Research Article
Volume 1 Issue 1 - 2016
Design and Evaluation of Cefixime Fast Dissolving Tablets Using Super Disintegrating Agents
Department of Pharmaceutics, Dr Samuel George Institute of Pharmaceutical sciences, Andhra Pradesh, India
*Corresponding Author: VL Narasaiah, Asst. Professor, Department of Pharmaceutics, Dr SGIPS, Andhra Pradesh, India.
Received: June 19, 2016; Published: August 31, 2016
Abstract
The aim of the present investigation was to formulate fast dissolving tablets of Cefixime by wet granulation using super disintegrants such as crospovidone, croscarmellose sodium, and sodium starch glycolate. The prepared tablets were evaluated for post compressional parameters like hardness, friability, weight variation, in-vitro disintegration time, in-vitro dispersion time, wetting time, in-vitro drug release studies. The drug-excipient interaction was studied by Fourier transform infrared spectroscopy (FTIR) studies. No chemical interaction between drug and excipients was confirmed by FTIR studies. Amongst all formulations, formulation F12 prepared by 24 mg of crospovidone showed less disintegrating time of 18.4 sec and faster dissolution. Enhancement of oral Bioavailability of cefixime can be increased by formulating it as a Fast Dissolving Tablet using crospovidone as super disintegrating agent.
Keywords: Cefixime; Fast dissolving; Super disintegrating agents
Abbreviations: FDT: Fast dissolving tablets; CCS: Croscarmellose sodium; SSG: Sodium starch glycolate; CP: Crospovidone; MCC: Microcrystalline cellulose; PVP: Poly vinyl pyrrolidine; SS: Sodium saccharine; MS: Magnesium stearate; FTIR: Fourier Transmitted Infrared Spectrophotometer
Introduction
Super disintegrants are the agents added to tablet formulations to encourage the break-up of the tablet into lesser fragments in an aqueous environment, thereby increasing the available surface area and promoting a more rapid release of the drug substance. In recent years, several newer disintegrants have been developed, are often called “super disintegrating agents”. [1,2] These newer substances can be used by lower levels than traditionally used disintegrants. There are three major mechanisms affecting tablet disintegration such as bulging, porosity, wicking and deformation. Three major groups of compounds have been developed as super disintegrants such as modified starches, cross-linked polyvinyl pyrrolidone and modified cellulose.
One of the major streams of application of super disintegrants is in the formulation of oral disintegrating tablets/mouth dissolving tablets. The oral fast dissolving tablet that disintegrates and dissolves in the mouth, either on or beneath the tongue or in the buccal cavity usually in a matter of seconds, without the need to take it water. [3]
The drug selected for the study was cefixime, which is used in the treatment of uncomplicated urinary tract infections. The biological half life is 3-4 hrs, oral bioavailability is 40-50% and its Protein binding approximately 60%. The main objective of the present study was to develop fast dissolving tablets of cefixime using sodium starch glycolate, croscarmellose sodium, and crospovidone as super disintegrating agents. It was also desired to study the effect of super disintegrants concentration. [4]
Materials and Methods
Materials: Cefixime a gift sample from M/S International Health care, Vijayawada, Andhra Pradesh, CCS, SSG, CP, MCC and Aerosil procured from Hetero drugs, Hyderabad, Mannitol, PVP, Sodium saccharin, Magnesium stearate obtained from SD Fine chemicals, Mumbai.
Methodology: Fast dissolving tablets were prepared by wet granulation technique by using super disintegrants such as CCS, SSG and CP. The list of ingredients is given in Table 1.
| Ingredients | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | F11 | F12 |
| Cefixime | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| CCS | 4 | 8 | 16 | 24 | - | - | - | - | - | - | - | |
| SSG | - | - | - | - | 4 | 8 | 16 | 24 | - | - | - | - |
| CP | - | - | - | - | - | - | - | - | 4 | 8 | 16 | 24 |
| MCC | 45 | 45 | 45 | 45 | 45 | 45 | 45 | 45 | 45 | 45 | 45 | 45 |
| Mannitol | 30 | 26 | 18 | 10 | 30 | 26 | 18 | 10 | 30 | 26 | 18 | 10 |
| PVP | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| SS | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Aerosil | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| MS | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
Table 1: Composition of Cefixime Fast Dissolving Tablets.
The components were blended and wet mass was prepared by adding ethanol. The wet mass was sieved (No.12) and dried at 45°C for 1 hr. The dried granules were again sieved (No.16) and lubricants, glidants were added. Tablets were compressed by using a Cadmach 16 station tablet machine with flat face punches and dies (9 mm in diameter).
Post Compressional Parameters: The prepared tablets were evaluated for thickness, weight variation, hardness, friability, Disintegration time, wetting time, water absorption ratio, and drug content studies. The thickness and diameter were measured by using Vernier calipers. In weight variation test twenty tablets were selected at a random and average weight was calculated. Then individual tablets were weighed and the weight was compared with an average weight. [5] The Pfizer hardness tester was used for the determination of hardness of tablets. A tablet was placed in contact between the plungers, and the handle was pressed, the force of fracture was recorded. The friability of the tablets was determined using Roche’s friabilator [6,7] (Cambel Electronics, Mumbai, India). The results were shown in Table 2.
| Formulation code | Thickness (mm) | Hardness (mm) | Friability (%) | Weight variation (mg) |
| F1 | 2.6 ± 0.01 | 4.20 ± 0.02 | 0.638 ± 0.03 | 199.25 |
| F2 | 3.4 ± 0.05 | 4.13 ± 0.04 | 0.744 ± 0.06 | 200.5 |
| F3 | 2.5 ± 0.03 | 4.12 ± 0.06 | 0.641 ± 0.09 | 205.75 |
| F4 | 3.1 ± 0.07 | 4.21 ± 0.08 | 0.579 ± 0.01 | 206.5 |
| F5 | 2.7 ± 0.06 | 4.19 ± 0.03 | 0.676 ± 0.04 | 202.25 |
| F6 | 2.9 ± 0.03 | 4.16 ± 0.05 | 0.686 ± 0.07 | 206.75 |
| F7 | 3.2 ± 0.03 | 4.18 ± 0.07 | 0.821 ± 0.02 | 207.5 |
| F8 | 3.3 ± 0.02 | 4.14 ± 0.09 | 0.783 ± 0.04 | 203.25 |
| F9 | 2.6 ± 0.04 | 4.15 ± 0.02 | 0.639 ± 0.05 | 201.75 |
| F10 | 3.0 ± 0.08 | 4.18 ± 0.01 | 0.698 ± 0.06 | 205.5 |
| F11 | 2.8 ± 0.03 | 4.13 ± 0.04 | 0.591 ± 0.08 | 199.75 |
| F12 | 3.5 ± 0.04 | 4.17 ± 0.05 | 0.599 ± 0.01 | 204.5 |
Table 2: Post Compressional Parameters for Cefixime Fast Dissolving Tablets.
Wetting Time: The wetting time of the tablets can be measured using a simple procedure. Five circular tissue papers of 10 cm diameter are placed in a petridish with a 10cm diameter. 10 ml of water-containing amaranth a water soluble dye is added to petridish. A tablet is carefully placed on the surface of the tissue paper. The time required for water to reach upper surface of the tablet is noted as a wetting time. [8] The results were shown in Table 3.
| Formulation code | Wetting time (sec) | Water absorption ratio | Disintegration time (sec) | Drug content (%) |
| F1 | 9.5 | 240.8 ± 0.05 | 22.3 | 99.4 ± 0.12 |
| F2 | 9.3 | 248.3 ± 0.04 | 20.9 | 98.3 ± 0.14 |
| F3 | 8.2 | 232.6 ± 0.02 | 25.5 | 99.8 ± 0.11 |
| F4 | 8.6 | 240.4 ± 0.03 | 19.7 | 99.6 ± 0.13 |
| F5 | 9.4 | 236.3 ± 0.03 | 22.6 | 100.2 ± 0.1 |
| F6 | 9.1 | 228.2 ± 0.01 | 25.4 | 98.9 ± 0.16 |
| F7 | 8.9 | 224.9 ± 0.07 | 21.3 | 99.5 ± 0.14 |
| F8 | 8.7 | 252.6 ± 0.06 | 23.1 | 98.5 ± 0.12 |
| F9 | 8.9 | 248.3 ± 0.05 | 19.5 | 98.7 ± 0.18 |
| F10 | 8.8 | 244.1 ± 0.07 | 26.3 | 101.2 ± 0.1 |
| F11 | 9.4 | 236.5 ± 0.05 | 23.3 | 99.7 ± 0.13 |
| F12 | 9.3 | 224.2 ± 0.05 | 18.4 | 98.2 ± 0.11 |
Table 3: Post Compressional Parameters for Cefixime Fast Dissolving Tablets.
Water Absorption Ratio: A piece of tissue paper folded twice was placed in a small petri dish (10 cm diameter) containing 6 ml of water. A tablet was put on the tissue paper and allowed to wet completely. The wetted tablet was then reweighed. Water absorption ratio, R was determined using following equation9. The results were shown in Table 3.
R = Wa – Wb × 100/Wb
Where, Wa = Weight of tablet after water absorption and
Wb = Weight of tablet before water absorption.
Wb = Weight of tablet before water absorption.
Disintegration Time: The test was carried out on 6 tablets using the apparatus specified in I.P.-1996 distilled water at 37ºC ± 2ºC was used as a disintegration media and the time in second taken for complete disintegration of the tablet with no palpable mass remaining in the apparatus was measured in seconds9. The results were shown in Table 3
Drug Content [10]: Twenty tablets were weighed and powdered. The quantity of powder equivalent to 50 mg of cefixime was dissolved in phosphate buffer pH 7.2 diluted to 100 ml with the same and the solution was filtered and suitably diluted. The drug content was estimated spectrophotometrically at 280 nm.
In vitro Dissolution Study: Dissolution study of cefixime fast dissolving tablets was carried out using USP type-II (paddles) dissolution apparatus using 900 ml of pH 7.2 maintained at 37 ± 0.5oC at a speed of 100 rpm. At known regular intervals, 5 ml of sample of dissolution medium were withdrawn by means of syringe fitted with pre-filter and analyzed for drug release by measuring the absorbance at 280 nm after suitable dilution with water. The volume of dissolution fluid was adjusted to 900 ml by replacing each 5 ml aliquot withdrawn with 5 ml of water. [11,12] The % drug release was calculated using an equation obtained from the calibration curve.
Drug – Excipient Compatibility Studies
FT-IR Spectroscopy: Infrared spectroscopy was conducted using thermo Nicol nexus 670 spectrophotometer and the spectrum was recorded in the wavelength region of 4000 to 500 cm-1. The procedure consisted of dispersing a sample (drug alone or mixture of drug and excipients) in KBr and compressing into discs by applying a pressure. The pellet was placed in the light path and the spectrum was obtained.
Results and Discussion
The present investigation was carried out on the design and evaluation of fast dissolving tablets of cefixime, which is having a short biological half-life, meant for the treatment of gonorrhea, tonsillitis and pharyngitis. In the present investigation fast dissolving tablets were prepared by wet granulation method. The use of super disintegrants for the preparation of fast dissolving tablets is highly effective and commercially useful. Prepared fast dissolving tablets are dispersed in the mouth quickly. The super disintegrants like CCS, SSG and CP alone in various concentrations were studied for achieving faster dissolving tablets.
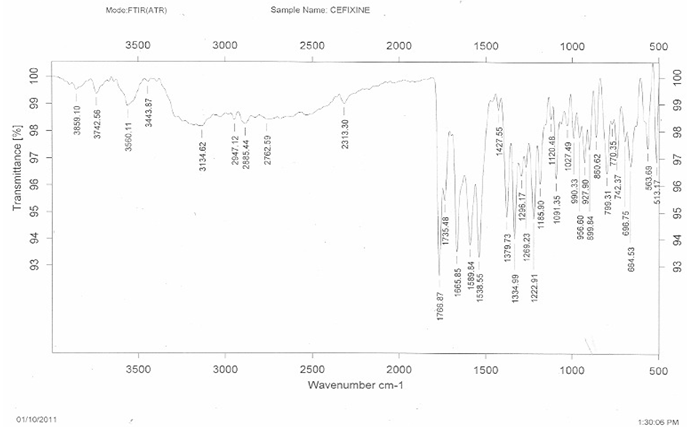
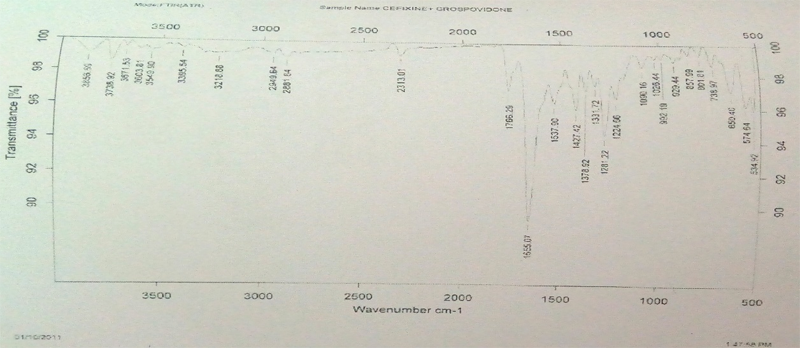
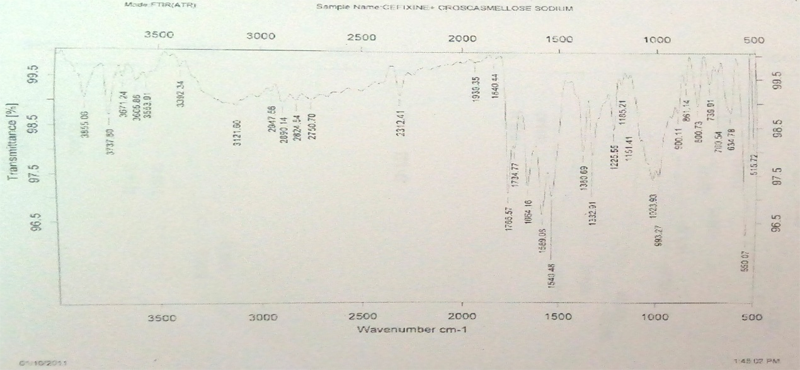
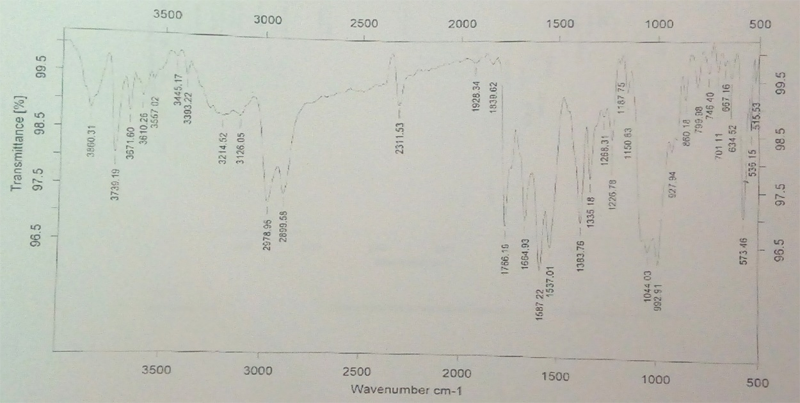
The FTIR spectra of pure cefixime and excipients are shown in Figure 1. The FTIR spectra of pure cefixime showed characteristic peaks at 2947.12 cm-1 (CH – stretching for alkanes), 1766.87 cm-1 (C = O – stretching for carboxylic acid), 3742.56 cm-1 (N-H - stretching), 1296.17 cm-1 (C-C - bending), 751.62 cm-1, 1427.55 cm-1 (C-H- bending). It might be the possibility of intermolecular hydrogen bonding between adjunct cefixime molecules. The spectrum of pure cefixime was equivalent to the spectra obtained by the addition of carrier. This indicated that no interaction occurred cefixime when mixed with excipients. These observations indicated that, the compatibility of excipients with cefixime.
Studies on CCS by Wet Granulation Method: The results of post compressional parameters tablets for the formulations F1, F2, F3 and F4 are shown in Table 2. The results of thickness (mm) for the formulations ranged from 2.5 ± 0.03 to 3.4 ± 0.05 and the hardness (kg/cm2) for the formulations ranged from 4.12 ± 0.06 to 4.21 ± 0.08. The results of friability (%) for the formulations ranged from 0.579 ± 0.01 to 0.744 ± 0.06 and the percentage weight variation ranged from 199.25 to 206.5 mg.
From the above results, all the formulations showed uniform thickness, hardness of the tablets was satisfactory and the percentage friability for all the formulations were below 1% indicating that friability was within the prescribed limits.
The results of drug content (%) for the formulation ranged from 98.3 ± 0.14 to 99.8 ± 0.11 good and uniform drug content (> 98%) was observed within the batches of different tablet formulations. The results of wetting time for the formulation ranged from 8.2 to 9.3. The results of Water absorption ratio for the formulation ranged from 232.6 ± 0.02 to 248.3 ± 0.04. The results were shown in Table 3.
To study the effect of CCS on release rate of cefixime from the tablets as shown in Figure 2A, different concentration of CCS (4, 8, 12, 16 mg) were employed by kneading the other process variables verses concentration of other excipients, method of preparation and hardness were kept constant. The drug release followed first order kinetics and Higuchi mechanism, the data was shown in Table 4.
| Formulation code | Correlation coefficient | % DE | ‘N’ value | |||
| Zero order | First order | Higuchi | Peppas | |||
| F1 | 0.9877 | 0.9807 | 0.9896 | 0.9765 | 20.38 | 0.223 |
| F2 | 0.9842 | 0.9867 | 0.9807 | 0.9738 | 24.87 | 0.325 |
| F3 | 0.9884 | 0.9866 | 0.9886 | 0.9647 | 29.76 | 0.419 |
| F4 | 0.9837 | 0.9910 | 0.9926 | 0.9591 | 33.42 | 0.502 |
| F5 | 0.9881 | 0.9862 | 0.9883 | 0.9697 | 23.05 | 0.215 |
| F6 | 0.9840 | 0.9858 | 0.9898 | 0.9703 | 24.88 | 0.317 |
| F7 | 0.9783 | 0.9855 | 0.9930 | 0.9638 | 28.92 | 0.444 |
| F8 | 0.9539 | 0.9905 | 0.9964 | 0.9480 | 35.58 | 0.494 |
| F9 | 0.9845 | 0.9743 | 0.9918 | 0.9649 | 29.6 | 0.202 |
| F10 | 0.9734 | 0.9813 | 0.9974 | 0.9515 | 34.71 | 0.241 |
| F11 | 0.9781 | 0.9591 | 0.9955 | 0.9568 | 37.25 | 0.332 |
| F12 | 0.9781 | 0.9921 | 0.9950 | 0.9595 | 42.54 | 0.407 |
Table 4: Dissolution Kinetics of Cefixime Fast Dissolving Tablets.
Application of korsmeyer peppas equation to the data showed that the mechanism of drug release of cefixime from CCS is governed by predominant fickian diffusion (slope > 0.5). It was also observed that the release rate was found to be influence of CCS employed in the preparation of fast dissolving tablets. Good correlation was observed in between the concentration of super disintegrating and release rate constant. The above results indicate that the increasing concentration of CCS, the drug release was enhanced.
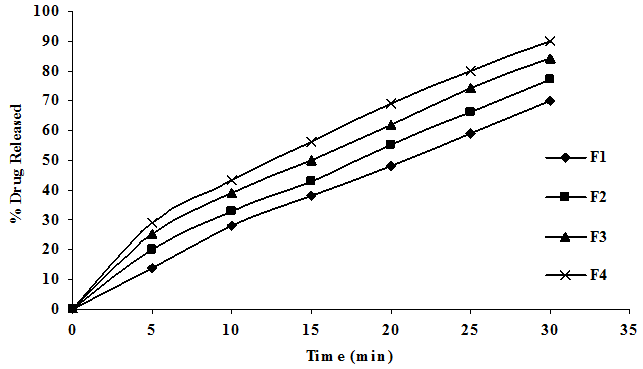
Figure 2A: Dissolution Profiles of Cefixime Fast Dissolving Tablets of Various Concentrations of CCS.
Studies on SSG by Wet Granulation Method: The results of physicochemical evaluation of tablets for the formulations F5, F6, F7 and F8 are shown in Table 2.
The results of thickness (mm) for the formulations ranged from 2.7 ± 0.06 to 3.3 ± 0.02 and the hardness (kg/cm2) for the formulations ranged from 4.14 ± 0.09 to 4.19 ± 0.03. The results of friability (%) for the formulations ranged from 0.676 ± 0.04 to 0.821 ± 0.02 and the weight variation ranged from 202.25 to 207.5.
From the above results, all the formulations showed uniform thickness, hardness of the tablets was satisfactory and the percentage friability for all the formulations were below 1% indicating that friability was within the prescribed limits.
The results of drug content (%) for the formulation ranged from 98.5 ± 0.12 to 100.2 ± 0.1. Good and uniform drug content (> 98%) was observed within the batches of different tablet formulations. The results of wetting time for the formulation ranged from 8.7 to 9.4. The results of Water absorption ratio for the formulation ranged from 224.9 ± 0.07 to 252.6 ± 0.06 mg. The results were shown in Table 3.
To study the effect of SSG on release rate of cefixime from the tablets as shown in Figure 3A, different concentration of SSG (4, 8, 12, 16 mg) were employed by kneading the other process variables verses concentration of other excipients method of preparation and hardness were kept constant. The drug release followed first order kinetics and Higuchi mechanism, the data was shown in Table 4. Application of korsmeyer peppas equation to the data showed that the mechanism of drug release of cefixime from SSG is governed by predominant fickian diffusion (slope > 0.5). It was also observed that the release rate was found to be influence of SSG employed in the preparation of fast dissolving tablets good correlation was observed in between the concentration of super disintegrating and release rate constant. The above results indicate that the increasing concentration of SSG, the drug release was enhanced.
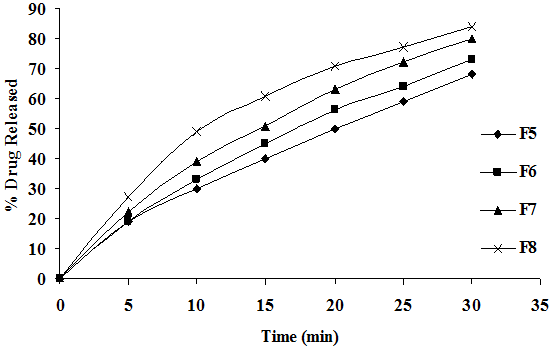
Figure 3A: Dissolution Profiles of Cefixime Fast Dissolving Tablets of Various Concentrations of SSG.
Studies on CP by Wet Granulation Method: The results of physicochemical evaluation of tablets for the formulations F9, F10, F11, and F12 are shown in Table 2. The results of thickness (mm) for the formulations ranged from 2.6 ± 0.04 to 3.5 ± 0.04 and the hardness (kg/cm2) for the formulations ranged from 4.13 ± 0.04 to 4.18 ± 0.01. The results of friability (%) for the formulations ranged from 0.591 ± 0.08 to 0.698 ± 0.06 and the percentage weight variation ranged from 199.75 to 205.5.
From the above results, all the formulations showed uniform thickness, hardness of the tablets was satisfactory and the percentage friability for all the formulations were below 1% indicating that friability was within the prescribed limits.
The results of drug content (%) for the formulation ranged from 98.2 ± 0.11 to 101.2 ± 0.15. Good and uniform drug content (> 98%) was observed within the batches of different tablet formulations. The results of wetting time for the formulation ranged from 8.8 to 9.4. The results of Water absorption ratio for the formulation ranged from 224.2 ± 0.05 to 248.3 ± 0.05 mg. The results were shown in Table 3.
To study the effect of CP on release rate of cefixime from the tablets as shown in Figure 4, different concentration of CP (4, 8, 12, 16 mg) were employed by kneading the other process variables verses concentration of other excipients, method of preparation and hardness were kept constant. The drug release followed first order kinetics and Higuchi mechanism, the data was shown in Table 4. Application of korsmeyer peppas equation to the data showed that the mechanism of drug release of cefixime from CP is governed by predominant fickian diffusion (slope > 0.5). It was also observed that the release rate was found to be influence of CP employed in the preparation of fast dissolving tablets good correlation was observed in between the concentration of super disintegrating and release rate constant. The above results indicate that the increasing concentration of CP, the drug release was enhanced.
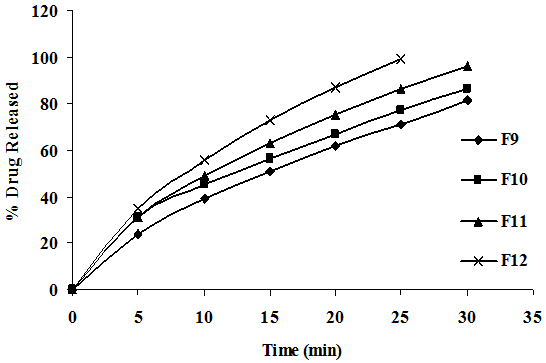
Figure 4A: Dissolution Profiles of Cefixime Fast Dissolving Tablets of Various Concentrations of CP.
Comparative Studies on CCS, CP and SSG: Disintegration time is very important for FDTs which is desired to be less than 60 seconds for orally disintegrating tablets. This rapid disintegration assists swallowing and also plays a role in drug absorption in buccal cavity, thus promoting bioavailability. Disintegration time of prepared FDTs was in the range of 18.4 to 26.3 seconds and the order was CP > CCS > SSG. This finding is in agreement with results obtained from wetting time, since SSG swells with more gelling than CCS and CP, which extend disintegration time as a result. As the concentration of super disintegrants in the formulations increased the disintegration time was found to decrease.
Wetting time is used as an indicator from the ease of the tablet disintegration in buccal cavity. It was observed that wetting time of tablets was in the range of 8.2 to 9.5 seconds. It was observed that type of the disintegrant affected the wetting of the tablets. On comparing super disintegrants the formulation containing SSG take more wetting time than CCS and CP. Wetting is related to the inner structure of the tablets and hydrophobicity of the components. This may be due to the fact that SSG is disintegrated by swelling mechanism leading to longer wetting time. Crospovidone and CCS perform their disintegrating action by wicking through capillary action and fibrous structure, respectively with minimum gelling.
Conclusion
The above results concluded that, although differences existed between the super disintegrants, the fast dissolving Cefixime tablets could be prepared by using any of the super disintegrants used. Overall results indicates that formulation F12, which contain 24 mg of CP was better one and satisfies all the criteria as fast dissolving tablet. Cefixime showing enhanced dissolution, may lead to improved bioavailability, improved effectiveness and hence better patient compliance.
References
- Lokesha Puttalin G and Kunchu Kavitha. “Fast Disintegrating Tablet: An Overview of Formulation, Technology and Evaluation”. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2.2 (2011): 589-601.
- Kumar S., et al. “A Review on Recent Trends in Oral Drug Delivery-Fast Dissolving Formulation”. Advances in Biological Research 6.1 (2012): 6-13.
- Sweetman SC. The Complete Drug Reference. 33th ed. London: Pharmaceutical Press; 2002.
- U.S. Pharmacopeial Convention. U.S. Pharmacopeia XXII/National Formulary 17; U.S. Pharmacopeial Convention: Rockville, MD, 1990.
- Sethi Vandana and Shrivastava Priyanka. “A Review Article on: Superdisintegrants”. Journal of Global Pharma Technology 10.4 (2012): 15-20.
- Bhagwati S T., et al. “Comparative Evaluation of Disintegrants by Formulating Cefixime Dispersible Tablets”. Indian Journal of Pharmaceutical Education and Research 39 (2005): 194-197.
- S B Shirsand., et al. “Formulation Design of Fast Disintegrating Tablets using Disintegrant Blends”. Indian Journal of Pharmaceutical Sciences 72.1 (2010): 130-133.
- Rawas-Qalaji M M., et al. “Fast-disintegrating sublingual tablets: effect of epinephrine load on tablet characteristics”. American Association of Pharmaceutical Scientists 7.2 (2006): E41.
- K S Remya., et al. “Formulation development, evaluation and comparative study of effects of superdisintegrants in cefixime oral disintegrating tablets”. Journal of Young Pharmacists 2.3 (2010): 234- 239.
- Kuchekar BS and Arumugam V. “Fast dissolving tablets”. Indian Journal of Pharmaceutical Education and Research 35 (2001): 150-152.
- N. G. Raghavendra Rao., et al. “Design And Evaluation Of Valsartan Fast Dissolving Tablets By Direct Compression Method”. American Journal of Pharmaceutical Research 5.1 (2015): 158-168.
- Sharma Vijay., et al. “Formulation and Evaluation of Mouth Dissolve Tablets of Cefixime”. International Research Journal of Pharmacy 2.5 (2011): 171-174.
Citation:
V L Narasaiah., et al. “Design and Evaluation of Cefixime Fast Dissolving Tablets Using Super Disintegrating Agents”. Chronicles of Pharmaceutical Science 1.1 (2016): 1-9.
Copyright: © 2016 VL Narasaiah., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.