Short Note
Volume 1 Issue 1 - 2017
Brief on -Hyphenated Methods of HPLC for Determining the Presence of Solutes.
Glocal School of Pharmacy, Glocal University, Mirzapur Pole, Saharanpur, 247121. U.P
*Corresponding Author: Mohamad Taleuzzaman, Glocal School of Pharmacy, Glocal University, Mirzapur Pole, Saharanpur, 247121. U.P.
Received: December 13, 2016; Published: February 20, 2017
Abstract
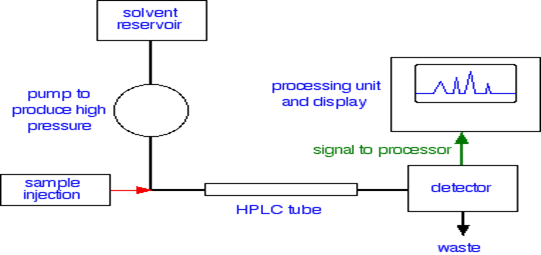
HPLC is the tool in liquid chromatography is unique because of particle size, smaller particle in the stationary phase, increase efficiency of a separation. However, if the particles are made smaller, capillary action increases and it becomes more difficult to drain the column under gravity. For quantitative analysis different types of detector is used in conjunction with HPLC which give precise and accurate result and it is apply according to the nature of the substance. Various types of detectors used in HPLC are mass spectrometry, infrared spectroscopy, visible spectroscopy, ultraviolet spectroscopy, fluorescence spectroscopy, nuclear magnetic resonance, conductivity measurement, and refractive index measurement. Each detector has its assets, limitations and sample types for which it is most effective. The recent development of the so-called hyphenated techniques has improved the ability to separate and identify multiple entities within a mixture.
Keywords: Hyphenated; Chromatography; Detector; Particle size; Quantitive analysis
Introduction
“Hyphenation” term introduce by Hirschfeld a couple of decades ago, to complete the chromatography analysis–separation, identification and quantification. Quantification has done by the hyphenated instrument like mass spectroscopy, infrared spectroscopy, visible spectroscopy, ultraviolet spectroscopy, fluorescence spectroscopy, conductivity measurement, refractive index measurement and nuclear magnetic resonance. In recent years, hyphenated techniques have received ever-increasing attention as the principal means to solve complex analytical problems. The combination power which better the identification and quantification in complex mixture. The unknown sample is also analyses in very precise and accurate method [1-3].
To give the structural information of a constituent present in sample high-performance liquid chromatography (HPLC), is linked to spectroscopic detection techniques, e.g., Fourier-transform infrared (FTIR), photodiode array (PDA) UV–VIS absorbance or fluorescence emission, mass spectroscopy (MS), and nuclear magnetic resonance spectroscopy (NMR),is apply. By this conjunction technique structural information of active molecules is obtain [2].
The conjunction between liquid chromatography and spectroscopy is basically a physical connection. In pharmaceutical industry hyphenated technique of HPLC with MS or NMR has increased the capability of solving structural problems of complex natural products [4-5]. Because of the greater sensitivity, LC-MS has been more extensively used than LC-NMR. The hyphenation does not always have to be between two techniques; the coupling of separation and detection techniques can involve more than one separation or detection techniques, e.g., LC-PDA-MS, LC-MS-MS, LC-NMR-MS, LC-PDA-NMR-MS [6].
The development of various hyphenated techniques has provided the natural product researchers with extremely powerful new tools that can provide excellent separation efficiency as well as acquisition of on-line complementary spectroscopic data on an LC or GC peak of interest within a complex mixture [7]. The main focus of this article is to provide an overview of basic operational principles of various modern hyphenated techniques and to present several literature examples of applications of these techniques. Here in brief on some hyphenated instrument like information on the principle, history, instrumentation, methodology and its application.
Advantages of Hyphenated Technique- To solve complex analytical problems, Shorter analysis time, Higher degree of automation, Higher sample throughput, Better reproducibility, Reduction of contamination because it is a closed system, Enhanced combined selectivity and therefore higher degree of information.
1. High Performance Liquid Chromatography –Mass Spectroscopy (HPLC-MS).
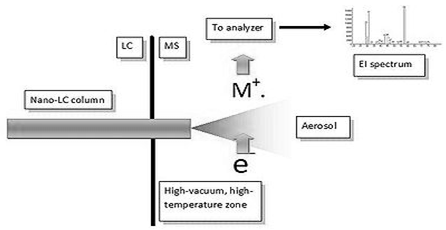
HPLC-MS hyphenated refers to the coupling of an HPLC with a mass spectrometer (MS). Sample passes through liquid chromatography that is HPLC column. The separated sample emerging from the column can be identified and quantified on the basis of its mass spectral data. Emerged sample from the HPLC is switching mass spectrometer. Mass spectrometry is a powerful analytical technique which basic principle is generates multiple ions from the sample, then separates them according to their specific mass-to-charge ratio (m/z), and then records the relative abundance of each ion type. A typical automated HPLC-MS system consists of double three-way diverter in-line with an auto sampler. It is used to quantify known materials, to identify unknown compounds within a sample, and to elucidate the structure and chemical properties of different molecules [8].
HPLC-MS hyphenated refers to the coupling of an HPLC with a mass spectrometer (MS). Sample passes through liquid chromatography that is HPLC column. The separated sample emerging from the column can be identified and quantified on the basis of its mass spectral data. Emerged sample from the HPLC is switching mass spectrometer. Mass spectrometry is a powerful analytical technique which basic principle is generates multiple ions from the sample, then separates them according to their specific mass-to-charge ratio (m/z), and then records the relative abundance of each ion type. A typical automated HPLC-MS system consists of double three-way diverter in-line with an auto sampler. It is used to quantify known materials, to identify unknown compounds within a sample, and to elucidate the structure and chemical properties of different molecules [8].
This technique basically studies the effect of ionizing energy on molecules. It depends upon chemical reactions in the gas phase in which sample molecules are consumed during the formation of ionic and neutral species. A mass spectrometer generates multiple ions from the sample under investigation; it then separates them according to their specific mass-to-charge ratio (m/z), and then records the relative abundance of each ion type. [8,30]
The application of HPLC-MS is quite very high in characterization and quantification of drugs. By this technique analysis have been done in drug metabolism studies, quantification and identification of impurities and degradation products. Drug metabolism studies considered for further development into a new therapeutic effect. For potential therapeutic effect the drug or metabolite must be stable. This technique is the choice for the pharmacokinetic and bioavailability profile of the drug. It also provides molecular weight information and fragmentation patterns for structure elucidation. Which ionization technique is used will depend on the structure and the acidity or basicity of the drug studied. In Vitro studies, Drug metabolism the liver is the primary organ that metabolizes drugs. Preliminary studies of drug metabolism The application of HPLC-MS in pharmaceutical is very wide here in brief can say to detect compounds from poly aromatic (non-polar) to peptide and proteins, for compounds identification have therefore commonly been carried out in vitro with liver microsomal preparations or hepatocytes. And purity, for determination of pesticides, herbicides& organic pollutant for environmental monitoring, analysis of food and proteome analysis is done by this technique [9-10].
2. High Performance Liquid Chromatography –Infrared spectroscopy (HPLC-IR).
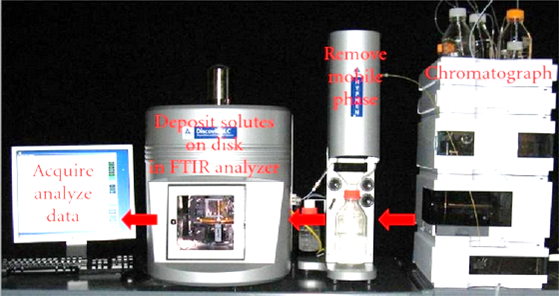
The conjunction technique developed by the coupling of an HPLC and the detection in respect of characterisation of unknown molecule with infrared spectrometry (IR) or FTIR is known as HPLC-IR. Or HPLC-FTIR. At temperatures above absolute zero, all the atoms in molecules are in continuous vibration with respect to each other. When the frequency of a specific vibration is equal to the frequency of the IR radiation directed on the molecule, the molecule absorbs the radiation. It is very specious spectroscopic technique for the identification of organic compounds, because in the mid-IR region the structures of organic compounds have many absorption bands that are characteristic of particular functional group, e.g., –OH, –COOH, and so on. But this technique has problem and found because the hyphenated technique's 237 absorption bands of the mobile phase solvent are so huge in the mid-IR region that they often obscure the small signal generated by the sample components. In addition, as a detection technique, IR is much less sensitive compared to various other detection techniques, e.g., UV and MS [7,8]. The solvent-elimination approach is the preferred option in most of the HPLC-IR operations for example, if one uses a mobile phase of a deuterated solvent such as heavy water or per-deuterated methanol, IR can monitor many organic compounds that have C–H structures in the molecules. After the mobile phase solvent is eliminated, IR detection is carried out in some medium that has a transparency for IR light. Generally, KBr or KCl salts are used for the collection of sample components in the eluent, and heating up the medium before IR detection eliminates the volatile mobile phase solvents.
The conjunction technique developed by the coupling of an HPLC and the detection in respect of characterisation of unknown molecule with infrared spectrometry (IR) or FTIR is known as HPLC-IR. Or HPLC-FTIR. At temperatures above absolute zero, all the atoms in molecules are in continuous vibration with respect to each other. When the frequency of a specific vibration is equal to the frequency of the IR radiation directed on the molecule, the molecule absorbs the radiation. It is very specious spectroscopic technique for the identification of organic compounds, because in the mid-IR region the structures of organic compounds have many absorption bands that are characteristic of particular functional group, e.g., –OH, –COOH, and so on. But this technique has problem and found because the hyphenated technique's 237 absorption bands of the mobile phase solvent are so huge in the mid-IR region that they often obscure the small signal generated by the sample components. In addition, as a detection technique, IR is much less sensitive compared to various other detection techniques, e.g., UV and MS [7,8]. The solvent-elimination approach is the preferred option in most of the HPLC-IR operations for example, if one uses a mobile phase of a deuterated solvent such as heavy water or per-deuterated methanol, IR can monitor many organic compounds that have C–H structures in the molecules. After the mobile phase solvent is eliminated, IR detection is carried out in some medium that has a transparency for IR light. Generally, KBr or KCl salts are used for the collection of sample components in the eluent, and heating up the medium before IR detection eliminates the volatile mobile phase solvents.
The application of this technique is dependent upon the region of IR. Near, Middle and Far IR are quantetively, Identification of functional group-Quantitative analysis-Detecting impurities and Analyse structure of molecule respectively. The broad area of Agricultural/Food, Polymer Petroleum and fuel industry, Environmental. Textiles Biomedical/Clinical [7,9].
3. High Performance Liquid Chromatography – Atomic Fluorescence Spectrometry (HPLC-AFS).
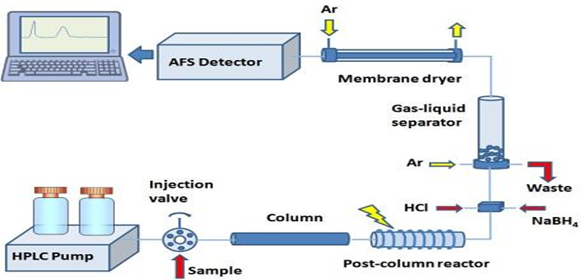
Atomic fluorescence is a spectroscopic process which is based upon the absorption of radiation of a certain wavelength by an atomic vapour and subsequent radiation deactivation of the excited atoms toward the detection device. Resonance fluorescence occurs when atoms absorb and re-emit radiation of the same wavelength. In HPLC there is mainly separation takes place of different constituent at different retention time. After that in detector quantitative elemental analysis takes place. The analytical features of AFS, such as detection limits below the mg and the wide linear calibration range, up to the mg, allow its application to a great variety of environmental, biological and food samples. AFS represents a suitable alternative to other atomic spectrometers commonly employed in speciation studies such as Atomic Absorption Spectrometry (AAS) and Inductively Coupled Plasma-Mass Spectrometry (ICP-MS). The instrumentation used for AFS and the design of the vapour generation and optical layouts required to sustain the full benefits of the AFS approach are also described.
Atomic fluorescence is a spectroscopic process which is based upon the absorption of radiation of a certain wavelength by an atomic vapour and subsequent radiation deactivation of the excited atoms toward the detection device. Resonance fluorescence occurs when atoms absorb and re-emit radiation of the same wavelength. In HPLC there is mainly separation takes place of different constituent at different retention time. After that in detector quantitative elemental analysis takes place. The analytical features of AFS, such as detection limits below the mg and the wide linear calibration range, up to the mg, allow its application to a great variety of environmental, biological and food samples. AFS represents a suitable alternative to other atomic spectrometers commonly employed in speciation studies such as Atomic Absorption Spectrometry (AAS) and Inductively Coupled Plasma-Mass Spectrometry (ICP-MS). The instrumentation used for AFS and the design of the vapour generation and optical layouts required to sustain the full benefits of the AFS approach are also described.
First described a method for the simultaneous speciation of several elements using an atomic fluorescence spectrometer as an element specific detector for chromatography. A three-channel on dispersive atomic fluorescence spectrometer with an HCL as the excitation source and a nitrogen-sheathed air–acetylene flame as the atomizer was used. It was interfaced to an HPLC system by direct connection of the column outflow to the nebulizer capillary of the burner. The column flow rate was compatible with the nebulizer flow rate. The technique was successfully applied to the simultaneous detection of several metals as their EDTA (copper, zinc, nickel), glycine (copper, zinc, nickel), and trien complexes. More research has been carried out to study metal speciation using different models of coupling of HPLC with AFS. HPLC-AFS hyphenated technique is an ideal for the detection of concerning hydride forming elements mainly As, Se, Sb and Hg. [14,15]
4. High Performance Liquid Chromatography – Ultra Violet/Visible (HPLC-UV/VIS).
HPLC-UV/VIS. detection is the most common technique applies for the analysis of chemical substance. High-performance liquid chromatography on-line with UV/Vis spectroscopy (HPLC-DAD) is the simplest and the most popular of hyphenated analytical techniques based on chromatography. Most of the applications of diode array detectors are restricted to the selection of a narrow wavelength range as recommended for quantitative analysis The Basics-Lambert–Beer’s law is UV/ VIS spectrometry is based on the comparison of incident light intensity (I°) and emergent intensity (I) after passing through the solution of interest. The ratio I/I° is the so-called transmittance (T), to obtain the absorbance (A -log 1/T, log I°/I) because A is directly proportional to the concentration of the absorbing analyte in the solution, if the path length ‘d’ of the measuring cell is held constant. This relationship is expressed by the Lambert–Beer law, a combination of two laws. The UV or UV/VIS detector is a simple device and the user should not have any trouble with it. The UV or UV/VIS detector is a simple device and the user should not have any trouble with it. The lamp must be replaced after the interval recommended by the manufacturer; in many instruments, the lamp hours are counted and a replacement demand appears on the display when the time has come. If detection is performed in the low UV region an earlier lamp replacement may be necessary.
HPLC-UV/VIS. detection is the most common technique applies for the analysis of chemical substance. High-performance liquid chromatography on-line with UV/Vis spectroscopy (HPLC-DAD) is the simplest and the most popular of hyphenated analytical techniques based on chromatography. Most of the applications of diode array detectors are restricted to the selection of a narrow wavelength range as recommended for quantitative analysis The Basics-Lambert–Beer’s law is UV/ VIS spectrometry is based on the comparison of incident light intensity (I°) and emergent intensity (I) after passing through the solution of interest. The ratio I/I° is the so-called transmittance (T), to obtain the absorbance (A -log 1/T, log I°/I) because A is directly proportional to the concentration of the absorbing analyte in the solution, if the path length ‘d’ of the measuring cell is held constant. This relationship is expressed by the Lambert–Beer law, a combination of two laws. The UV or UV/VIS detector is a simple device and the user should not have any trouble with it. The UV or UV/VIS detector is a simple device and the user should not have any trouble with it. The lamp must be replaced after the interval recommended by the manufacturer; in many instruments, the lamp hours are counted and a replacement demand appears on the display when the time has come. If detection is performed in the low UV region an earlier lamp replacement may be necessary.
Strategies of interpreting UV/VIS spectra the calculation of wavelength corresponding to the absorbance maximum is the most simple among protocols of the processing of zero order spectra. The absorbance maximum is one of the key elements of colorants characteristics.
HPLC-UV/VIS serves as a tool for the identification of compounds containing conjugated double bonds, mainly aromatic compounds, and the evaluation of their purity. Application of this technique enables identification of desired compound after chromatographic separation if its spectrum differs significantly from the spectra of other compounds present in the analysed system. It is reported the strategies of UV/VIS spectroscopy-based identification of substances separated via HPLC. Determination of absorbance maximum location in zero order spectra, Chemometrical analysis of zero order determination of convexity intervals on the basis of the first derivatives of UV spectra, determination of minima and/or zero crossing location in the second derivatives of UV spectra, Calculation of amplitudes of derivatives of UV spectra, Calculation of parameters including amplitudes of the second derivative of a spectrum and absorbance at selected wavelength in zero order spectrum. Identification of peptides on the basis of retention times obtained via RP-HPLC and parameters describing the first and second derivatives of UV spectra. Chemo-metrical analysis of UV spectra derivatives, Chemo metrical analysis including characteristics of chromatograms at selected wavelength and spectra of individual peaks [15,17].
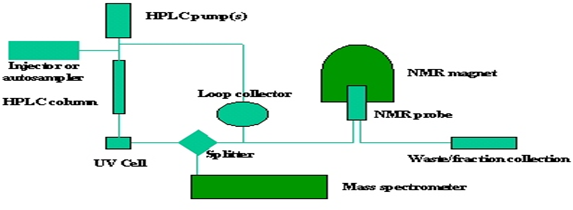
5. High Performance Liquid Chromatography – Nuclear Magnetic Resonance (HPLC-NMR).
The application of the HPLC-NMR technique is mainly in natural chemistry. After separation and identification the structure elucidation performed in NMR. The coupling of NMR with HPLC reported in the early of 1980s, however the use of this hyphenated technique in the analytical field is later. Also the analysis by this technique is reported in complex mixture of all types particularly natural product and drug related metabolites in bio-fluids. This technique generally insensitive for the analysis of organic compound in environmental sample.
The application of the HPLC-NMR technique is mainly in natural chemistry. After separation and identification the structure elucidation performed in NMR. The coupling of NMR with HPLC reported in the early of 1980s, however the use of this hyphenated technique in the analytical field is later. Also the analysis by this technique is reported in complex mixture of all types particularly natural product and drug related metabolites in bio-fluids. This technique generally insensitive for the analysis of organic compound in environmental sample.
The coupling of the technique is on two basic parameter, first is that the flow cell must be design as that the laminar flow of HPLC is achieved to maintain the chromatographic resolution. Such arrangement gives an adequate resolution. Second is coupling HPLC with NMR using proton carrying solvent which gives the mobile phase signal so far from an intense spectrum signal.
With the experiment H1 NMR the values of chemical shift, peak multiplicities, coupling constant and retention time are help to chemical structure elucidation. Quantification by this technique observed particularly in environmental analysis. For this internal standard compound is added to the sample. Structural information of the molecule got but some time identification of unknowns will not possible. Apart from the above, technique is powerful for the differentiation of isomer, functional group which leads the technique importance [18-19].
6. High Performance Liquid Chromatography – Refractive Index (HPLC-RI).
The refractive index detector was one of the first online detectors to be developed and was described by Tiselius and Claesson in 1942. And also one of the first online liquid chromatography (LC) detectors to be made commercially for general use. The refractive index detector is a bulk property detector. This detector responds to some physical property of the total column eluent and not some specific property of the solute. Bulk property detectors have an inherently limited sensitivity, irrespective of the instrumental technique used. Consider a hypothetical bulk property detector that is to monitor the density of the column eluent. Assume it is required to detect the concentration of a dense material, such as carbon tetrachloride (specific gravity-1.595), at a level of 1 -µg/ml in n-heptane (specific gravity- 0.684).
The refractive index detector was one of the first online detectors to be developed and was described by Tiselius and Claesson in 1942. And also one of the first online liquid chromatography (LC) detectors to be made commercially for general use. The refractive index detector is a bulk property detector. This detector responds to some physical property of the total column eluent and not some specific property of the solute. Bulk property detectors have an inherently limited sensitivity, irrespective of the instrumental technique used. Consider a hypothetical bulk property detector that is to monitor the density of the column eluent. Assume it is required to detect the concentration of a dense material, such as carbon tetrachloride (specific gravity-1.595), at a level of 1 -µg/ml in n-heptane (specific gravity- 0.684).
Refractive index detector in chromatography is very least sensitive detector. Major disadvantage of the detector is its ambient nature; sensitivity loses with change of temperature, pressure and flow rate, also can not apply this in gradient flow due to continuous change in mobile phase refractive index change. It reported useful for detecting those compounds that are nonionic, do not absorb in the UV and do not fluoresce (e.g. aliphatic alcohols, fatty acids, ethers, etc.). Even if the refractive index value calculated at thermostat condition, the heat release due to adsorption and desorption to and from the stationary phase it change the value. The density of the contents of the cell will also change with pressure and, if there is a significant pressure drop across the cell. These factors restricted the application of HPLC-RI and less sensitivity [26-27].
The numerical value of refractive index is decrease with increasing temperature the value is usually quoted at 20°C or 25°C in literature and actually it is a mean value measured for the two sodium lines. It is ratio of due to refraction phenomenon and the value is dimensionless. Even the oldest and less sensitivity it is important in analytical field because of universal response [20-21].
7. High Performance Liquid Chromatography –Conductivity Detector (HPLC-CD).
This is one of the most important conjunction techniques that are applied in chromatographic analysis. Here Ion exchanges chromatography where ions are separated and quantitatively detected by electric conductivity measurement of a solution. After the targeted ions are eluted, the change in electric current is detected, with a constant voltage imposed between the electrodes. A conductivity detector is employed as a detector in an ion chromatograph, which is a system dedicated to measuring ions. This detector is used mainly to measure inorganic ions and small organic substances, including organic acids and amines [22-23].
This is one of the most important conjunction techniques that are applied in chromatographic analysis. Here Ion exchanges chromatography where ions are separated and quantitatively detected by electric conductivity measurement of a solution. After the targeted ions are eluted, the change in electric current is detected, with a constant voltage imposed between the electrodes. A conductivity detector is employed as a detector in an ion chromatograph, which is a system dedicated to measuring ions. This detector is used mainly to measure inorganic ions and small organic substances, including organic acids and amines [22-23].
Solutions containing ionic components will conduct electricity. Conductivity detector measures electronic resistance and measured value is directly proportional to the concentration of ions present in the solution. Thus it is generally used for ion chromatography. The sensor of the electrical conductivity detector is the simplest of all the detector sensors and consists of only two electrodes situated in a suitable flow cell. The sensor consists of two electrodes sealed into a glass flow cell. In the electric circuit, the two electrodes are arranged to be the impedance component in one arm of a Wheatstone bridge [24-25].
It is a routine analytical method and a versatile. The concept of Ion chromatography with conductivity detector successively with advancements of rapid development in separation for the compounds having functional group that either oxidized or reduced and ions that is cation or anion. Moreover, it could include other separation methods (e.g., ion interaction and ion exclusion) for simultaneous separation of analyte components. IC analysis has matured to a well-established rugged, sensitive and reliable analysis technique for a wide variety of chemical compounds present in various matrices. On this manner, many papers have been published during the last few years dealing with new modalities in sample pretreatment, separation, detection, etc., for improving samples analysis [28,29].
Conclusion
HPLC itself is most widely used method for quantitative analysis in the pharmaceutical industry and in pharmaceutical analysis laboratories. Conjunction technique made more -Fast and accurate analysis, higher degree of automation, higher sample throughput, better reproducibility, reduction of contamination due to its closed system, separation and quantification achieved at same time.
Conflict of Interest
We declare that we have no conflict of interest.
We declare that we have no conflict of interest.
References
- Piotr M., et al. “Application of high-performance liquid chromatography on-line with ultraviolet/visible spectroscopy in food science”. Polish journal of food and nutrition sciences 15.56(Suppl. 1) (2006): 145–153.
- Acquistucci R., et al. “Application of the microwave hydrolysis to furosine determination in cereal and dairy foods”. Journal of Agricultural and Food Chemistry 44.12 (1996): 3855–3857.
- Andrews GR. “Distinguishing pasteurized, UHT and sterilized milks by their lactulose content”. Journal of the Society of Dairy Technology 37.3 (1984): 92–95.
- Bertrand D and Scotter CNG. “Application of multivariate analyses to NIR spectra of gelatinized starch”. Applied Spectroscopy 46.9 (1992): 1420–1425.
- Birlouez-Aragon I., et al. “A rapid fluorimetric method to estimate the heat treatment of liquid milk”. International Dairy Journal 8.9 (1998): 771–777.
- Buser W and Erbersdobler HF. “Determination of furosine by gas-liquid chromatography”. Journal of Chromatography A 346 (1985): 363–368.
- Patel K.N., et al. “Introduction to Hyphenated Techniques and Their Applications in Pharmacy”. Pharmaceutical Methods 1.1 (2010): 2-13.
- Joachim E. “The Use of Hyphenated LC–MS Technique for Characterization of Impurity Profiles during Drug Development”. Journal of Pharmaceutical and Biomedical Analysis 18.4-5 (1998): 707-714.
- Patel K.N., et al. “Introduction to Hyphenated Techniques and Their Applications in Pharmacy”. Pharmaceutical Methods 1.1(2010): 2-13.
- John CL., et al. “Directly Coupled HPLC-NMR and HPLC-NMR-MS in Pharmaceutical Research and Development”. Journal of Chromatography B: Biomedical Sciences and Applications 748.1 (2000): 233-58.
- Albert K. “On-line LC-NMR and Related Techniques”. London: Wiley, 2002.
- John KR and Richard JS. “Use of Liquid Chromatography-Nuclear Magnetic Resonance Spectroscopy for the Identification of Impurities in Drug Substances”. Journal of Chromatography A 677.2 (1994): 385-389.
- Ahuja S and Scypinski S. Handbook of Modern Pharmaceutical Analysis. 1st Edition Volume 3, USA; Academic Press (2001): 149-152.
- Corzo N., et al. “Changes in furosine and proteins of UHT-treated milks stored at high ambience temperatures”. Zeitschrift für Lebensmittel-Untersuchung und -Forschung 198.4 (1994): 302–306.
- Rachana R., et al. “HYPHENATED TECHNIQUE- A BOON TO ANALYTICAL WORLD”. International Journal of Pharmaceutical Sciences and Research 1.11 (2015): 4184-4191.
- Corzo N., et al. “Ratio of lactulose to furosine as indicator of quality of commercial milks”. Journal of Food Protection 57.8 (1994): 737–739.
- De Rafael D., et al. “Determination of low levels of lactulose in milk”. Milchwissenschaft 51.10 (1996): 552–553.
- DeRafael D., et al. “Formation of lactulose and furosine during heat treatment of milk at temperatures of 100–120oC”. Milchwissenschaft 52.2 (1997): 76–78.
- Delgado T., et al. “Determination of furosine in milk samples by ion-pair reversed phase liquid chromatography”. Chromatographia 33.7 (1992): 374–376.
- Ding CH and Chang TC. “Detection of reconstituted milk in fresh milk”. Journal of the Chinese Agricultural Chemical Society 24 (1986): 406–411.
- Ahmed NE., et al. “Evaluation of methods used to determine ochratoxin A in coffee beans”. Journal of Agricultural and Food Chemistry 55.23 (2007): 9576-9580.
- Almeida APJ., et al. “Ochratoxin A in Brazilian instant coffee”. Brazilian Journal of Microbiology 38.2 (2007): 300-303.
- Becker M., et al. “Column liquid chromatography electrospray ionization-tandem mass spectrometry for the analysis of ochratoxin”. Journal of Chromatography A 818.2 (1998): 260-264.
- Handbook of Ion Chromatography Third, Completely Revised and Enlarged Edition, Joachim Weiss (2004).
- Tswett M. S., Khromofilly. V Rastitel’nom i Zhivotnom Mire ( Chromophylls in the Plant and Animal World). Karbasnikov Publishers, Warsaw (1910).
- Amin M., et al. “Determination of common inorganic anions and cations by non-suppressed ion chromatography with column switching”. Journal of Chromatography A 1182.2 (2008): 169-175.
- Yasser M., et al. “Ion Exchange Chromatography - An Overview”.
Citation:
Mohamad Taleuzzaman and Sadaf Jamal Gilani. “Brief on -Hyphenated Methods of HPLC for Determining the Presence of Solutes”. Chronicles of Pharmaceutical Science 1.1 (2017): 35-42.
Copyright: © 2017 Mohamad Taleuzzaman and Sadaf Jamal Gilani. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.