Research Article
Volume 1 Issue 4 - 2017
Nano suspensions: A Prospective Approach
Department of Pharmaceutics, Dr Samuel George Institute of Pharmaceutical sciences
*Corresponding Author: Dr Samuel George Institute of Pharmaceutical sciences, Tarlupadu Road, Markapur, Prakasam (dt), Andhra Pradesh, India.
Received: May 23, 2017; Published: August 16, 2017
Abstract
Nano refers to particles size range of 1-1000 nm. Nano-suspensions are part of nanotechnology. Nano-suspensions contain submicron colloidal dispersion of pharmaceutical active ingredient particles in a liquid phase stabilized by surfactants. Nano-suspension technology is a unique and economical approach to overcome poor bioavailability that is related with the delivery of hydrophobic drugs, including those that are poorly soluble in aqueous media. Nano-suspensions are important carriers to develop novel drug formulations. Few techniques such as precipitation methods, milling methods and homogenization methods are developed to produce Nano-suspension (NS) and have been successfully employed in large- scale production.
They are administered by Parenteral, per oral, ocular and pulmonary routes. Now their application also extended to site-specific delivery. Nano-suspensions are prepared by using wet mill, high pressure homogenizer, emulsion‐solvent evaporation, melt emulsification method and super critical fluid techniques. Nano-suspension technology can be used to improve the stability as well as bioavailability of poorly soluble drug. Nano-suspensions are also use in various dosage forms, including specialized drug delivery system such as mucoadhesive hydrogel. Rapid strides have been made in the delivery of nano-suspensions by parenteral, per-oral, ocular and pulmonary routes. Currently, efforts are being directed to extending their applications in site-specific drug delivery.
Keywords: Nano-suspension; Bioavailability; Poorly soluble drugs; Precipitation methods; Milling methods; homogenization methods; High pressure homogenization
Introduction
Various formulation parameters that play a crucial role in the successful formulation of drugs are aqueous solubility, stability at surrounding temperature and humidity, photo stability, compatibility with solvent and excipient. Among this aqueous solubility became a overcome for the formulation of new molecular entities or material. More than 40% of the new chemical entities being generated through drug discovery programmes are poorly water‐soluble or lipophilic compounds. Formulating a poorly water soluble drug has always been a challenging problem come up against by the pharmaceutical scientist.
The formulation of nano‐sized particles can be implemented to all drug compounds belonging to biopharmaceutical classification system (BCS) classes II and IV to increase their solubility and hence partition into gastrointestinal barrier. Micronization is used for class II drugs of (BCS), i.e. drugs having a good permeability and poor solubility. There are many conventional methods for increasing the solubility of poorly soluble drugs, which include micronization, solubilisation using co‐solvents, salt form, surfactant dispersions, precipitation technique, and oily solution. Other techniques are like liposomes, emulsions, microemulsion, solid dispersion and inclusion complexation using cyclodextrins show sensible achiever, but they lack in universal applicability to all drugs.
These techniques are not applicable for those drugs which are not soluble in aqueous and organic solvents. Nanotechnology can be used to solve the problems associated with these conventional approaches for solubility and bioavailability enhancement. Nano suspension is favoured for compounds that are insoluble in water (but are soluble in oil) with high log P value, high melting point and high doses. Nano suspension technology can also be used for drugs which are insoluble in both water and organic solvents. Hydrophobic drugs such as naproxen, clofazomine, bupravaquone, nimesulide, mitotane, amphotericin B27, omeprazole, nifedipine and spironolactone are formulated as nanosuspension [1,2].
Nano-suspensions
Nano suspensions are colloidal dispersions of Nano sized drug particles stabilized by surfactants. They can also be defined as a biphasic system consisting of pure drug particles dispersed in an aqueous vehicle in which the diameter of the suspended particle is less than 1 μm in size. Reduction of drug particles to Nano meter range leads to an enhanced dissolution rate not only because of increased surface area but also because of saturation solubility [3].
Nano suspensions are colloidal dispersions of Nano sized drug particles stabilized by surfactants. They can also be defined as a biphasic system consisting of pure drug particles dispersed in an aqueous vehicle in which the diameter of the suspended particle is less than 1 μm in size. Reduction of drug particles to Nano meter range leads to an enhanced dissolution rate not only because of increased surface area but also because of saturation solubility [3].
The increase in the saturation solubility and solution velocity of Nano particle is due to increase of vapour pressure of the particles. Nano-suspension have disclosed the problems associated with the delivery of poorly water‐soluble and poorly water‐soluble and lipid soluble drugs and are unequalled because of their simplicity and rewards they confer over other actions or strategies.
Need of Nano-suspension for Bioavailability Enhancement
Nevertheless, pharmacokinetic studies of BCS class–II drugs showed that they have a low oral bioavailability, which may be due to poor water solubility of drug. There are many classical pharmaceutical ways to improve drug dissolution rate such as dissolution in aqueous mixtures with an organic solvent, formation of ß-cyclodextrin complexes, solid dispersions and drug salt form [4].
Nevertheless, pharmacokinetic studies of BCS class–II drugs showed that they have a low oral bioavailability, which may be due to poor water solubility of drug. There are many classical pharmaceutical ways to improve drug dissolution rate such as dissolution in aqueous mixtures with an organic solvent, formation of ß-cyclodextrin complexes, solid dispersions and drug salt form [4].
During last 20 years a new technology, reducing drug particle size, has been developed to increase drug dissolution rate. According to Noyes–Whitney equation, drugs with smaller particle size have enlarged surface areas which lead to increase dissolution velocity. Higher the dissolution rate together with the resulting higher concentration gradient between gastrointestinal lumen and systemic circulation could further increase oral bioavailability of drugs. A Nano-suspension is a submicron colloidal dispersion of drug particles which are stabilized by surfactants.
A pharmaceutical Nano-suspension is defined as very finely dispersed solid drug particles in an aqueous vehicle for oral, topical, parenteral or pulmonary administration. The particle size distribution of the solid particles in Nano-suspensions is usually less than one micron with an average particle size ranging between 200 and 600 nm [5]. In nanosuspension technology, the drug is maintained in the required crystalline state with reduced particle size, leading to an increased dissolution rate and therefore improved bioavailability. An increase in the dissolution rate of micronized particles (particle size < 10 μm) is related to an increase in the surface area and consequently the dissolution velocity.
Nano sized particles can increase solution velocity and saturation solubility because of the vapor pressure effect. In addition; the diffusional distance on the surface of drug nanoparticles is decreased, thus leading to an increased concentration gradient. Increase in surface area as well as concentration gradient lead to a much more pronounced increase in the dissolution velocity as compared to a micronized product. Another possible explanation for the increased saturation solubility is the creation of high energy surfaces when disrupting the more or less ideal drug microcrystals to nanoparticles. Dissolution experiments can be performed to quantify the increase in the saturation solubility of a drug when formulated into a nano-suspension [6].
Advantages of Nano-suspensions [7,8]
- The major advantages of nano-suspension technology are:
- Provides ease of manufacture and scale-up for large scale production,
- Long-term physical stability due to the presence of stabilizers,
- Oral administration of nano-suspensions provide rapid onset, reduced fed/fasted ratio and improved bioavailability,
- Rapid dissolution and tissue targeting can be achieved by IV route of administration,
- Reduction in tissue irritation in case of subcutaneous/intramuscular administration,
- Higher bioavailability in case of ocular administration and inhalation delivery,
- Drugs with high log P value can be formulated as nano-suspensions to increase the bioavailability of such drugs,
- Improvement in biological performance due to high dissolution rate and saturation solubility of the drug,
- Nanosuspensions can be incorporated in tablets, pellets, hydrogels and suppositories are suitable for various routes of administration,
- Interesting Special Features of Nano-Suspensions [9,10]
- Increase in saturation solubility and consequently an increase in the dissolution rate of the drug.
- Increase in adhesive nature, thus resulting in enhanced bioavailability.
- Increasing the amorphous fraction in the particles, leading to a potential change in the crystalline structure and higher solubility.
- Absence of ostwald ripening, producing physical long term stability as an aqueous suspension.
- Possibility of surface-modification of nano-suspensions for site specific delivery.
- Criteria for selection of drugs for nano-suspensions [11,12]
- Nano-suspension can be prepared for the API that is having either of the following characteristics:
- Water insoluble but which are soluble in oil (high log P) or API are insoluble in both water and oils
- Drugs with reduced tendency of the crystal to dissolve, regardless of the solvent
- API with very large dose
Formulation Consideration for Nano-suspension [13]
1. Stabilizer
Stabilizer plays an important role in the formulation of Nano-suspensions. In the absence of an appropriate stabilizer, the high surface energy of Nano-sized particles can induce agglomeration or aggregation of the drug crystals. The main functions of a stabilizer are to wet the drug particles thoroughly, and to prevent Ostwald’s ripening and agglomeration of nanosuspensions in order to yield a physically stable formulation by providing steric or ionic barriers. The type and amount of stabilizer has a pronounced effect on the physical stability and in-vivo behavior of Nano-suspensions. In some cases, a mixture of stabilizers is required to obtain a stable Nanosuspension.
1. Stabilizer
Stabilizer plays an important role in the formulation of Nano-suspensions. In the absence of an appropriate stabilizer, the high surface energy of Nano-sized particles can induce agglomeration or aggregation of the drug crystals. The main functions of a stabilizer are to wet the drug particles thoroughly, and to prevent Ostwald’s ripening and agglomeration of nanosuspensions in order to yield a physically stable formulation by providing steric or ionic barriers. The type and amount of stabilizer has a pronounced effect on the physical stability and in-vivo behavior of Nano-suspensions. In some cases, a mixture of stabilizers is required to obtain a stable Nanosuspension.
2. Organic solvents
Organic solvents may be required in the formulation of Nano-suspensions if they are to be prepared using an emulsion or microemulsion as a template [14]. As these techniques are still in their infancy, elaborate information on formulation considerations is not available. The acceptability of the organic solvents in the pharmaceutical area, their toxicity potential and the ease of their removal from the formulation need to be considered when formulating a no suspensions using emulsions or microemulsions as templates. The pharmaceutically acceptable and less hazardous water miscible solvents, such as ethanol and isopropanol, and partially water-miscible solvents, such a ethyl acetate, ethyl format, butyl lactate, triacetin, propylene carbonate and benzyl alcohol.
Organic solvents may be required in the formulation of Nano-suspensions if they are to be prepared using an emulsion or microemulsion as a template [14]. As these techniques are still in their infancy, elaborate information on formulation considerations is not available. The acceptability of the organic solvents in the pharmaceutical area, their toxicity potential and the ease of their removal from the formulation need to be considered when formulating a no suspensions using emulsions or microemulsions as templates. The pharmaceutically acceptable and less hazardous water miscible solvents, such as ethanol and isopropanol, and partially water-miscible solvents, such a ethyl acetate, ethyl format, butyl lactate, triacetin, propylene carbonate and benzyl alcohol.
3. Co-surfactants
The choice of co-surfactant is critical when using microemulsions formulate Nano-suspensions. Since co-surfactants can greatly influence phase behavior, the effect of co-surfactant on uptake of the internal phase for selected microemulsions composition and on drug loading should be investigated. Although the literature describes the use of bile salts and dipotassium glycerrhizinate as co-surfactants, various solubilizers, such as Transcutol, glycofurol, ethanol and isopropanol, can be safely used as co-surfactants in the formulation of microemulsions [15,16].
The choice of co-surfactant is critical when using microemulsions formulate Nano-suspensions. Since co-surfactants can greatly influence phase behavior, the effect of co-surfactant on uptake of the internal phase for selected microemulsions composition and on drug loading should be investigated. Although the literature describes the use of bile salts and dipotassium glycerrhizinate as co-surfactants, various solubilizers, such as Transcutol, glycofurol, ethanol and isopropanol, can be safely used as co-surfactants in the formulation of microemulsions [15,16].
4. Other additives
Formulation considerations Nano-suspensions may contain additives such as buffers, salts, polyols, osmogent and cryoprotectant, depending on either the route of administration or the properties of the drug moiety.
Formulation considerations Nano-suspensions may contain additives such as buffers, salts, polyols, osmogent and cryoprotectant, depending on either the route of administration or the properties of the drug moiety.
Preparation of Nano-suspension
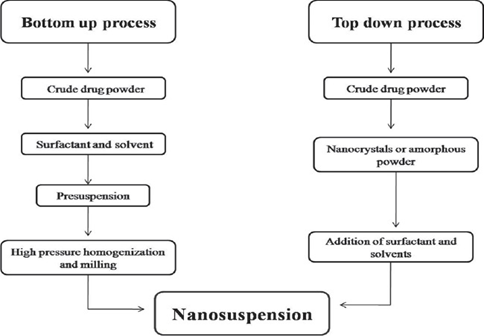
There are two methods for preparation of Nano suspension [17]. They are ‘Bottom up technology’ and ‘Top down technology’. For the production of nanoparticles in Bottom up technology the drug is dissolved in a solvent, which is then added to non‐solvent that causes precipitation of the fine drug particles. All‐Trans retinoic acid Nano-suspensions were prepared with a precipitation method.
There are two methods for preparation of Nano suspension [17]. They are ‘Bottom up technology’ and ‘Top down technology’. For the production of nanoparticles in Bottom up technology the drug is dissolved in a solvent, which is then added to non‐solvent that causes precipitation of the fine drug particles. All‐Trans retinoic acid Nano-suspensions were prepared with a precipitation method.
Use of simple and low cost equipment and also benefit for higher saturation solubility is the advantage for precipitation technique compared to other methods of nano-suspension preparation. Precipitation technique is not applicable to drugs which are poorly soluble in aqueous and non-aqueous media. In this technique, the drug needs to be soluble in at least one solvent which is miscible with non-solvent. The major challenge is to avoid crystal growth due to Ostwald ripening beings caused by different saturation solubilities in the vicinity of differently sized particles.
The top-down process follows disintegration approach from large particles, microparticles to Nano sized particles [18].
Examples are:
Examples are:
- High pressure homogenization
- Nano edge
- Nano pure
- Media milling (Nanocrystals).
Bottom-up process is an assembly method forms nanoparticles from molecules. An example includes [19]:
- Solvent-Anti-solvent method
- Super critical fluid process
- Emulsification Solvent evaporation technique
- Lipid emulsion/Micro-emulsion template.
The principle techniques used in recent years for preparing Nano-suspensions are:
Milling Techniques
Media milling [20,21] (Nanocrystals or Nano systems)
In this method the Nano-suspensions are produced using high‐shear media mills or pearl mills. The media mill consists of a milling chamber, a milling shaft and a recirculation chamber. The milling medium is framed of glass, zirconium oxide or highly cross‐linked polystyrene resin. The milling chamber is charged with the milling media, water, drug and stabilizer, and the milling media or pearls are then rotated at a very high shear rate.
Media milling [20,21] (Nanocrystals or Nano systems)
In this method the Nano-suspensions are produced using high‐shear media mills or pearl mills. The media mill consists of a milling chamber, a milling shaft and a recirculation chamber. The milling medium is framed of glass, zirconium oxide or highly cross‐linked polystyrene resin. The milling chamber is charged with the milling media, water, drug and stabilizer, and the milling media or pearls are then rotated at a very high shear rate.
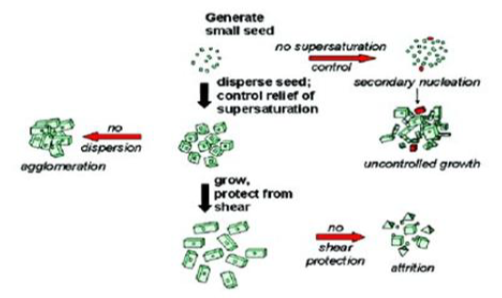
The milling process is performed under controlled temperatures. The high energy and shear forces generated as a result of the impaction of the milling media with the drug provide the energy input to break the microparticulate drug into Nano‐sized particles. The unimodal distribution profile and mean diameter of < 200, require a time profile of 30‐60 min. The media milling procedure can successfully process micronized and non‐micronized drug crystals. Once the formulation and the process are optimized, very short batch‐to‐batch variation is observed in the quality of the dispersion. A Nano-suspension of Naproxen with a mean particle size of 300‐600 nm was prepared using pearl milling technique as shown in figure: 1 & 2.
Dry co-grinding [22]
Since many years, Nano-suspensions are prepared through wet grinding processes by using pearl ball mill. Nowadays, Nano-suspensions can be prepared by dry milling methods. Stable Nano-suspensions are prepared by using dry grinding of poorly soluble drug with soluble polymers and copolymers after dispersing in liquid medium. Many poorly water-soluble drugs like nifedipine, Griseofulvin, and glibenclamide with sodium dodecyl sulfate and poly vinylpyrrolidone as stabilizer.
Since many years, Nano-suspensions are prepared through wet grinding processes by using pearl ball mill. Nowadays, Nano-suspensions can be prepared by dry milling methods. Stable Nano-suspensions are prepared by using dry grinding of poorly soluble drug with soluble polymers and copolymers after dispersing in liquid medium. Many poorly water-soluble drugs like nifedipine, Griseofulvin, and glibenclamide with sodium dodecyl sulfate and poly vinylpyrrolidone as stabilizer.
Applications
- Paint
- Ink
- Coatings
- Food grade
- Nutraceuticals
- Ceramics
- Pharmaceuticals
- Environmental
- Biotechnical products
- Pulp and paper products
- Bio fuels
- Crop protection
Homogenization [23]
High pressure homogenization
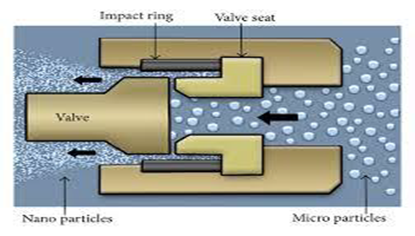
It is most widely used method for preparing Nano-suspensions of many poorly aqueous soluble drugs. It involves three steps. First drug powders are dispersed in stabilizer solution to form presuspension, and then the presuspension is homogenized in high pressure homogenizer at a low pressure for premilling (Figure 3), and finally homogenized at high pressure for 10 to 25 cycles until the Nano-suspensions of desired size are formed. Different methods are developed based on this principle for preparations of Nano-suspensions are Dissocubes, Nanopure, Nanoedge and Nanojet.
High pressure homogenization
It is most widely used method for preparing Nano-suspensions of many poorly aqueous soluble drugs. It involves three steps. First drug powders are dispersed in stabilizer solution to form presuspension, and then the presuspension is homogenized in high pressure homogenizer at a low pressure for premilling (Figure 3), and finally homogenized at high pressure for 10 to 25 cycles until the Nano-suspensions of desired size are formed. Different methods are developed based on this principle for preparations of Nano-suspensions are Dissocubes, Nanopure, Nanoedge and Nanojet.
Homogenisation in aqueous media [24] (Dissocubes)
Homogenization involves the forcing of the suspension under pressure through a valve having a narrow aperture. Dissocube technology was developed by Muller et al. in which, the suspension of the drug is made to pass through a small orifice that results in a reduction of the static pressure below the boiling pressure of water, which leads to boiling of water and formation of gas bubbles. When the suspension leaves the gap and normal air pressure is reached again, the bubbles shrink and the surrounding part containing the drug particles rushes to the center and in the process colloids, causing a reduction in the particle size. Most of the cases require multiple passes or cycles through the homogenizer, which depends on the hardness of drug, the desired mean particle size and the required homogeneity.
Homogenization involves the forcing of the suspension under pressure through a valve having a narrow aperture. Dissocube technology was developed by Muller et al. in which, the suspension of the drug is made to pass through a small orifice that results in a reduction of the static pressure below the boiling pressure of water, which leads to boiling of water and formation of gas bubbles. When the suspension leaves the gap and normal air pressure is reached again, the bubbles shrink and the surrounding part containing the drug particles rushes to the center and in the process colloids, causing a reduction in the particle size. Most of the cases require multiple passes or cycles through the homogenizer, which depends on the hardness of drug, the desired mean particle size and the required homogeneity.
Homogenization in Non-Aqueous Media [25] (Nanopure)
Nanopure is the technology in which suspension is homogenized in water-free media or water mixtures. In the Dissocubes technology the cavitation is the determining factor of the process. But, in contrast to water, oils and oily fatty acids have very low vapor pressure and a high boiling point. Hence, the drop of static pressure will not be sufficient enough to initiate cavitation. In nanopure technology, the drug suspensions in the non- aqueous media were homogenized at 0°C or even below the freezing point and hence are called "deep-freeze" homogenization. The results obtained were comparable to Dissocubes and hence can be used effectively for thermo labile substances at milder conditions.
Nanopure is the technology in which suspension is homogenized in water-free media or water mixtures. In the Dissocubes technology the cavitation is the determining factor of the process. But, in contrast to water, oils and oily fatty acids have very low vapor pressure and a high boiling point. Hence, the drop of static pressure will not be sufficient enough to initiate cavitation. In nanopure technology, the drug suspensions in the non- aqueous media were homogenized at 0°C or even below the freezing point and hence are called "deep-freeze" homogenization. The results obtained were comparable to Dissocubes and hence can be used effectively for thermo labile substances at milder conditions.
Advantages [26,27]
- Drugs that are poorly soluble in both aqueous and organic media can be easily formulated into Nano-suspensions.
- Ease of scale-up and little batch-to-batch variation.
- Narrow size distribution of the nanoparticulate drug present in the final product.
- Allows aseptic production of Nano-suspensions for parenteral administration.
- Flexibility in handling the drug quantity, ranging from 1 to 400 mg mL-1, thus enabling formulation of very dilute as well as highly concentrated Nano-suspensions.
Disadvantages [28,29]
- Prerequisite of micronized drug particles.
- Prerequisite of suspension formation using high-speed mixers before subjecting it to homogenization.
Nano edge [30,31,32]
The principle involved in Nanoedge is same that of the precipitation and homogenization techniques. This technique has an advantage of getting smaller particle size and greater stability in short period of time. In this technique the precipitated suspension is further homogenized to get smaller particle size and to avoid crystal growth (figure 4). Precipitation is performed in water using water miscible solvent, such as methanol, ethanol, and isopropanol. It is desired to remove the solvent completely by including evaporation step to provide a solvent free modified starting material followed by high pressure homogenization.
The principle involved in Nanoedge is same that of the precipitation and homogenization techniques. This technique has an advantage of getting smaller particle size and greater stability in short period of time. In this technique the precipitated suspension is further homogenized to get smaller particle size and to avoid crystal growth (figure 4). Precipitation is performed in water using water miscible solvent, such as methanol, ethanol, and isopropanol. It is desired to remove the solvent completely by including evaporation step to provide a solvent free modified starting material followed by high pressure homogenization.
Nanojet technology [33,34,35]
Nanojet technology is also called as opposite stream technology. In this technique a stream of suspension in two or more divided parts were passed with high pressure were made to colloid with each other, due to the high shear forces produced during the process leads to results in the reduction of particle size.
Nanojet technology is also called as opposite stream technology. In this technique a stream of suspension in two or more divided parts were passed with high pressure were made to colloid with each other, due to the high shear forces produced during the process leads to results in the reduction of particle size.
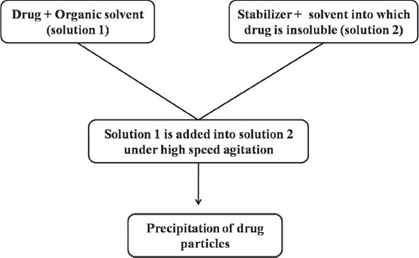
Precipitation Method [36]
Using a precipitation technique, the drug is dissolved in an organic solvent and this solution is mixed with a miscible anti-solvent. In water-solvent mixture the solubility is low and the drug precipitates. Mixing processes vary considerably. Precipitation has also been coupled with high shear processing. The Nano edge process (is a registered trademark of Baxter International Inc. and its subsidiaries) relies on the precipitation of friable materials for subsequent fragmentation under conditions of high shear and/or thermal energy.
Using a precipitation technique, the drug is dissolved in an organic solvent and this solution is mixed with a miscible anti-solvent. In water-solvent mixture the solubility is low and the drug precipitates. Mixing processes vary considerably. Precipitation has also been coupled with high shear processing. The Nano edge process (is a registered trademark of Baxter International Inc. and its subsidiaries) relies on the precipitation of friable materials for subsequent fragmentation under conditions of high shear and/or thermal energy.
Emulsion solvent diffusion method [37]
A part from the use of emulsion as drug delivering vehicle they can also be used as templates to produce Nano-suspension. The use of emulsions as templates is applicable for those drugs that are soluble in either volatile organic solvent or partially water‐miscible solvent. Such solvents can be used as the dispersed phase of the emulsion. An organic solvent or mixture of solvents loaded with the drug is dispersed in the aqueous phase containing suitable surfactants with stirring to form an emulsion.
A part from the use of emulsion as drug delivering vehicle they can also be used as templates to produce Nano-suspension. The use of emulsions as templates is applicable for those drugs that are soluble in either volatile organic solvent or partially water‐miscible solvent. Such solvents can be used as the dispersed phase of the emulsion. An organic solvent or mixture of solvents loaded with the drug is dispersed in the aqueous phase containing suitable surfactants with stirring to form an emulsion.
The obtained emulsion was further homogenized by high pressure homogenization. After homogenization cycles the emulsion was diluted with water, homogenized by homogenizer to diffuse the organic solvent and convert the droplets into solid particles. Since one particle is formed in each emulsion droplet, it is possible to control the particle size of the Nano-suspension by controlling the size of the emulsion. Optimizing the surfactant composition increases the intake of organic phase and ultimately the drug loading in the emulsion. Originally methanol, ethanol, ethyl acetate, chloroform are used as organic solvents. Nano-suspension of ibuprofen, diclofenac, acyclovir were prepared by this method.
Melt emulsification method [38,39]
In this method drug is dispersed in the aqueous solution of stabilizer and heated above the melting point of the drug and homogenized to give an emulsion. During this process, the sample holder was enwrapped with a heating tape fitted with temperature controller and the temperature of emulsion was maintained above the melting point of the drug. The emulsion was then cooled down either slowly to room temperature or on an ice‐bath. The main advantage of melt emulsification technique relative to the solvent diffusion method is total avoidance of organic solvents during the production process. Nano-suspension of ibuprofen was prepared by this method.
In this method drug is dispersed in the aqueous solution of stabilizer and heated above the melting point of the drug and homogenized to give an emulsion. During this process, the sample holder was enwrapped with a heating tape fitted with temperature controller and the temperature of emulsion was maintained above the melting point of the drug. The emulsion was then cooled down either slowly to room temperature or on an ice‐bath. The main advantage of melt emulsification technique relative to the solvent diffusion method is total avoidance of organic solvents during the production process. Nano-suspension of ibuprofen was prepared by this method.
Supercritical fluid method [40,41]
The organic solvents used in the preparation of conventional methods such as solvent extraction‐evaporation, solvent diffusion and organic phase separation methods are hazardous to environment and physiological systems. To rectify the problem occurred through the conventional method supercritical fluid technology has been investigated for the preparation of biodegradable micro and nanoparticles, because supercritical fluids are environmentally safe.
The organic solvents used in the preparation of conventional methods such as solvent extraction‐evaporation, solvent diffusion and organic phase separation methods are hazardous to environment and physiological systems. To rectify the problem occurred through the conventional method supercritical fluid technology has been investigated for the preparation of biodegradable micro and nanoparticles, because supercritical fluids are environmentally safe.
The most common techniques using supercritical fluids are supercritical anti‐solvent (SAS), precipitation with compressed anti‐solvent process (PCS) and rapid expansion of supercritical solution (RESS). The process of SAS employs a liquid solvent, e.g. methanol, which is completely miscible with the supercritical fluid (SC CO2), to dissolve the solute to be micronized; at the process condition, because the solute is insoluble in the supercritical fluid, the extract of the liquid solvent by supercritical fluid leads to the instantaneous precipitation of the solute, resulting in the formation of nanoparticles.
Solvent evaporation [42,43]
In the solvent evaporation method, the solutions of polymer are prepared in volatile solvents and emulsions. But from the past year’s dichloromethane and chloroform were used which was now replaced by ethyl acetate which has a better profile of toxicology. The emulsion is converted into a nanoparticle suspension on evaporation of the solvent for the polymer, which is allowed to diffuse through the continuous phase of the emulsion.
In the solvent evaporation method, the solutions of polymer are prepared in volatile solvents and emulsions. But from the past year’s dichloromethane and chloroform were used which was now replaced by ethyl acetate which has a better profile of toxicology. The emulsion is converted into a nanoparticle suspension on evaporation of the solvent for the polymer, which is allowed to diffuse through the continuous phase of the emulsion.
In the conventional methods, two main strategies are being used for the formation of emulsions, the preparation of single-emulsions, e.g., oil-in-water (o/w) or double-emulsions, e.g., (water-in-oil)-in-water, (w/o/w). These methods require high-speed homogenization or ultrasonication, followed by evaporation of the solvent, either by continuous magnetic stirring at room temperature or under reduced pressure. By ultracentrifugation the solidified nanoparticles are collected which was washed with distilled water to remove the additives like surfactants, and then it was lyophilized. The particle size was influenced by the concentration of polymer, stabilizer and the speed of homogenizer.
Evaluation of Nano-suspensions
Particle size and size distribution [44]
It is the most important parameter in the evaluation of the suspensions as it is having the direct effect on the solubility and dissolution rate and the physical stability of the formulation. The mean particle size and the width of particle size can be determined by Photon Correlation Spectroscopy (PCS), laser diffraction and coulter current multisizer.
Particle size and size distribution [44]
It is the most important parameter in the evaluation of the suspensions as it is having the direct effect on the solubility and dissolution rate and the physical stability of the formulation. The mean particle size and the width of particle size can be determined by Photon Correlation Spectroscopy (PCS), laser diffraction and coulter current multisizer.
Particle charge (Zeta Potential) [45]
The particle charge is of importance in the study of the stability of the suspensions. Usually the zeta potential of more than ± 40 mV will be considered to be required for the stabilisation of the dispersions. For electrostatically stabilized Nano-suspension a minimum zeta potential of ± 30 mV is required and in case of combined steric and electrostatic stabilization it should be a minimum of ± 20 mV of zeta potential is required.
The particle charge is of importance in the study of the stability of the suspensions. Usually the zeta potential of more than ± 40 mV will be considered to be required for the stabilisation of the dispersions. For electrostatically stabilized Nano-suspension a minimum zeta potential of ± 30 mV is required and in case of combined steric and electrostatic stabilization it should be a minimum of ± 20 mV of zeta potential is required.
Crystalline Sate and Particle Morphology [46]
It is of importance as there are chances of the polymorphism during the storage of the Nano-suspensions. Hence it is necessary to study the crystal morphology of the drug in suspension. Differential Scanning Calorimetry (DSC) is most commonly used for such studies.
It is of importance as there are chances of the polymorphism during the storage of the Nano-suspensions. Hence it is necessary to study the crystal morphology of the drug in suspension. Differential Scanning Calorimetry (DSC) is most commonly used for such studies.
Saturation solubility and Dissolution Velocity [47]
The main advantage associated with the Nano-suspensions is improved saturation solubility as well as dissolution velocity. These are studied in different physiological solutions at different pH. Kelvin equation and the Ostwald-Freundlich equations can explain increase in saturation solubility. Determination of these parameters is useful to assess in vivo performance of the formulation.
The main advantage associated with the Nano-suspensions is improved saturation solubility as well as dissolution velocity. These are studied in different physiological solutions at different pH. Kelvin equation and the Ostwald-Freundlich equations can explain increase in saturation solubility. Determination of these parameters is useful to assess in vivo performance of the formulation.
Stability of Nano-suspensions [48]
Stability of the suspensions is dependent on the particle size. As the particle size reduces to the nanosize the surface energy of the particles will be increased and they tend to agglomerate. So stabilizers are used which will decrease the chances of Ostwald ripening and improving the stability of the suspension by providing a steric or ionic barrier.
Stability of the suspensions is dependent on the particle size. As the particle size reduces to the nanosize the surface energy of the particles will be increased and they tend to agglomerate. So stabilizers are used which will decrease the chances of Ostwald ripening and improving the stability of the suspension by providing a steric or ionic barrier.
In-vitro drug release studies [49]
Dissolution tests were performed in a USP typeII (paddle method - TDT-08 L, Electro lab, Mumbai, India) at 37°C ± 0.5°C and paddle was rotated at a speed of 100 rpm. Open ended tube was tightened with semi-permeable membrane at one end and 8.5 ml of CMNS was added into it. This setup was immersed on the dissolution medium. Samples of 5 ml were collected at every 30min intervals and required dilution with dissolution medium then detected at 425 nm using UV spectrophotometer. The peak area of standard solution and sample collected at every 30 min intervals was recorded and the percentage drug release was calculated.
Dissolution tests were performed in a USP typeII (paddle method - TDT-08 L, Electro lab, Mumbai, India) at 37°C ± 0.5°C and paddle was rotated at a speed of 100 rpm. Open ended tube was tightened with semi-permeable membrane at one end and 8.5 ml of CMNS was added into it. This setup was immersed on the dissolution medium. Samples of 5 ml were collected at every 30min intervals and required dilution with dissolution medium then detected at 425 nm using UV spectrophotometer. The peak area of standard solution and sample collected at every 30 min intervals was recorded and the percentage drug release was calculated.
In-vivo biological performance [50]
The establishment of an in-vitro/in-vivo correlation and the monitoring of the in-vivo performance of the drug is an essential part of the study, irrespective of the route and the delivery system employed. It is of the utmost importance in the case of intravenously injected nanosuspensions since the in-vivo behaviour of the drug depends on the organ distribution, which in turn depends on its surface properties, such as surface hydrophobicity and interactions with plasma proteins.
The establishment of an in-vitro/in-vivo correlation and the monitoring of the in-vivo performance of the drug is an essential part of the study, irrespective of the route and the delivery system employed. It is of the utmost importance in the case of intravenously injected nanosuspensions since the in-vivo behaviour of the drug depends on the organ distribution, which in turn depends on its surface properties, such as surface hydrophobicity and interactions with plasma proteins.
Application of Nano-suspensions [51]
Nanosuspensions have wide range of applications especially in the case of low solubility and low bioavailability drugs. They are mentioned below.
Nanosuspensions have wide range of applications especially in the case of low solubility and low bioavailability drugs. They are mentioned below.
Oral Drug Delivery
Nano-suspensions for Oral Drug Delivery Because of the numerous advantages oral route is the most preferable route for many of the drugs (figure 3) especially in the case of orally administering antibiotics such as atovaquone and bupravaquone. By making it in nanosize, its solubility and bioavailability will increase.
Nano-suspensions for Oral Drug Delivery Because of the numerous advantages oral route is the most preferable route for many of the drugs (figure 3) especially in the case of orally administering antibiotics such as atovaquone and bupravaquone. By making it in nanosize, its solubility and bioavailability will increase.
Bioavailability enhancement
Drug with poor solubility, poor permeability or poor solubility in gastrointestinal tract will leads to poor oral bioavailability. Nano-suspension resolves the problem of poor bioavailability by solving the problem of poor solubility, and poor permeability across the membranes. Dissolution rate was increased.
Drug with poor solubility, poor permeability or poor solubility in gastrointestinal tract will leads to poor oral bioavailability. Nano-suspension resolves the problem of poor bioavailability by solving the problem of poor solubility, and poor permeability across the membranes. Dissolution rate was increased.
Ocular administration
For delivery of poorly soluble drug in cul‐de‐sac suspensions and ointments are recommended. Suspensions have advantages of prolonged residual time in cul‐de‐sac and avoidance of higher tonicity produced by water soluble drugs. The ocular bioavailability of suspensions depends on the dissolution rate of the drug in lachrymal fluid. However the inflow and outflow of lacrimal fluid causes variation in the dissolution rate of the drug.
For delivery of poorly soluble drug in cul‐de‐sac suspensions and ointments are recommended. Suspensions have advantages of prolonged residual time in cul‐de‐sac and avoidance of higher tonicity produced by water soluble drugs. The ocular bioavailability of suspensions depends on the dissolution rate of the drug in lachrymal fluid. However the inflow and outflow of lacrimal fluid causes variation in the dissolution rate of the drug.
Intravenous administration
The parenteral route is an invading route. Despite all these limitations, the parenteral route still retains its value because of its special advantages, such as quick onset of action in case of emergency, reduction in dose of the drug and the ability to target the drug quickly to the desired site of action, especially in the case of severe infections. The parenteral route is often employed as a substitute when the drug is either not absorbed through the gastrointestinal tract or undergoes extensive first‐pass metabolism.
The parenteral route is an invading route. Despite all these limitations, the parenteral route still retains its value because of its special advantages, such as quick onset of action in case of emergency, reduction in dose of the drug and the ability to target the drug quickly to the desired site of action, especially in the case of severe infections. The parenteral route is often employed as a substitute when the drug is either not absorbed through the gastrointestinal tract or undergoes extensive first‐pass metabolism.
Pulmonary administration
Aqueous Nano-suspension can be nebulized using mechanical or ultrasonic nebulizer for lung delivery. The Nano-particulate nature of the drug allows the rapid diffusion and dissolution of the drug at the site of action. At the same time, the increased adhesiveness of the drug to mucosal surfaces offers a prolonged residence time for the drug at the absorption site. This ability of Nano-suspensions to offer quick onset of action initially and then controlled release of the active moiety is highly beneficial and is required by most pulmonary diseases.
Aqueous Nano-suspension can be nebulized using mechanical or ultrasonic nebulizer for lung delivery. The Nano-particulate nature of the drug allows the rapid diffusion and dissolution of the drug at the site of action. At the same time, the increased adhesiveness of the drug to mucosal surfaces offers a prolonged residence time for the drug at the absorption site. This ability of Nano-suspensions to offer quick onset of action initially and then controlled release of the active moiety is highly beneficial and is required by most pulmonary diseases.
Targeted drug deliver [52]
Nano-suspensions can also be used as targeted drug delivery. It can be designed by incorporating the drug into the mononuclear phagocytic system. Targeted drug delivery can be used for the anti mycobacterial, fungal or leishmanial drugs to macrophages if the infectious pathogen is persisting intracellular. The further plan of action for targeted drug delivery system is by using various surface coatings for active or passive targeting.
Nano-suspensions can also be used as targeted drug delivery. It can be designed by incorporating the drug into the mononuclear phagocytic system. Targeted drug delivery can be used for the anti mycobacterial, fungal or leishmanial drugs to macrophages if the infectious pathogen is persisting intracellular. The further plan of action for targeted drug delivery system is by using various surface coatings for active or passive targeting.
Mucoadhesion of the nanoparticles
A nanoparticle has an ability to adhere to the mucosa surface due to small particles. The adhesion of the particles is the first step before particle absorption. To further increase the adhesive time nano-suspensions are formulated with hydrogels made from mucoadhesive polymers, e.g. different types of carbopol and chitosan. The adhesiveness of the nano-suspension not only helps to improve the bioavailability but also improves targeting of the parasites persisting in the GIT.
A nanoparticle has an ability to adhere to the mucosa surface due to small particles. The adhesion of the particles is the first step before particle absorption. To further increase the adhesive time nano-suspensions are formulated with hydrogels made from mucoadhesive polymers, e.g. different types of carbopol and chitosan. The adhesiveness of the nano-suspension not only helps to improve the bioavailability but also improves targeting of the parasites persisting in the GIT.
Topical formulations
Drug nanoparticles can also be incorporated into water free ointments and creams, which have an increased saturation solubility and enhanced diffusion of drug into the skin.
Drug nanoparticles can also be incorporated into water free ointments and creams, which have an increased saturation solubility and enhanced diffusion of drug into the skin.
Marketed Products Based on Nano-suspension [53]
All the products based on Nano-suspension have been approved by the FDA from the year 2000 on. All listed products are based on top-down approaches, eight relying on media milling and one on high- pressure homogenization. Although the bottom-up approaches hold tremendous potential with respect to improving bioavailability in obtaining smaller particle sizes (< 100 nm) and amorphous drug particles, no commercial application of these systems has yet been realized.
All the products based on Nano-suspension have been approved by the FDA from the year 2000 on. All listed products are based on top-down approaches, eight relying on media milling and one on high- pressure homogenization. Although the bottom-up approaches hold tremendous potential with respect to improving bioavailability in obtaining smaller particle sizes (< 100 nm) and amorphous drug particles, no commercial application of these systems has yet been realized.
A third remarkable point is that all commercial products are intended for oral delivery. This is an illustration of the general preference of the oral route, since it avoids the pain and discomfort associated with injections and is more attractive from a marketing and patient compliance perspective. Finally, the major advantage of nanocrystals for oral delivery is generally regarded as being on the increased specific surface area of the particles.
| Product | Drug compound | Indication |
| RAPAMUNE® | Sirolimus | Immunosuppressant |
| EMEND® | Aprepitant | Antiemetic |
| TriCor® | Fenofibrate | Treatment of hypercholesterolemia |
| MEGACE® ES | Megestrol acetate | Appetite stimulant |
| Triglide™ | Fenofibrate | Treatment of hypercholesterolemia |
References
- Elaine Merisko‐Liversidge., et al. “a formulation approach for poorly water‐soluble compounds”. European Journal of Pharmaceutical Sciences 18.2 (2003):113‐120.
- Dubey R. “Impact of nanosuspension technology on drug discovery and development”. Journal of Drug Delivery Science and Technology 6 (2006): 65-67.
- Amidon GL., et al. “A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability”. Pharmaceutical Research 12.3 (1995): 413-420.
- Yu LX., et al. “Biopharmaceutics classification system: the scientific basis for biowaiver Extensions”. Pharmaceutical Research 19.2 (2002):921-925.
- Lennerna¨s H and Abrahamsson B. “The use of biopharmaceutic classification of drugs in drug discovery and development: current status and future extension”. Journal of Pharmacy and Pharmacology 57.3 (2005): 273-285.
- Varshosaz J., et al. “Dissolution enhancement of gliclazide using in situ micronization by solvent change method”. Powder Technology 187.3 (2008): 222- 230.
- Pahala S., et al. “Solubilization of rapamycin”. International Journal of Pharmaceutics 213.1 (2001): 25-29.
- Abu Serajuddin TM. “Salt formation to improve drug solubility”. Advanced Drug Delivery Reviews 59.7 (2007): 603-616.
- Wong SM., et al. “Enhancement of the dissolution rate and oral absorption of a poorly water soluble drug by formation of surfactant‐containing microparticles”. International Journal of Pharmaceutics 317.1 (2006): 61‐68.
- Marazban S., et al. “Enhanced drug dissolution using evaporative precipitation into aqueous solution”. International Journal of Pharmaceutics 243.1.2 (2002):17‐31.
- True LR., et al. “Development and characterization of a scalable controlled precipitation process to enhance the dissolution of poorly soluble drugs”. Pharmaceutical Research 21.11 (2004): 2048‐2057.
- Nakano M. “Places of emulsions in drug delivery”. Advanced Drug Delivery Reviews 45 (2000): 1-4.
- Jadhav KR., et al. “Applications of microemulsion based drug delivery system”. Current Drug Delivery 3.3 (2006): 267‐273.
- Lawrence MJ and Rees GD. “Microemulsion‐based media as novel drug delivery systems”. Advanced Drug Delivery Reviews 45.1 (2000): 89-121.
- Marcela L., et al. “Solubilization of naphthoquinones by complexation with hydroxypropyl‐bcyclodextrin”. International Journal of Pharmaceutics 159 (1997): 13-18.
- Liversidge GG and Conzentino P. “Drug particle size reduction for decreasing gastric irritancy and enhancing absorption of naproxen in rats”. International Journal of Pharmaceutics 125.2 (1995): 309-313.
- Peters K., et al. “Preparation of clofazamine nanosuspension for intravenous use and evaluation of its therapeutic efficacy in Mycobacterium avium infection”. Journal of Antimicrobial Chemotherapy 45.1 (2000): 77-83.
- Jacobs C., et al. “Production and characterization of mucoadhesive nanosuspensions for the formulation of bupravaquone”. International Journal of Pharmaceutics 214.1.2 (2001): 3-7.
- Debuign F., et al. “Synthesis of nimesulid nanoparticles in the microemulsion epikuron/isopropyl myristate/water/n‐butanol (or isopropanol)”. Journal of Colloid and Interface Science 243.1 (2001): 90-101.
- Trotta M., et al. “Emulsions containing partially water‐miscible solvents for the preparation of drug nanosuspensions”. Journal of Controlled Release 76.1.2 (2001): 119-128.
- Kayser O., et al. “Formulation of amphotericin B as nanosuspension for oral administration”. International Journal of Pharmaceutics 254.1 (2003): 73-75.
- Hecq J., et al. “Preparation and characterisation of nanocrystals for solubility and dissolution rate enhancement of nifedipine”. International Journal of Pharmaceutics 299.1.2 (2005): 167-177.
- Langguth P., et al. “Nanosuspension formulations for low‐soluble drugs: pharmacokinetic evaluation using spironolactone as model compound”. Drug Development and Industrial Pharmacy 31.3 (2005): 319-329.
- Patravale VB., et al. “A promising drug delivery strategy”. Journal of Pharmacy and Pharmacology 56.7 (2004): 827-840.
- Kesisoglou F., et al. “Nanosizing—oral formulation development and biopharmaceutical evaluation”. Advanced Drug Delivery Reviews 59.7 (2007): 631-644.
- Keck CM and Muller RH. “Drug nanocrystals of poorly soluble drugs produced by high pressure homogenization”. European Journal of Pharmaceutics and Biopharmaceutics 62.1 (2006): 3-16.
- Rabinow B. “Nanosuspensions in drug delivery”. Nature 3 (2004): 785-793.
- Kocbek P and Kristl J. “Preparation and evaluation of nanosuspensions for enhancing the dissolution of poorly soluble drugs”. International Journal of Pharmaceutics 312.1.2 (2006): 179-186.
- Trotta M., et al. “Preparation of griseofulvin nanoparticles from water‐dilutable microemulsions”. International Journal of Pharmaceutics 254.2 (2003): 235-242.
- Zhang X., et al. “Preparation of All‐Trans Retinoic Acid Nanosuspensions Using a Modified Precipitation Method”. Drug Development and Industrial Pharmacy 32.7 (2006): 857-863.
- Liversidge GG and Cundy K. “Particle size reduction for improvement of oral bioavailability of hydrophobic drugs: I. Absolute oral bioavailability of nanocrystalline danazol in beagle dogs”. International Journal of Pharmaceutics 125.1 (1995): 91-97.
- Merisko Liversidge E., et al. “Nanosizing. A formulation approach for poorly‐water‐soluble compounds”. European Journal of Pharmaceutical Sciences 18.2 (2003): 113-120.
- Muller RH., et al. “Pharmaceutical nanosuspensions for medicament administration as systems with increased saturation solubility and rate of solution”. (1999).
- Jacobs C., et al. “Nanosuspensions as a new approach for the formulation for the poorly soluble drug tarazepide”. International Journal of Pharmaceutics 196.2 (2000): 161-164.
- Anchalee AA and Pardeep KG. “Effect of arginine hydrochloride and hydroxypropyl cellulose as stabilizers on the physical stability of high drug loading nanosuspensions of a poorly soluble compound”. International Journal of Pharmaceutics 351.1.2 (2008): 282‐288.
- Ruolan X., et al. “Preparation and characterization of intravenously injectable nimodipine nanosuspension”. International Journal of Pharmaceutics 350.1.2 (2008): 338-343.
- Muller RH., et al. “Modified‐Release Drug Delivery Technology”. New York Basel (2003): 135-149.
- Muller RH and Peters K. “Nanosuspensions for the formulation of poorly soluble drugs”. International Journal of Pharmaceutics 160.2 (1998): 229‐237.
- Francesco L., et al. “Diclofenac nanosuspensions. Influence of preparation procedure and crystal form on drug dissolution behavior”. International Journal of Pharmaceutics 373.1.2 (2009): 124-132.
- Panchaxari D., et al. “Polymeric ocular nanosuspension for controlled release of acyclovir: in vitro release and ocular distribution”. Iranian Journal of Pharmaceutical Research 8.2 (2009): 79‐86.
- Thote AJ and Gupta RB. “Formation of nanoparticles of a hydrophilic drug using supercritical carbon dioxide an microencapsulation for sustained release”. Nanomedicine: Nanotechnology, Biology and Medicine 1.1 (2005): 85‐90.
- Chattophadhyay P and Gupta RP. “Production of griseofulvin nanoparticles using supercritical CO2 antisolvent with enhanced mass transfer”. International Journal of Pharmaceutics 228.1.2 (2001): 19‐31.
- Sun Y., et al. “Polymeric nanoparticles from rapid expansion of supercritical fluid solution”. Chemistry 11.5 (2005): 1366‐1373.
- Young TJ., et al. “Rapid expansion from supercritical to aqueous solution to produce submicron suspension of water insoluble drugs”. Biotechnology Progress 16.3 (2000): 402‐407.
- Muller RH. “Zetapotential und Partikelladung–Kurze Theorie, praktische MeBdurchfuhrung, Dateninterpretation”. Wissenschaftliche Verlagsgesellschaft, Stuttgart. 1996.
- Andrej D., et al. “Advantages of celecoxib nanosuspension formulation and transformation into tablets”. International Journal of Pharmaceutics 376.1.2 (2009):204-212.
- Lenhardt T., et al. “Evaluation of Nanosuspensions for Absorption Enhancement of Poorly Soluble Drugs: In Vitro Transport Studies across Intestinal Epithelial Monolayers”. The AAPS Journal 10.3 (2008): 435-438
- HanafyA., et al. “Pharmacokinetic evaluation of oral fenofibrate nanosuspension and SLN in comparison to conventional suspension of micronized drug”. Advanced drug delivery reviews 59.6 (2007): 419‐ 426.
- Liversidge GC. “Workshop on particulate drug delivery systems. Paper presented at the 23 International symposium of the controlled release bioactive maerial society”. (1996).
- Chen Y., et al. “Oleanoic acid nanosuspension: preparation, in vitro characterization and enhanced hepato protective effect”. Journal of Pharmacy and Pharmacology 57.2 (2005): 259‐64.
- Schöler N., et al. “Atovaqu one nanosuspensions show excellent therapeutic effect in a new murine model of reactivated toxoplasmosis”. Antimicrobial Agents and Chemotherapy 45.6 (2001): 1771‐1779.
- Sagar MA and Pradeep RV. “Diclofenac‐loaded biopolymeric nanosuspensions for ophthalmic application”. Nanomedicine: Nanotechnology, Biology, and Medicine 5.1 (2009): 90-95.
- Hany SMA., et al. “Preparation of hydrocortisone nanosuspension through a bottom‐up nanoprecipitation technique using microfluidic reactors”. International Journal of Pharmaceutics 375.1.2 (2009): 107-113.
Citation:
VL. Narasaiah., et al. “ Nano suspensions: A Prospective Approach”. Chronicles of Pharmaceutical Science 1.4 (2017): 180-192.
Copyright: © 2017 VL. Narasaiah., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.