Research Article
Volume 3 Issue 2 - 2019
Skull Base Osteomyelitis - An Institutional Experience
Consultant – Neurosurgery and Spine Surgery, Manipal Hospital, Whitefield, Bangalore, India
*Corresponding Author: Dr Sibhi Ganapathy, Consultant – Neurosurgery and Spine Surgery, Manipal Hospital, Whitefield, Bangalore, India.
Received: March 29, 2019; Published: April 17, 2019
Abstract
Skull base osteomyelitis usually occurs in the background of severe immunosuppression, but may rarely occur in immune competent individuals as well. It is usually seen in association with sinusitis or persistent otitis media with erosion of the bony septae separating the infected cavity from the intracranial compartment. The Process also leads to infection of the bone, which spreads through the diploe and the intra-osteon vasculature to involve multiple skull bones. A specific problem remains invasive malignant otitis media that involves the temporal bone. Fungal sinusitis often spreads to the frontal bone from the frontal sinus. Other sites include the cavernous sinus and surrounding central skull base due to the drainage of veins from the face and mouth.
We present our series of cases at a tertiary care center in Bangalore, India where 7 such patients were diagnosed treated and followed up for a year. Their presentations, location and type of organism as well as treatment parameters were studied, tabulated and compared with standard data available.
Our series emphasizes the need for early diagnosis and aggressive treatment as the condition is not only chronic, but also very difficult to treat with deficits irreversible and morbid. Sometimes treatment arrives too late as a diagnosis can only be made once irreversible damage has been already caused. Further study regarding better imaging modalities as well as better antibiotic therapy is required before success in management can be declared.
Keywords: Skull base osteomyelitis; Temporal; Frontal; Sphenoid; Fungal; Bacterial
Introduction
Skull base osteomyelitis has always been a condition evoking despair amongst clinicians and patients in equal measure owing to the difficulty in diagnosis, high mortality and the persistant nature of the disease. The incidence of this condition is rising, especially due to the increased number of immunocompramised patients presenting late with sinusitis, mastoiditis, Otitis Media or other skull base infections that eventually spread to the bone [1-3]. The incidence of denovo osteomyelitis has also increased owing atleast in part to the injudicious use of antibiotics in treatment of ear and sinus infections leading to the creation of organisms of greater virulance and tenacity [2,3].
Another frequently encountered problem with skull base osteomyelitis is the variability of resenting complaints and signs. Various cobinations of cranial nerve palsies, cerebellar signs, papilloedema, symptoms of raised ICP, ear and nasal discharge, as well as brainstem signs [1,3,4,6]. These maybe associated with features of raised ICP, and general inflammation as well. A good knowledge of the anatomy of the skull base as well as a high index of suspicion can assist in early diagnosis. Imaging plays a major role in the detection of the disease with the MRI at the forefront [4-6]. The detection of early dural enhancement, collections, inflammations, displacement of structures and even abscess inside the parenchyma of the brain can be visualized early.
However, the gold standard of diagnosis always remains the histopathological and microbioloical analysis of a biopsy [2,4-7]. This helps in 2 ways, it assists in differentiating an infection from a noninfectious inflammatory condition such as sarcoidosis or vasculitis, and second it allows for the detection of the causative organism allowing for specific targeted treatment. This assumes particular importance, as the primary offender in skull base osteomyelitis is varied with equal number falling prey to virulent bacteria as well as fungi.
Treatment regimens are also varied due to the infrequent incidence of the disease and variability of aetiology. Hence the study documentation and follow up of patients with skull base osteomyelitis is of great importance. We present our clinical experience of 7 patients treated for proved skull base osteomyelitis and followed up for 3 years in a tertiary care Neurosurgical centre in India.
Materials and Methods
All patients with diagnosed skull base osteomyelitis were included in the study over a period of 2 years (From february 2016 to february 2018). The study was a retrospective cohort analysis with long term follow up used to identify prognosis and response to therapy.
Demographics, patient data, were tabulated and analysed. Response was inferred as a symptomatic improvement of the patient after initiation of treatment. No response was inferred as no improvement with progression of the deficets within 1 year of treatment.
Results
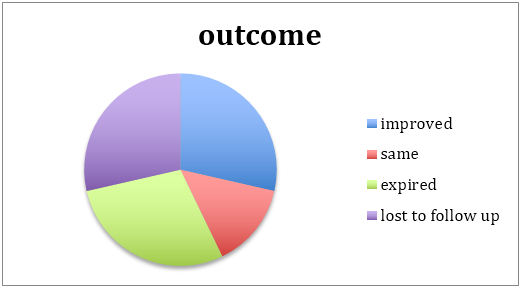
The median duration of treatment was for 6weeks. However 2 patients were lost to follow up afterwards (beyond the 6 weeks) hence their outcomes couldn’t be plotted out.
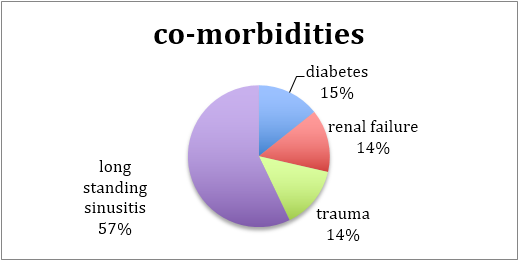
In general, the patients were debilitated elderly people with comorbidities that lead to immunospuuression that aggravated the spread of virulent aggressive pathogens. Only in one patient was there an early onset (in terms of age), which could be attributed to direct penetrating head trauma, which lead to meningo-encephalitis and an abscess progressing into osteomyelitis.
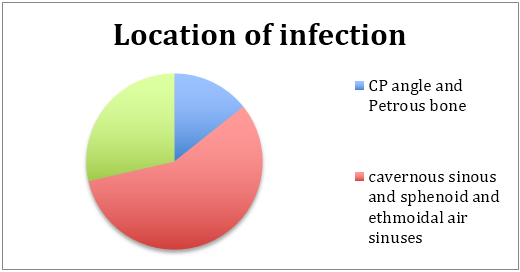
As expected the foci of infection are in close proximity to air sinuses. A large majority were in the central skull base (associated with the sphenoid and ethmoidal sinuses extending into the cavernous sinus as well), while another important subgroup were confined to the CP angle region and temporal fossa in close proximity to the mastoid sinuses.
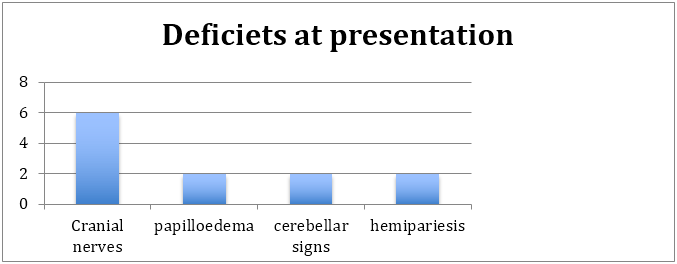
The patients presented with multiple cranial nerve palsies resembling a cordoma. The commonest nerves involved were lower craial nerves [9-11] and temporal fossa located nerves [3-6] extending onto the orbital apex. 1 patient presented with optic nerve compression with primary optic atrophy. Posterior fossa lesions presented with cerebellar signs as well as some brainstem signs [5,7,8 cranial nerve signs with hemipariesis/plegia] 1 patient had a fungal vasculitis which made him suffer an internal capsule lacunar infarct leading to hemipariesis. The focusof infection was near the frontal snus and was secondary to fungal sinusitis. Only large masses near the cavernous sinus presented with papilloedema and signs of raised ICP. Headache was common, but proably due to dural stretch or dural irritation of the phlegmon itself rather than ICP itself (as demonstrated by low incidence of papilloedema and secondary optic atrophy).
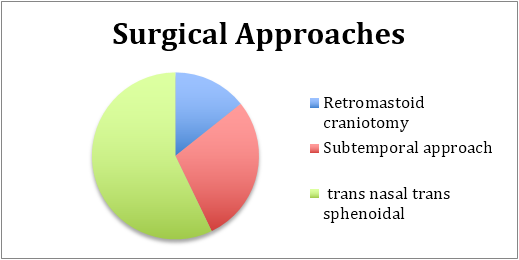
Due to the unclear nature of the mass and the surrounding reaction of the brain and dura, biopsies were considered critical to a diagnosis. Surgical approaches were as demonstrated. Procedures involved dural biopsies along with a biopsy of the lesion if possible.
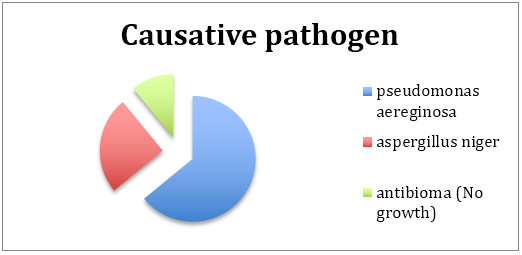
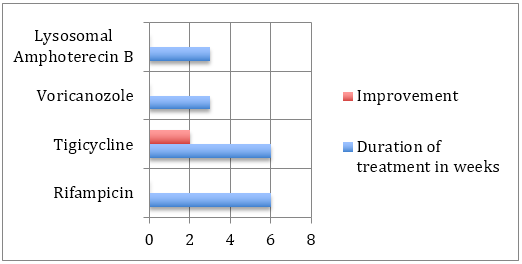
The causative pathogen was mainly of 2 types. The patients with better immune responses were colonised by an aggressive strain of pseudomonas aereginosa which on improper and inadequate treatment spread to the bone. These infections were controlled by high level antibiotics, often with uncomfortable side effects, making drug compliance a difficult proposition for the patient and their caregivers. Response was optimal for Tigicycline and Rifampicin. It is however improtant to note that despite the aggressive nature of the infection, improvement was most commonly seen when drug therapy was faithfully carried out.
The other group of infections were fungal in nature and were predominantly aspergillus. These were seen in elderly debilitated patients with poor immunity. The infection was multicentric, tenacious and poorly responsive to antifungal therapy. The important difference in therpay is that amphoterecin B, the most trusted and effective antifungal was a source of tremendous conern as it created devastating side effects which worsenned the overall condition of the patient, making drug compliance impossible. An alternative was Voricanozole which although not as effective was better tolerated. Cost of medical therapy expected to proceed for many months also hampered drug compliance, and outcomes.
Outcomes were a difficult statistic to decifer owing to the complex web of medical, microbiological, pharmacological and last but not least, social factors which complicated the drug compliance and follow up model expected by us to assess the patients progress.
Most of the improvement happened with bacterial disease when drug compliance, both by motivation and financial permission were allowed. Fungal osteomyelits with its morbid patient set, aggressive disease and difficult medical management accounted for drug defaults, and death.
An important case was the young patient with penetrating head trauma. He had a sterile growth (owing probably to excessive use of antibiotics), and was treated with empirical antibiotcs. No improvement occurred desite multiple pus cultures and biopsies. The decompression of the mass surgically reduced the headache, but his cranial nerve deficets never improved.
Discussion
Other case reports have been written on unique presentations of central skull base infection similar to those presented here [1,2,4,6,7,9,11,12]. The specific details of an individual case can be expected to vary, given the rarity of the condition. The region in which this condition arises is an anatomical “minefield,” and presenting symptoms will vary according to the precise location of infection. However, there are common findings among the different cases that should raise the possibility of this diagnosis. These cases also highlight the problem of differentiating inflammatory pathology from a malignant cause. It is widely reported that elderly, diabetic, predominantly male patients are most at risk [1]. Case reports on this subject mainly describe diabetic or immune compromised patients (17 of 24).
Severe infection can adversely affect diabetic control, and there may well be need to use a sliding scale of insulin to achieve this. Of the 7 cases referenced and presented here, the average age was 58 years and the sex distribution was 5:1 (M : F). An initial complaint of headache, often intractable, with symptoms related to individual CN palsies, is common to almost all cases. Involvement of both CN VI and the lower CNs (IX, X, XI) should in particular raise the suspicion of clival pathology [2]. Ophthalmoplegia (most often sixth nerve paresis) and paresis of CN XII has been considered in the past to be pathognomonic of a malignant neoplasm of the nasopharynx. Although it is now clear that this combination is not exclusive to nasopharyngeal carcinoma, malignant tumours of some sort, often metastases, are still the most common cause reported in other series [3].
Blood tests consistently demonstrate elevated acute-phase reactants, in particular the ESR (reported in 18 of 24 cases), whereas leucocytosis is less reliable (as is a lack of fever), although often seen at least to a moderate degree. One would not expect elevated acute-phase reactants with malignancy, so, given the potentially similar radiological findings, this may be a useful discriminator. Monitoring of the ESR is one of the key investigations that can help to guide how long antibiotic therapy is continued, and its normalization would appear to be a good indicator that the infection has resolved.
The imaging findings are of particular interest. The bone erosion and marrow infiltration of the basiocciput and/or petrous apex, as well as mass-like soft tissue swelling below the skull base, often raise concern that there is an underlying malignant process such as nasopharyngeal carcinoma or skull base metastases. This can lead to a delay in diagnosis because tissue samples from surgical biopsies may be sent for histology only and yield non diagnostic results. Because of the dramatic imaging findings, an initial negative biopsy may be put down to sampling error. An important lesson is to always send biopsy material for microbiological analysis (including fungal and mycobacterial cultures) as well as histology; ultimately, this may lead to the correct diagnosis (and obviously dictate the antibiotic treatment regime).
Different imaging modalities may be helpful in the assessment of these patients, although MRI is probably the most important. Computed tomography findings may be lacking initially but may later demonstrate clear bony erosion. In one of our cases, and in a patient described by Rowlands., et al. [4] an initial CT scan failed to demonstrate bony destruction. Seabold., et al. found no CT evidence of bone erosion in 13 out of 35 patients with biopsy-confirmed cranial osteomyelitis [5]. When present, the findings may fail to resolve for a long time after treatment is finished, so the use of CT for follow-up is questioned. Magnetic resonance imaging is a better soft tissue discriminator, offering good imaging of soft tissue planes around the skull base and abnormalities of the medullary cavity of bone [6]. Malone., et al. [6] describe typical findings of SBO to include marrow T1 hypo intensity and T2 hyper intensity, although these are not specific.
Clival enhancement can be seen both with SBO and malignant processes but is never normal. In terms of the differential diagnoses, the primary concern is of a malignant process, be it primary or secondary. Squamous cell carcinoma, lymphoma, nasopharyngeal carcinoma and haematogenous metastases may all have similar MRI findings to those described for SBO. Also, non-neoplastic conditions such as Wegener's granulomatosis, tuberculosis, sarcoidosis, fibrous dysplasia, and Paget's disease can have similar MRI findings—the appearance on CT can help make the differentiation (particularly for the latter two conditions). Given that neither test is diagnostic, nor are these cases common, we advocate the use of both imaging modalities to get as much information as possible.
Various nuclear medicine imaging techniques have been advocated in suspected SBO including gallium-67 scintigraphy, indium-111 white blood cell scans, technetium-99m methylene diphosphonate (MDP) bone scans, and single-photon emission computed tomography (SPECT). The latter two techniques may have an advantage over CT and MRI in detecting postoperative osteomyelitis and may be more useful in the follow-up of patients on antibiotics because marrow signal change may persist for up to 6 months after successful treatment [5] Although some centers advocate monitoring disease progression by the use of these scans, this is not currently the policy in our department. There is currently no imaging technique to replace the role of biopsy for microbiological culture in cases of suspected SBO.
Pseudomonas aeruginosa is the most common pathogen implicated in osteomyelitis secondary to malignant otitis external [7]. This too seems true for central SBO, although other organisms have been reported, including Aspergillus, [8] Gram-positive organisms [6], mycobacterium, and Candida [9]. Given that antibiotic treatment for SBO is often required for prolonged periods, microbiological advice is recommended. Culture sensitivities will guide the choice of antibiotics, influenced by local prescribing policies. The length of time antibiotics are administered is variable among the cases reported, but in each case treatment was given for at least 1 month and up to 6 months.
The Bone Infection Unit in Oxford, United Kingdom, often recommends up to 6 weeks of intravenous treatment followed by 6 to 12 months of oral medication, guided by clinical response. Adjuvant hyperbaric oxygen may also provide benefit. In cases of MOE, this may improve successful treatment by reversing tissue hypoxia, enhancing phagocytic killing of aerobic microorganisms, and stimulating neomicroangiogenesis [10]. However, the very limited availability of this treatment modality makes it impractical for widespread consideration.
The patient may have a history of previous otitis externa, which was treated with apparent success, yet have no obvious activity at the time that SBO is present. None of our cases showed evidence of active otitis externa or granulations in the external ear canal. Where the two infections occur sequentially, the diagnosis would be considered sequelae of malignant otitis externa, and would therefore have been excluded from the patient group under discussion here. In the case described by Rowlands., et al. [4] resolution seemed apparent several months before the development of CN palsies and SBO. We, too, found this in two of our cases. It may well be that the initial otitis externa still represents the focus of infection, which could have lain quiescent within the temporal bone until later reactivated. Other authors conclude that the infection of central SBO may originate from paranasal sinus inflammatory disease or be haematogenous in origin [6].
We report cases in which CN function has returned to a greater or lesser extent. Analyzing this can be difficult because function may return due to compensation of the contralateral side rather than true resolution of the affected side. The literature implies that this recovery is variable, and certainly not universal [6, 11]. However, this potential is relevant when counselling the patient and in aiding therapy (such as speech and language therapy) involved in the aftercare of the patient.
Some authors have advocated that with typical clinical picture and imaging findings together with a positive response to ciprofloxacin, the diagnosis can be made without the need for biopsies [1]. However, we feel that even in a tertiary referral setting, this situation arises rarely and in varied form, such that it is not easy to diagnose clinically, and because the imaging findings can be so alarming, biopsy is inevitably required to ensure both the absence of malignancy and to aid subsequent microbiological advice. Clival biopsies can be taken endoscopically via the nose, the technique for which is well described [1].
This is a serious condition, with potential for serious complication including the CN palsies with which it may present, cavernous sinus thrombosis, meningeal and brain extension, and death.
Conclusion
Cranial nerve palsies in the elderly diabetic or immunocompromised patient, with imaging findings of a lesion causing bony destruction in the central skull base, should raise the concern of a diagnosis of central SBO as well as of malignancy, and investigation for each should be concurrent. A past history of otitis externa, even if apparently resolved before the onset of the presenting symptoms, should further raise suspicion of an underlying infective cause.
Prompt commencement of antibiotic therapy is desirable to lower the risk of potential sequelae, which can prove to be fatal. The length of time that this therapy is continued should be guided by clinical findings, normalization of inflammatory markers, and resolution on MRI. Surgery has limited but very important roles: (1) providing tissue that helps exclude a neoplastic pathology and (2) allowing reliable culture of the microorganism responsible.
References
- Seabold J E., et al. “Cranial osteomyelitis: diagnosis and follow-up with In-111 white blood cell and Tc-99m methylene diphosphonate bone SPECT, CT, and MR imaging”. Radiology 196.3 (1995): 779–788.
- Malone D G., et al. “Osteomyelitis of the skull base”. Neurosurgery 30.3 (1992): 426–431.
- Huang K-L and Lu C-S. “Skull base osteomyelitis presenting as Villaret's syndrome”. Acta Neurologica Taiwanica 15.4 (2006): 255–258.
- Kountakis S E., et al. “Osteomyelitis of the base of skull secondary to Aspergillus”. American Journal of Otolaryngology 18.1 (1997): 19–22.
- Chang P C., et al. “Central skull base osteomyelitis in patients without otitis externa: imaging findings”. American Journal of Neuroradiology 24.7 (2003): 1310–1316.
- Davis J C., et al. “Adjuvant hyperbaric oxygen in malignant external otitis”. Archives of Otolaryngology–Head & Neck Surgery 118.1 (1992): 89–93.
- Grobman L R., et al. “Atypical osteomyelitis of the skull base”. Laryngoscope 99 (1989): 671–676.
- Magliulo G., et al. “Osteomyelitis of the skull base with atypical onset and evolution”. Annals of Otology, Rhinology & Laryngology 109 (2000): 326–333.
- Cavel O., et al. “The role of the otorhinolaryngologist in the management of central skull base osteomyelitis”. American Journal of Rhinology & Allergy 21.3 (2007): 281–285.
- Kulkarni S., et al. “Sixth and tenth nerve palsy secondary to pseudomonas infection of the skull base”. American Journal of Ophthalmology 139.5 (2005): 918–920.
- Keane J R. “Combined VIth and XIIth cranial nerve palsies: A clival syndrome”. Neurology 54 (2000): 1540–1541.
- Rowlands R G., et al. “Masked pseudomonal skull base osteomyelitis presenting with a bilateral Xth cranial nerve palsy”. The Journal of Laryngology & Otology 116.7 (2002): 556–558.
Citation:
Sibhi Ganapathy. “Skull Base Osteomyelitis - An Institutional Experience”. Current Opinions in Neurological Science 3.2 (2019): 661-668.
Copyright: © 2019 Sibhi Ganapathy. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.






























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.