Case Report
Volume 3 Issue 2 - 2019
Clinical Use of Combined Transcranial Magnetic Stimulation with Intravenous Ketamine for Treatment-Resistant Depression with Comorbid Pain, Substance Misuse, and Grief: A Case Report
1The Neuroscience Center, Deerfield, Illinois, USA
2PathFinder Brain SPECT Imaging, Deerfield, Illinois, USA
2PathFinder Brain SPECT Imaging, Deerfield, Illinois, USA
*Corresponding Author: Steven Richard Devore Best, The Neuroscience Center, Deerfield, Illinois, USA.
Received: April 15, 2019; Published: May 02, 2019
Abstract
Treatment-resistant depression (TRD) is a major public health problem, with approximately one third of patients with depression failing to respond to multiple antidepressant medications. It can also be associated with suicidal ideation and when it occurs alongside chronic pain and neurotoxicity (substance misuse/ abuse whether iatrogenic or illicit) it is especially likely to be resistant to treatment. One factor underlying TRD is the dysregulation of a thalamo-cortical circuit including the anterior cingulate cortex (ACC). Recent research has shown the efficacy of both transcranial magnetic stimulation (TMS) and of intravenous ketamine, as therapeutic approaches in TRD and anxiety. We hypothesized that stimulating the ACC with TMS would facilitate entrainment of this circuit, thereby improving response to ketamine. We describe the novel therapy of combination TMS with ketamine (CTK) for an adult female patient with severe, long-term depression, with recent suicidal ideation triggered by the death of her spouse, all comorbid with chronic pain, anxiety and iatrogenic neurotoxicity. Pre- and post-treatment assessments indicated that the CTK therapy resulted in substantial symptom reductions in all disorders. This was corroborated at follow-up by the significant functional improvement observed on brain imaging with Single Photon Emission Computed Tomography (SPECT).
Keywords: Treatment-Resistant Depression (TRD); Transcranial Magnetic Stimulation (TMS); ketamine; combination; comorbidity; Biomarker
Abbreviations: ACC: Anterior Cingulate Cortex; BPI-SF: Brief Pain Inventory-Short Form; Ceretec: 99mTechnetium- HMPAO; CTK: combination TMS with ketamine; HMPAO: Hexamethyl, propylene amine oxime; MDD: Major Depressive Disorder; MRI: Magnetic Resonance Imaging; NMDA: N- Methyl-D-Aspartate; OASIS: Overall Anxiety Severity and Impairment Scale; PFC: Prefrontal Cortex; PHQ-9: Patient Health Questionnaire; SPECT: Single Photon Emission Computed Tomography; SWLS: Satisfaction with Life Scale; TRD: Treatment-Resistant Depression; TMS: Transcranial Magnetic Stimulation.
Introduction
Treatment-resistant depression (TRD) refers to a chronic major depressive disorder (MDD) in spite of the administration of an adequate dose of an antidepressant medication for sufficient duration, with good treatment adherence, and yet resulting in nonresponse or lack of remission [1]. Mental health experts commonly agree that TRD should only be diagnosed in patients who have not been helped by two or more antidepressant treatment trials of adequate dose and duration [1,2]. TRD is a major public health problem, with approximately one third of patients with depression failing to respond satisfactorily to multiple antidepressant medications [1]. In addition, TRD is associated with general medical costs estimated to be 20 times greater than those stemming from treatment-responsive depression [3]. Depression occurring alongside a comorbid anxiety disorder and/or pain may contribute to treatment resistance [1-4].
To address these challenges, recent research investigated the efficacy of Transcranial Magnetic Stimulation (TMS) and its variant, repetitive Transcranial Magnetic Stimulation (rTMS), as a treatment for TRD and anxiety [5,6]. Most studies have used TMS to apply electromagnetic stimulation to the left dorsolateral prefrontal cortex (DLPFC), a region implicated in both depression and anxiety [7-9]. Research has shown a strong anticorrelation between functional MRI signals in the subgenual anterior cingulate cortex (ACC) and the dorsolateral prefrontal cortex (PFC) [10, 11].
Unfortunately, few studies have demonstrated full remission with TMS alone. A parallel body of research indicates a positive effect of ketamine, an N-Methyl-D-Aspartate (NMDA) antagonist, on depression [12, 13] and pain [14]. A primary benefit of ketamine is that it provides rapid, albeit short-term, relief from TRD symptoms relating to suicidality within approximately two hours. To date, little research has been carried out to explore the possible synergistic effects of combining TMS with ketamine for the treatment of TRD alone or comorbid with pain.
Research suggests that one factor involved with TRD is the dysregulation of a thalamocortical circuit, including the ACC, among other areas [15]. Research has also shown abnormalities in ACC neuronal functioning for anxiety [16]. Accordingly, we hypothesized that stimulating the ACC with TMS would facilitate improved reactivity of this circuit, thereby improving response to ketamine. In addition, the ketamine effect facilitates the increase of TMS intensity above the standard level, which further helps to re-establish normal oscillatory rhythms in this region, leading to a decrease in depression and anxiety symptoms.
Brain imaging using single-photon emission computed tomography (SPECT) can be used to evaluate the functional status of gray matter areas via the measurement of relative changes in brain perfusion and can be a useful tool to assess cerebral dysfunction [17-20]. In addition, a systematic review of neurobiological changes in 52 studies showed that neuroimaging, including SPECT imaging, were good predictors of response to TMS in MDD [21].
We report on a patient with TRD comorbid with chronic pain, iatrogenic neurotoxicity, anxiety and suicidal ideation. The patient had not responded to numerous types of psychotropic and pain medicine but showed remarkable results after treatment with the novel combination therapy of TMS with intravenous ketamine infusions (CTK), first proposed in recent papers from our group [4, 22,23]. A recent clinical review of 28 patients demonstrated the potential long-term benefits of CTK for patients with depressive syndromes [24].
Methods
The patient was a 62-year-old European-American woman working in the nursing field and presenting on the verge of suicide. Following episodes of ethanol abuse, skeletal pain and prolonged family stressors and intense grief (eventual death of husband after a long battle with cancer), together with a history of medication trials and multiple treatment failures, she had been considered as TRD.
Her formal diagnoses were TRD and the effects of polypharmacy, which had been prescribed to address her suffering. The neurological exam was within normal limits and did not suggest focal or coarse dysfunction of the central nervous system. She had been treated with varied pharmacological interventions but, during this time, her symptoms did not respond to algorithm-driven pharmacologic management.
Before beginning CTK therapy, the patient was assessed using well-validated measures of anxiety (OASIS), depression and suicidality (PHQ-9) and Satisfaction With Life Scale (SWLS). Marked impairment was indicated (OASIS = 20; PHQ-9 = 25 and SWLS = 12). She also underwent functional brain imaging with SPECT using 99mTc-Ceretec (HMPAO) before CTK therapy and following 5 months of treatment [17-19]. The SPECT images taken prior to CTK therapy showed extensive bilateral hypo perfusion involving all lobes (see upper row of Figure 1).
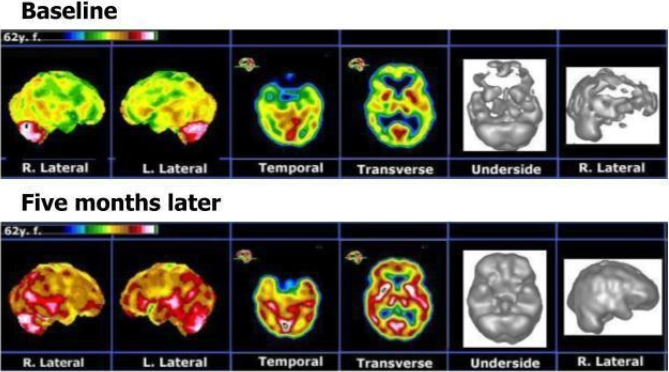
Figure 1: Brain SPECT functional imaging of an individual TRD patient, pre- and post-combination TMS and ketamine infusion (CTK) therapy. The upper row represents selected images from the baseline display. The lower row represents the corresponding images five months after CTK therapy began. Post-treatment there were major perfusion improvements involving all cortical and subcortical structures.
The treatment initiated was based on the patented CTK therapy developed by the first author of this case study [25]. The treatment protocol included two days of TMS pre-treatment with four TMS sessions daily (30 minutes duration with 45 minutes of rest between treatments). The following day, CTK treatment delivered continuous TMS (40 minutes, 1 Hz) with a simultaneous intravenous ketamine infusion (30 minutes), resulting in five minutes of TMS alone pre- and post-infusion [4,22,23]. To attenuate anxiety during treatment, anxiolytics were given on an as-needed basis (Versed, 1-2 mg).
During pre-treatment and CTK, the TMS head coil was positioned at the midline of the anterior scalp to achieve maximal stimulation of the medial prefrontal area that overlays the ACC and commonly described as Fz on the International 10-20 System, a region implicated in depression and anxiety [7,8,16]. TMS treatments were administered at 115% of motor threshold at 1 Hz continuous pulsation settings, established to be within safety guidelines and consistent with our protocol in use for over three years [4,22,23]. The TMS equipment used was Neotonus®, which was identical to the equipment sold by Neuronetics.
The IV ketamine infusion was delivered in a standard commercial formulation using a biomarker-dependent dosing strategy, whereby ketamine (beginning at 100 mg) was gradually titrated in small increments until the patient entered a mildly cataleptic state. The ketamine used was Ketalar®, which is a Registered Trademark of PAR Sterile Products LLC.
Treatment began the following day and continued 2 or 3 times per week for the following months. The dosage of infused ketamine increased gradually from 100 mg at the first session to a peak of 1,000 mg between 21st and 32nd sessions, before it was tapered down to 175 mg at the 58th session (five months later). At this time, Brain SPECT functional imaging was repeated. The CTK treatment continued at lower doses of ketamine for a further four weeks, at which time the battery of psychological tests was also repeated.
Results and Discussion
Following five months of treatment (58 sessions), the patient reported markedly improved symptoms and a dramatic clinical improvement, leading to major changes in her daily life: enthusiastic, rational, planning for future, taking charge of her financial and family situation and a renewed religious sentiment. At this time, the post-treatment brain SPECT imaging showed markedly improved perfusion across the cortical and sub-cortical structures (Figure 1).
Figure 1 provides the SPECT images prior to treatment (upper row) and after five months of CTK therapy (lower row). The first two columns show 2/8 images from the stereotactic surface projections obtained via the Neurostat software [18] and applying our own color code. The next two columns are representative of the orthogonal sections display. The last two columns show volumetric displays (threshold=67%). These SPECT images show major perfusion increases involving all cortical and subcortical structures following CTK treatments.
Following an additional month of treatment, the repeated psychological testing also reflected major improvements. She reported being free of anxiety, depression, and suicidality (OASIS = 0, PHQ-9 = 1). Moreover, she reported herself to be extremely satisfied with her life circumstances (SWLS = 35). An additional observation was that she no longer complained about pain. At a long-term follow-up, the patient had been practically free of suffering for almost three years.
This case report presents the therapeutic strategy aimed at addressing a complex cluster of symptoms occurring in the context of comorbid psychiatric and somatic disorders (depression, anxiety, grief, neurotoxicity and pain). While existing research has indicated that TMS is somewhat effective in treating depression and anxiety [5,6,9], and that ketamine produces short-term relief from depression and anxiety [12,13], it is the CTK therapy using TMS simultaneously with ketamine infusion that achieved the effective and long-term results in this case.
CTK therapy resulted in tremendous relief from the otherwise intractable depression and comorbid anxiety, as well as improvement of the somatic component (neurotoxicity and chronic pain). The favorable effect of CTK is further emphasized by the substantial and widespread improvement of cortical perfusion seen on the brain SPECT functional imaging following therapy, shown in Figure 1. It is hypothesized that modulation of the known dysfunctional thalamocortical circuit, via the entraining effect of electromagnetic stimulation, rendered this patient more responsive to the ketamine infusion. This positive result appears to be enduring, as the patient has been free of suffering for almost 3 years, at the time of this writing.
This long-term relief is further substantiated by our present clinical experience with similar cases, as well as by a recent literature review that points to the relation between mental disorders and chronic physical conditions, as well as the need to integrate their treatment [24, 26]. Future work will continue to evaluate the efficacy, as well as the response predictors of this treatment on a larger patient population.
Conclusion
This case presentation points to the clinical long-term efficacy of the novel CTK therapy of combination TMS with ketamine infusion in a case of TRD, with anxiety, suicidal ideation and comorbid with chronic pain and iatrogenic neurotoxicity. The major clinical improvements were durable and also substantiated by the positive changes upon routine questionnaire-based psychometric assessment and by the major increases in cortical and subcortical perfusion, as seen on the brain SPECT functional imaging. The favorable results were obtained despite years of unsuccessful previous treatments and at the time of writing, appears to be enduring as the patient has been free from suffering for almost 3 years.
References
- Sackeim HA. “The definition and meaning of treatment-resistant depression”. The Journal of Clinical Psychiatry62 (2001): 10-17.
- Fava M and Davidson KG. “Definition and epidemiology of treatment-resistant depression”. Psychiatric Clinics of North America 19.2 (1996): 179-200.
- Crown WH., et al. “The impact of treatment-resistant depression on health care utilization and costs”. The Journal of Clinical Psychiatry63.11 (2002): 963-971.
- Best SRD. “Rapid relief of treatment resistant depression by facilitated ketamine infusion: A preliminary report”. Activitas Nervosa Superior 56.1-2 (2014): 28-36.
- Gross M., et al. “Has repetitive transcranial magnetic stimulation (rTMS) treatment for depression improved? A systematic review and meta analysis comparing the recent vs. the earlier rTMS studies”. Acta Psychiatrica Scandinavica 116.3 (2007): 165-173.
- Zwanzger P., et al. “Transcranial magnetic stimulation for panic”. American Journal of Psychiatry159 (2014): 315-326.
- Chang CC., et al. “Reduction of dorsolateral prefrontal cortex gray matter in late-life depression”. Journal of Psychiatric Research193.1 (2011): 1- 6.
- Smith R., et al. “Antidepressant effects of sertraline associated with volume increases in dorsolateral prefrontal cortex”. Journal of Affective Disorders146.3 (2013): 414-419.
- Cohen H., et al. “Repetitive transcranial magnetic stimulation of the right dorsolateral prefrontal cortex in posttraumatic stress disorder: a double-blind, placebo-controlled study”. American Journal of Psychiatry161.3 (2004): 515-524.
- Fox MD., et al. “Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate”. Biological Psychiatry72.7 (2012): 595–603.
- Cash RFH., et al. “Subgenual Functional Connectivity Predicts Antidepressant Treatment Response to Transcranial Magnetic Stimulation: Independent Validation and Evaluation of Personalization”. Biological Psychiatry(2019).
- Berman RM., et al. ”Antidepressant effects of ketamine in depressed patients”. Biological Psychiatry47.4 (2000): 351-354.
- Ionescu DF., et al. “A single infusion of ketamine improves depression scores in patients with anxious bipolar depression”. Bipolar Disorders 17.4 (2014): 438-443.
- Niesters M., et al. “Ketamine for chronic pain: risks and benefits”. Journal of Clinical Pharmacology 77.2 (2014): 357-367.
- Llínas RR., et al. “Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography”. Proceedings of the National Academy of Sciences of the United States of America96.26 (1999): 15222-15227.
- Etkin A., et al. “Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder”. American Journal of Psychiatry167.5 (2010): 545-554.
- Catafau AM. “Brain SPECT in clinical practice. Part I: perfusion”. Journal of Nuclear Medicine 42.2 (2001): 259-271.
- Minoshima S., et al. “Anatomical standardization: linear scaling and nonlinear warping of functional brain images”. Journal of Nuclear Medicine35.9 (1994): 1528-1537.
- Juni JE., et al. “Procedure guidelines for brain perfusion SPECT using 99mTc radiopharmaceuticals 3.0”. Journal of Nuclear Medicine Technology37.3 (2009): 191-195.
- Pavel D and Best SRD. “Brain SPECT as imaging biomarker for evaluating effects of novel treatments in psychiatry”. Journal of Nuclear Medicine58.1 (2017): 1298.
- Fidalgo TM., et al. “Biological markers in noninvasive brain stimulation trials in major depressive disorder: A systematic review”. Journal of ECT 30.1 (2014): 47-61
- Best SRD. “Combined ketamine/transcranial magnetic stimulation treatment of severe depression in bipolar I disorder”. Journal of ECT 30.4 (2014): 50-51.
- Best SRD. “Combined ketamine and transcranial magnetic stimulation for treatment resistant depression in the context of chronic OCD: A case report”. Neuropsychiatric Electrophysiology 1.2 (2015): 1-2.
- Best SRD., Pavel, DG., Haustrup, N., Raskas, EJ. “Combined treatment of transcranial magnetic stimulation and ketamine (CTK) for therapy of treatment-resistant depression: A long-term retrospective review of clinical use” Heliyon (submitted for review)
- Best SRD. “Treatment of thalmocortical dysryhthmia” United States Patent (2017), Patent number US9649501B”
- Scott KM., et al. “Association of mental disorders with subsequent chronic physical conditions: World mental health surveys from 17 countries”. JAMA Psychiatry 73.2 (2016): 150-158.
Citation:
Steven R D Best MD and Dan G Pavel MD. “Clinical Use of Combined Transcranial Magnetic Stimulation with Intravenous Ketamine for Treatment-Resistant Depression with Comorbid Pain, Substance Misuse, and Grief: A Case Report”. Current Opinions in Neurological Science 3.2 (2019): 672-677.
Copyright: © 2019 Steven Richard Devore Best. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.






























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.