Case Report
Volume 1 Issue 2 - 2017
Resolution of Refractory Non-Infectious Chronic Uveitic Cystoid Macular Oedema with a Single Intravitreal Etamsylate Injection
1Universidad Alfonso X el Sabio, Madrid
2Hospital de Día Pio XII, Madrid
3Departamento de Investigación, Hospital Universitario Ramón y Cajal, Madrid
4Laboratoire National de Santé, Dudelange, Luxembourg
5Departamento de Estructura y Función de Proteínas, Centro de Investigaciones Biológicas, (CSIC) Madrid, Spain
2Hospital de Día Pio XII, Madrid
3Departamento de Investigación, Hospital Universitario Ramón y Cajal, Madrid
4Laboratoire National de Santé, Dudelange, Luxembourg
5Departamento de Estructura y Función de Proteínas, Centro de Investigaciones Biológicas, (CSIC) Madrid, Spain
*Corresponding Author: Pedro Cuevas, Facultad de Medicina, Universidad Alfonso X el Sabio, 28691-Villanueva de la Cañada. Madrid.
Received: April 07, 2017; Published: April 13, 2017
Abstract
Uveitic (or inflammatory) macular oedema is one of the most common cause of visual impairment in patients with uveitis and the most frequent structural complications of uveitis. This study showed efficacy and safety of intravitreal etamsylate injection in the
treatment of macular oedema due to posterior chronic uveitis. In parallel with resolution of the macular oedema, visual acuity was
significantly improved after treatment. It is concluded that, in such a condition, etamsylate may be an effective treatment option to
control macular oedema.
Keywords: Refractory uveitic macular oedema; Fibroblast growth factor; Intravitreal etamsylate
Introduction
Uveitis is a group of ocular inflammatory conditions that can lead to severe vision loss. Macular oedema is one of the clinical manifestation
of uveitis leading to reduced visual acuity. Uveitic macular oedema most commonly occurs because of chronic intraocular inflammation
[1]. Macular oedema causes an inflammatory response releasing mediators which damage the retinal pigment epithelium (RPE).
This results in leakage into the retina, especially at the macula [2]. Chronic macular oedema in uveitis patients may lead to macular cysts
and macular holes, resulting in no reversible loss of visual acuity. Development of an epiretinal membrane is also a consequence of chronic
uveitic macular oedema. Refractory macular oedema usually occurs in patients with chronic or recurrent uveitis.
Therapeutic interventions are targeted to resolve the inflammatory response in uveitis as well as to treat or prevent the recurrence
of macular oedema. In this sense, steroids (by means of injections or implants) are the standard treatment of uveitic macular oedema but
they may be associated with risk of intraocular pressure elevation as well as predisposition to cataract formation [3].
Furthermore, anti-VEGF agents, administered as a single or multiple intravitreal injections, have been used to treat uveitic macular
oedema but no long-term prospective studies have proven to be effective in uveitic macular oedema [4-7]. In this scenario, research on
new anti-inflammatory compounds with safety profiles in toxicity has been salient. Previously, we have reported the efficacy and safety
of etamsylate, an inhibitor of fibroblast growth factor (FGF) [8] in several inflammatory diseases, including both types of age-related
macular degeneration (AMD) [9,10]. Etamsylate is the N-Ethylethanamine salt of the dobesilic acid, an analogous of gentisic acid, a main
catabolite of aspirin [8]. The objective of this study was to investigate the effects of intravitreal administration of etamsylate in a patient
with refractory non-infectious chronic uveitis.
Case Presentation
A 32-year-old female who has a history of right eye posterior uveitis for two years presented with decreased vision in the affected eye.
She had previously received intravitreal dexamethasone implant (Ozurdex) and 6 Lucentis injections in her right eye. A full general and
ophthalmic examination (including anterior segment bio-microscopy, fundoscopy, intraocular pressure measurement, best-corrected
visual acuity (BCVA) and a spectral-domain optical coherence tomography (SD-OCT) were performed. BCVA was obtained with a projected
Snellen chart. According to fundoscopy and SD-OCT data patient showed morphological features of non-infectious uveitic macular
oedema. BCVA was 0.10 and central macular thickness (CMT) was 402 μm. After discussion with the patient regarding the benefits, risks
and alternatives of treatment, she chose intravitreal etamsylate administration. Informed consent was obtained. The study was approved
by the institution review board and it followed the principles outlined in the Declaration of Helsinki.
Treatment
Intravitreal etamsylate (Dycinone®, Sanofi. France) injection (150 μl) was administered in the operating room under complete aseptic conditions with topical anesthesia. Topical ciprofloxacin was given 4 times a day for 5 days postoperatively. After treatment, patient was examined at day 1 and 7, and 3 months thereafter. At day 1 and day 7, injected eye underwent an ophthalmic examination for anterior chamber reaction and intraocular pressure rise. All ocular and systemic adverse events, including information on their relationship to drug and procedure, were recorded at each visit.
Intravitreal etamsylate (Dycinone®, Sanofi. France) injection (150 μl) was administered in the operating room under complete aseptic conditions with topical anesthesia. Topical ciprofloxacin was given 4 times a day for 5 days postoperatively. After treatment, patient was examined at day 1 and 7, and 3 months thereafter. At day 1 and day 7, injected eye underwent an ophthalmic examination for anterior chamber reaction and intraocular pressure rise. All ocular and systemic adverse events, including information on their relationship to drug and procedure, were recorded at each visit.
Results and Discussion
A substantial reduction of CMT after intravitreal etamsylate injection (402 μm vs 270 μm) was observed. Furthermore, BCVA improves
significantly from 0.10 at baseline (treatment start) to 0.70 at last follow-up visit (3 months). No adverse effects were referred.
Chronic inflammatory process has a significant, if no primary role, in ophthalmic diseases. The goal of uveitis treatment should not
only be to suppress inflammation when it recurs but also to attain complete remission of inflammation and prevent complications such
as cystoid macular oedema.
An inflammatory agent has been described to be closely related to several ocular diseases, which is the protein known as fibroblast
growth factor (FGF) [11]. Inflammation elicited by FGF is prone to the consolidation of a positive inflammatory feedback loop typical of
chronic diseases since it induces the upregulation of the synthesis of COX-2 and phospholipase A2 which reciprocally promote the expression
of FGF [12]. Inhibition of FGF obviously interrupts these loops, which probably explain the success of the clinical benefits in treating
chronic inflammatory diseases [13], as shown in the results presented here with a single punctual treatment of etamsylate.
Conclusion
Intravitreal etamsylate for the management of uveitis can favourably modify the course of this condition, indicating that blockade of
FGF may restore the integrity of the blood-retinal barrier, reduce central macular thickness and significantly improve visual function. Additional
studies are needed to confirm the results.
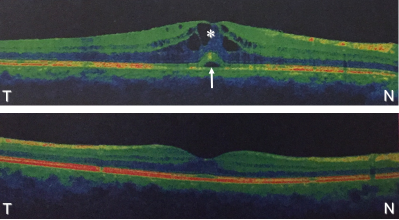
Figure 1: Comparison Cross-sectional spectral-domain optical coherence scans in a patient with uveitic macular oedema.
Upper image: pre-treatment scan showing central macular thickness of 402 μm. Arrow indicates neurosensory detachment; asterisk
denotes, cystic oedema. Lower image: post-treatment scan showing normal retina with central macular thickness reduction to 270 μm.
Note the resolution of macular oedema as well as neurosensory detachment. N, nasal; T, temporal.
References
- Dick AD. “The treatment of chronic uveitic macular oedema”. British Journal of Ophthalmology 78.1 (1994): 1-2.
- Ossewaarde-van Norel A and Rothova A. “Clinical review: Update on treatment of inflammatory macular edema”. Ocular Immunology and Inflammation 19.1 (2011): 75-83.
- Wang JK., et al. “An updated review of long-term outcomes from randomized controlled trials in approved pharmaceuticals for diabetic macular edema”. Eye Science 30.4 (2015):176-183.
- Lasave AF., et al. “Short-term results of a single intravitreal bevacizumab (avastin) injection versus a single intravitreal triamcinolone acetonide (kenacort) injection for the management of refractory noninfectious uveitic cystoid macular edema”. Ocular Immunology and Inflammation 17.6 (2009):423-430.
- Weiss K., et al. “Intravitreal VEGF levels in uveitis patients and treatment of uveitic macular oedema with intravitreal bevacizumab”. Eye (London, England) 23.9 (2009):1812-1818.
- Mackensen F., et al. “Intravitreal bevacizumab (avastin) as a treatment for refractory macular edema in patients with uveitis: a pilot study”. Retina (Philadelphia, Pa.) 28.1 (2008): 41-45.
- Cervantes-Castañeda RA., et al. “Intravitreal bevacizumab in refractory uveitic macular edema: one-year follow-up”. European Journal of Ophthalmology 19.4 (2009): 622-629.
- Fernández IS., et al. “Gentisic acid, a compound associated with plant defense and a metabolite of aspirin, heads a new class of in vivo fibroblast growth factor inhibitors”. The Journal of Biological Chemistry 285.15 (2010):11714-29.
- Cuevas P., et al. “Treatment of dry age-related macular degeneration with dobesilate”. BMJ Case Reports 21 (2012): doi: 10.1136/bcr.02.2012.5942.
- Cuevas P., et al. “Case Report: Resolution of submacular haemorrhage secondary to exudative age-related macular degeneration after a single intravitreal dobesilate injection”. F1000Research 2. 271 (2013): (doi: 10.12688/f1000research.2-271.v1)
- Hou J., et al. “Design of a superior cytokine antagonist for topical ophthalmic use”. Proceedings of the National Academy of Sciences of the United States of America 110.10 (2013): 3913-3918.
- Angulo J., et al. “Diacetyloxyl derivatization of the fibroblast growth factor inhibitor dobesilate enhances its anti-inflammatory, anti-angiogenic and anti-tumoral activities”. Journal of Translational Medicine 13 (2015): 48.
- Cuevas P., et al. “Long-term effectiveness of dobesilate in the treatment of papulopustular rosacea”. BMJ Case Reports 16 (2011). pii: bcr0820114579.
Citation:
Pedro Cuevas., et al. “Resolution of Refractory Non-Infectious Chronic Uveitic Cystoid Macular Oedema with a Single Intravitreal
Etamsylate Injection”. Chronicles of Pharmaceutical Science 1.2 (2017): 70-72.
Copyright: © 2017 Pedro Cuevas., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.