contrast was reported as being unremarkable and did not reveal any para-meningitic focus of infection causing weakness.
The patient was transferred to our hospital for further workup. On initial evaluation, the patient had bilateral, symmetrical, proximal upper-extremity weakness with absent reflexes in both upper limbs. Strength in both lower extremities was near normal with preserved reflexes. Plantar reflexes were extensor bilaterally. Later on she lost lower limb reflexes. The patient also had a mild right facial droop with flattening of the right nasolabial fold. There was appreciable horizontal nystagmus that had the appearance of ocular flutter as well as downbeat nystagmus in left lateral gaze. CBC was remarkable for a mild thrombocytosis. A basic metabolic panel showed hypokalemia, and hyponatremia. B12 and folate were normal, and RPR was negative. Repeat T2 MRI of the cervical spine with contrast showed hyper intensity in the anterior horn of the cervical cord, as seen in figure 1. Thoracic and lumbar spine MRI with contrast were unremarkable.
A repeat Lumbar Puncture showed 146 nucleated cells (95% lymphocytes), 0 red blood cells, mildly elevated protein (53.8 mg/dL) and normal glucose. Flow cytometry for acute lymphoma was negative. IgG index was elevated, and oligo clonal bands were positive at two. Repeat CSF meningitis encephalitis PCR panel was negative. Antibiotics were discontinued and the patient was continued on supportive care with IV fluids and antipyretics. CSF from the outside hospital returned strongly positive for WNV with high titers of both IgG and IgM, which was confirmed with repeat testing. CSF aerobic and anaerobic bacterial cultures finalized negative and cultures for acid fast bacilli and fungi were negative. Histoplasma antigen was negative in serum and urine. A PCR for Mycobacterium tuberculosis in CSF was negative.
Electromyography (EMG) and nerve conduction study (NCS) of the bilateral upper limbs was performed. Right median compound muscle action potentials (CMAPs) were decreased and there was mild to moderately reduced recruitment suggestive of motor neuron cell involvement. Conduction velocity was normal. Right median and ulnar sensory nerve action potentials (SNAPs) were normal. Concentric needle EMG of right upper limb muscles showed increased insertional activity (IA) of the right deltoid, 2+ fibrillations (fibs)/positive sharp waves (psw), and no motor unit action potential (MUAP). The right biceps showed an increased IA, 2+ fib/psw's, normal MUAP’s and moderately reduced recruitment. The right flexor carpi radialis and flexor digitorum sublimis showed an increased IA, 2+ fib/psw's, normal MUAP’s and mildly reduced recruitment. Right first dorsal interossei showed an increased IA, 1+ fib/psw's, normal MUAP’s and mildly reduced recruitment. The study is consistent with an acute to subacute disease of motor neurons or their axons with ongoing denervation.
Discussion
West Nile virus (WNV) is an RNA virus from the flavivirus family that is transmitted via the Culex mosquito bite. It is indigenous to Africa, Asia, Europe and Australia. [1] The first cases of WNV in the United Stated were detected in New York City during the 1999, which later spread to the whole United States [1,3] Infection occurs more commonly in summer and fall months in North America [5]
Symptoms only occur in 20-40% of people with WNV infection. It’s incubation period is 2-14 days [4,6]. WNV commonly presents with fever, headache, malaise, myalgia, fatigue, skin rash, lymphadenopathy, diarrhea and vomiting. One percent of individuals with WNV develop WNND, which is characterized by meningitis, encephalitis and/or acute flaccid paralysis [4]. In European WNV hospitalized cases neuromuscular weakness occurs in 15 to 20%, but in North America approximately 50% of cases have neuromuscular weakness.7 WNND is more common in the immunocompromised and elderly. The mechanism by which WNV enters the central nervous system is unknown, however, it is thought to result from elevated inflammatory cytokines such as tumor necrosis factor-α, which alters the permeability of the blood brain barrier [2].
The patient’s symptoms of fever and headache along with the MRI imaging, which showed meningeal enhancement in the base of the brain, and CSF findings were suggestive of meningitis without signs of overt meningeal irritation. Similarly there were symptoms and signs of encephalitis including fever, headache, right facial palsy, brain stem involvement and bilateral positive Babinski reflexes. Her CSF picture was suggestive of viral meningoencephalitis with an elevated lymphocyte count, elevated protein, but negative cultures, PCR’s, cytology, VDRL, encephalitis panel. CSF WNV IgG and IgM antibody came back positive, confirming the diagnosis of WNND.
The Patient also had ocular manifestation in the form of ocular flutter and horizontal nystagmus, suggestive of Omni pause neuron involvement. She had no sensory system involvement. She had weakness in all four limbs, upper extremity involvement being worse than lower extremity involvement. She had severe bilaterally symmetrical upper limb weakness, with absent reflexes, positive bilateral Babinski reflexes and normal sensory conduction with motor neuropathy on EMG/NCV, which is suggestive of both upper and lower motor neuron involvement. Her Anterior Horn cells in the cervical spine were hyper-intense suggesting Polio-like involvement of WNV rather than GBS like picture.
Most WNV cases present with asymmetrical extremity weakness accompanying meningoencephalitis. [7,8] Our patient presented with rapidly progressive severe proximal greater than distal bilaterally symmetrical upper greater than lower limb weakness. Our patient did not have GBS-like presentation as there was no albumino-cytological dissociation and the anterior horn cells were affected. The patient was treated conservatively with supportive care and rehabilitation. Her negative inspiratory forces (NIF’s) remained normal throughout the course. She improved slightly in her limb strength when seen in the outpatient clinic.
Conclusion
WNV can be a fatal disease with varied and unusual neurological manifestations. WNV should be considered in patients with unusual neurological manifestations including rapidly progressive, bilaterally symmetrical, proximal and distal limb weakness with muscle atrophy, especially in summer and fall months. Prompt recognition and diagnosis is crucial for these patients to get rapid treatment before there is a chance of disease progression and untimely death of the patient, as well as adequate measures to be taken to eradicate mosquitos from the area.
Acknowledgement
I am thankful to Pooja Pokharna for helping with the formatting.
I am thankful to Pooja Pokharna for helping with the formatting.
References
- Campbell GL., et al. “West Nile virus”. The Lancet infectious diseases 2.9 (2002): 519-529.
- Davis LE., et al. “West Nile virus neuroinvasive disease”. Annals of neurology 60.3 (2006): 286-300.
- Afzal A., et al. “Opsoclonus myoclonus syndrome: an unusual presentation for West Nile virus encephalitis”. Proceedings (Baylor University. Medical Center) 27.2 (2014): 108-110.
- Kramer LD., et al. “West Nile virus”. The Lancet Neurology 6.2 (2007): 171-181.
- Centers for Disease Control and Prevention. West Nile virus. (2017):
- Moudgal V., et al. “West Nile virus neuroinvasive disease”. Journal of Clinical Outcomes Management 16.11 (2009): 515-524.
- Madden K. “West Nile virus infection and its neurological manifestations”. Clinical Medicine & Research 1.2 (2003): 145-150.
- Sejvar JJ., et al. “West Nile virus–associated flaccid paralysis”. Emerging Infectious Diseases 11.7 (2005): 1021-1027.
Citation:
Renu Pokharna., et al. “West Nile Neuroinvasive Disease: A Case Report”. Current Opinions in Neurological Science 2.2 (2018):
441-444.
Copyright: © 2018 Renu Pokharna., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.






























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.