Research Article
Volume 1 Issue 4 - 2017
Treatment of Parkinson’s Disease with Phenolic Antioxidant Drugs: Oxidative Stress, Reactive Oxygen Species and Selectivity.
1Peter Kovacic, Department of Chemistry and Biochemistry, San Diego State University, San Diego, CA, USA
2Wil Weston, Library and Information Access, San Diego State University, San Diego, CA, USA
2Wil Weston, Library and Information Access, San Diego State University, San Diego, CA, USA
*Corresponding Author: Peter Kovacic, Department of Chemistry and Biochemistry, San Diego State University, San Diego, CA, USA.
Received: August 15, 2017; Published: August 23, 2017
Abstract
Parkinson’s is one of the most prevalent brain diseases characterized by the presence of oxidative stress and reactive oxygen species. It is also classified as a type of dementia. Phenolic compounds comprise one of the class of drugs for this disease. However, there is no cure available; the drugs act as antioxidants which alleviate the toxic effects. The drugs involved are the following: L-dopa, opicapone, apomorphine, catechin, entacapone, rotigotine, safinamide, and piperine.
Keywords: Parkinson’s disease; Phenolic antioxidants; Oxidative stress
Abbreviations: (AGE): advanced glycation end products; (OS): oxidative stress; (ET): electron transfer; (ROS): reactive oxygen species; (AO): antioxidant; (4-HNE): 4-hydroxynonenal; (RNS): reactive nitrogen species; (COMT): catechol- O-methyltransferase; (SAR): structure–activity relationship (SAR)
Introduction
Parkinson’s disease is a neurological condition involving the destruction of dopaminergic neurons (Filograna., et al. 2016) resulting in reduction of striatal dopamine levels leading to characteristic motor symptoms. The drugs are used mainly to treat symptoms; currently the most effective treatment remains a dopamine replacement therapy with L-dopa, along with an inhibitor of aromatic amino acid decarboxylase (Bonifácio., et al. 2007). Oxidative stress (OS) is involved in the dopaminergic neurotoxicity. However, Parkinson’s disease is a sporadic disease, in which exposure to environmental factors, such as neurotoxins, pesticides, head trauma, genetic mutations, and inflammation are potential factors (Sarrafchi., et al. 2016). Antioxidant (AO) molecules are discussed which combat OS. Reactive oxygen species (ROS) are responsible for damage or death of neuronal cells. Oxidation of unsaturated lipids generates malondialdehyde and 4– hydroxy–2, 3–nonenal; whereas, nucleic acid oxidation leads to 8-hydroxyguanosine. Iron accumulation takes place in the brain of Parkinson’s disease patients, which can produce OS in a number of ways. Additionally, the psychotic symptoms present in many Parkinson’s disease patients are associated with many other factors in a complicated manner, which can complicate the treatment of motor symptoms by limiting the use of medications (Divac., et al. 2016).
Discussion
Reviews of Parkinson’s disease and OS mechanisms have been published as recently as 2015 (Katunina., et al. 2015). A more recent 2016 article deals with various treatment options, such as use of drugs that elevate dopamine levels like L-dopa (Farzanehfar, 2016). Additional attention has been paid to AO aspects; a 2016 report is concerned with therapeutic targets of AO agents in Parkinson’s disease and Alzheimer’s disease (Jiang, Sun & Chen, 2016). Furthermore, there is evidence that stress from ROS and reactive nitrogen species (RNS) plays a role in Parkinson’s neurodegeneration (de Farias., et al. 2016).
Biomarkers of Parkinson’s disease include malondialdehyde, lipid hydroperoxide and superoxide dismutase. Lipid peroxidation
should be a target for treatment of Parkinson’s, in addition to dopamine. The bioactive components of various plants are known to possess
AO and anti-inflammatory properties, in addition to iron chelating potential (Morgan & Grundmann, 2017). One study suggests that
the complementary use of these herbal supplements may improve the therapeutic effects of pharmaceutical drugs (Morgan & Grundmann,
2017). A previous review focuses on anti-inflammation, where the evidence of the involvement of ROS and OS is discussed along
with mechanisms (Kovacic & Somanathan, 2014).
A review deals with important causes of neurodegeneration, such as OS and mitochondrial damage, and the complexity of dopamine metabolism is reviewed (Meiser, Weindl, & Hiller, 2013). Increased levels of lipid peroxidation are observed in the brains of Parkinson’s disease patients. Catecholamine reactions might lead to excess ROS resulting in cell death. Dopamine and L-dopa are prone to oxidation of the catechol portion leading to o-quinones which are electron transfer (ET) entities capable of generating ROS and OS. Monomethylation of the catechol via catechol- O-methyltransferase (COMT) prevents oxidation to o-quinone while maintaining AO power.
In Parkinson’s disease, levels of natural AOs are decreased in patients, rendering greater chance of OS. Reactive nitrogen species (RNS) can also be involved, such as peroxynitrite (NO3-), a powerful oxidizing agent that can induce damage. Marked species differences exist with animal models, making for limited use in application to human brain diseases.
A 2014 review deals with L-dopa and dopamine neurotoxicity (Segura-Aguilar, Ahumada-Castro, & Paris, 2014). L-Dopa does not produce neurotoxicity in vivo because of rapid decarboxylation to dopamine. In vivo, the only metabolite detected is L-3-O-methyldopa. Under certain conditions, oxidation to an o-quinone species can occur which generates ROS and OS with resultant toxicity. The o-quinone cyclizes to aminochrome which is able to undergo formation of other products, many of which are neurotoxic. Also, L-dopa is known to induce dyskinesia (difficulty in moving) via a complex mechanism. In addition, the properties of the generated dopamine are discussed.The compound appears to be toxic to neurons because of oxidation to the o-quinone aminochrome. The AO N-acetyl-L-cysteine, a thiol, prevents dopamine induced apoptosis which otherwise leads to cell death (Segura-Aguilar, Ahumada-Castro, & Paris, 2014).
There is extensive additional literature dealing with AOs in Parkinson’s disease. A 2016 review addresses AOs in Parkinson’s disease therapy, evaluating the current literature that links oxidative stress and mitochondrial dysfunction to Parkinson’s (Filograna., et al. 2016). In another study, plasma AO status was studied in Chinese Parkinson’s patients, which demonstrated that the plasma antioxidant status is impaired in de novo Parkinson’s disease patients (Yuan., et al. 2016). One study deals with intake of AO vitamins and risk of Parkinson’s disease (Hughes., et al. 2016). They found that the intake of antioxidant vitamins did not reduce the risk of Parkinson’s and indicated that the intake of AO vitamins was unrelated to Parkinson’s disease risk.
This report involves the following AO compounds: L-dopa, opicapone, apomorphine, catechin, entacapone, rotigotine, safinamide, and piperine.
L-Dopa
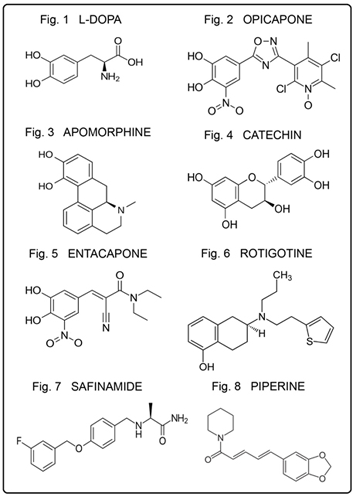
L-Dopa (Figure 1), a catechol type, is the principal drug in the treatment of Parkinson’s disease (Kianirad & Simuni, 2016). However, adverse reactions complicate the picture. Novel approaches to therapy are presented. The AO property protects DNA against oxidative attack (Colamartino., et al. 2015). The drug helps to maintain dopamine levels by decarboxylation. The AO activity was evaluated in connection with OS in Parkinson’s disease in which L-dopa demonstrated a protective effect (Colamartino., et al. 2015). Additional treatment of L-dopa and dopamine is presented in the introduction.
L-Dopa (Figure 1), a catechol type, is the principal drug in the treatment of Parkinson’s disease (Kianirad & Simuni, 2016). However, adverse reactions complicate the picture. Novel approaches to therapy are presented. The AO property protects DNA against oxidative attack (Colamartino., et al. 2015). The drug helps to maintain dopamine levels by decarboxylation. The AO activity was evaluated in connection with OS in Parkinson’s disease in which L-dopa demonstrated a protective effect (Colamartino., et al. 2015). Additional treatment of L-dopa and dopamine is presented in the introduction.
Opicapone
Opicapone (Figure 2) is used together with L-dopa, acting as a catechol drug and a COMT inhibitor in order to prevent methylation of hydroxyl (Annus & Vécsei, 2017). It is an adjunctive therapy in adults who cannot be stabilized on L-dopa. Many COMT inhibitors carry a risk for toxic effects to hepatic cells; opicapone was designed to be effective without these adverse toxic effects (Lees., et al. 2017)
Opicapone (Figure 2) is used together with L-dopa, acting as a catechol drug and a COMT inhibitor in order to prevent methylation of hydroxyl (Annus & Vécsei, 2017). It is an adjunctive therapy in adults who cannot be stabilized on L-dopa. Many COMT inhibitors carry a risk for toxic effects to hepatic cells; opicapone was designed to be effective without these adverse toxic effects (Lees., et al. 2017)
Apomorphine
Apomorphine (Figure 3), containing a catechol structure, displays activity as a dopamine agonist (Millan., et al. 2002). The clinical effect is similar to that of L-dopa, being employed as a rescue drug. The strong dopaminergic action makes it effective for therapeutic use and is comparable to L-dopa in other respects (Chaudhuri & Clough, 1998). Additional aspects will be addressed in a forthcoming structure–activity relationship (SAR) report.
Apomorphine (Figure 3), containing a catechol structure, displays activity as a dopamine agonist (Millan., et al. 2002). The clinical effect is similar to that of L-dopa, being employed as a rescue drug. The strong dopaminergic action makes it effective for therapeutic use and is comparable to L-dopa in other respects (Chaudhuri & Clough, 1998). Additional aspects will be addressed in a forthcoming structure–activity relationship (SAR) report.
Catechin
Catechin (Figure 4), a flavonoid catechol type, displays AO behavior in vitro (Pietta, 2000; Tournaire., et al. 1993). The effect can be attributed to the presence of phenolic hydroxyl groups. In the case of catechin, hydroxyl groups in the ortho position of the B ring can greatly enhance the AO capacity (Huang., et al. 2012).
Catechin (Figure 4), a flavonoid catechol type, displays AO behavior in vitro (Pietta, 2000; Tournaire., et al. 1993). The effect can be attributed to the presence of phenolic hydroxyl groups. In the case of catechin, hydroxyl groups in the ortho position of the B ring can greatly enhance the AO capacity (Huang., et al. 2012).
Entacapone
Entacapone (Figure 5), a catechol derivative, is commonly used with Parkinson’s disease medication. It was developed specifically as an inhibitor of L-dopa methylation; additionally, it is a powerful inhibitor of COMT (De Santi., et al. 1998).
Entacapone (Figure 5), a catechol derivative, is commonly used with Parkinson’s disease medication. It was developed specifically as an inhibitor of L-dopa methylation; additionally, it is a powerful inhibitor of COMT (De Santi., et al. 1998).
Rotigotine
Rotigotine (Figure 6), a phenolic drug, is a dopamine agonist (Wood., et al. 2015). It was introduced as a non-ergoline dopamine receptor agonist for the treatment of idiopathic Parkinson’s disease and for countering of restless legs syndrome (Perez Lloret & Rascol, 2016). Also, like some other dopamine agonists, rotigotine possesses some antidepressant effects which could also be useful in the treatment of depression (Bertaina-Anglade, La Rochelle, & Scheller, 2006).
Rotigotine (Figure 6), a phenolic drug, is a dopamine agonist (Wood., et al. 2015). It was introduced as a non-ergoline dopamine receptor agonist for the treatment of idiopathic Parkinson’s disease and for countering of restless legs syndrome (Perez Lloret & Rascol, 2016). Also, like some other dopamine agonists, rotigotine possesses some antidepressant effects which could also be useful in the treatment of depression (Bertaina-Anglade, La Rochelle, & Scheller, 2006).
Safinamide
Safinamide (Figure 7), an add-on for Parkinson’s disease treatment, acts as a monoamine oxidase inhibitor (Perez-Lloret & Rascol, 2016). It is a phenolic ether with the potential of undergoing dealkylation to the phenolic form. In fact, O-debenzylation has been reported (European Medicines Agency, 2015). The drug displays dopaminergic properties and inhibits monoamine oxidase B (Fabbri., et al. 2015). This 2015 article summarizes safinamide’s pharmacological properties, noting its specific use as an add-on therapy to stabilize L-dopa levels and also for the treatment of motor symptoms. A more current 2016 review addresses the safety and efficacy of use for the treatment of Parkinson’s disease, involving no specific issues other than those already known with monoamine oxidase inhibitors (Merck Index Online, 2013).
Safinamide (Figure 7), an add-on for Parkinson’s disease treatment, acts as a monoamine oxidase inhibitor (Perez-Lloret & Rascol, 2016). It is a phenolic ether with the potential of undergoing dealkylation to the phenolic form. In fact, O-debenzylation has been reported (European Medicines Agency, 2015). The drug displays dopaminergic properties and inhibits monoamine oxidase B (Fabbri., et al. 2015). This 2015 article summarizes safinamide’s pharmacological properties, noting its specific use as an add-on therapy to stabilize L-dopa levels and also for the treatment of motor symptoms. A more current 2016 review addresses the safety and efficacy of use for the treatment of Parkinson’s disease, involving no specific issues other than those already known with monoamine oxidase inhibitors (Merck Index Online, 2013).
Piperine
Piperine (Figure 8), a pepper alkaloid, has been used in traditional medicine as an insecticide, and to impart pungent taste to brandy [30]. It inhibits enzymes involved in metabolism of xenobiotics (Bhardwaj., et al. 2002; Srinivasan, 2007), where in a 2002 study, piperine inhibited the major drug-metabolizing enzyme cytochrome P450 3A4 (Bhardwaj., et al. 2002). The dealkylation to a catechol derivative would be comparable to that of safinamide. Additionally, a study in 2013 reports anti-apoptotic and anti-inflammatory effects, in addition to AO properties (Shrivastava., et al. 2013). The anti-inflammation mechanism is further discussed in another article (Kovacic & Somanathan, 2014).
Piperine (Figure 8), a pepper alkaloid, has been used in traditional medicine as an insecticide, and to impart pungent taste to brandy [30]. It inhibits enzymes involved in metabolism of xenobiotics (Bhardwaj., et al. 2002; Srinivasan, 2007), where in a 2002 study, piperine inhibited the major drug-metabolizing enzyme cytochrome P450 3A4 (Bhardwaj., et al. 2002). The dealkylation to a catechol derivative would be comparable to that of safinamide. Additionally, a study in 2013 reports anti-apoptotic and anti-inflammatory effects, in addition to AO properties (Shrivastava., et al. 2013). The anti-inflammation mechanism is further discussed in another article (Kovacic & Somanathan, 2014).
This commentary is part of a planned series on diseases based on the unifying theme of ROS-OS-AO. In addition, a forthcoming report deals with structure-activity relationship (SAR).
Acknowledgement
The assistance by Thelma Chavez is acknowledged.
The assistance by Thelma Chavez is acknowledged.
References
- Annus Á and Vécsei L. “Spotlight on opicapone as an adjunct to levodopa in Parkinson’s disease: design, development and potential place in therapy”. Drug Design, Development and Therapy 11 (2017): 143-151.
- Bertaina Anglade., et al. “Antidepressant properties of rotigotine in experimental models of depression”. European Journal of Pharmacology 548.1.3 (2006): 106-114.
- Bhardwaj., et al. “Piperine, a major constituent of black pepper, inhibits human P-glycoprotein and CYP3A4”. Journal of Pharmacology and Experimental Therapeutics 302.2 (2002): 645-650.
- Bonifácio MJ., et al. “Catechol-O-methyltransferase and Its Inhibitors in Parkinson's Disease”. CNS Drug Reviews 13.3 (2007): 352-379.
- Chaudhuri KR and Clough C. “Subcutaneous apomorphine in Parkinson's disease: Effective yet underused”. British medical journal 316 (1998): 641.
- Colamartino M., et al. “Evaluation of levodopa and carbidopa antioxidant activity in normal human lymphocytes in vitro: implication for oxidative stress in Parkinson's disease”. Neurotoxicity Research 27.2 (2015): 106-17.
- De Farias CC., et al. “Highly specific changes in antioxidant levels and lipid peroxidation in Parkinson's disease and its progression: Disease and staging biomarkers and new drug targets”. Neuroscience Letters 617 (2016): 66-71.
- De Santi C., et al. “Catechol-O-methyltransferase: variation in enzyme activity and inhibition by entacapone and tolcapone”. European Journal of Clinical Pharmacology 54.3 (1998): 215-219.
- Divac N., et al. “The efficacy and safety of antipsychotic medications in the treatment of psychosis in patients with Parkinson's disease”. Behavioural Neurology 4938154 (2016).
- European Medicines Agency “Summary of Product Characteristics for Xadago. European public assessment reports”. (2015).
- Fabbri M., et al. “Clinical pharmacology review of safinamide for the treatment of Parkinson's disease”. Neurodegenerative Disease Management 5.6 (2015): 481-496.
- Farzanehfar P. “Towards a better treatment option for Parkinson's disease: A review of adult neurogenesis”. Neurochemical Research 41.12 (2016): 3161-3170.
- Filograna R., et al. “Anti-oxidants in Parkinson’s disease therapy: A critical point of view”. Current Neuropharmacology 14.3 (2016): 260-271.
- Huang W., et al. “Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing”. Journal of Zhejiang University Science B 13.2 (2012): 94-102.
- Hughes KC., et al. “Intake of antioxidant vitamins and risk of Parkinson's disease”. Movement Disorders Journal 31.12 (2016): 1909-1914.
- Jiang T., et al. “Oxidative stress: A major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson's disease and Alzheimer's disease”. Progress in Neurobiology 147 (2016): 1-19.
- Katunina E.A., et al. “Oxidative stress and Parkinson's disease: mechanisms and perspectives of treatment”. Zhurnal Nevrologii I Psikhiatrii Imeni S.S. Korsakova 115.7 (2015): 141-145.
- Kianirad Y and Simuni T. “Novel approaches to optimization of levodopa therapy for Parkinson's disease”. Current Neurology and Neuroscience Reports 16.4 (2016): 34.
- Kovacic P and Somanathan R. “Inflammation and Anti-Inflammatory Agents – Reactive Oxygen Species and Toxicity, in: I. Laher (Ed.). Systems Biology of Free Radicals and Antioxidants”. Springer-Verlag Berlin Heidelberg (2014): 3197-3216.
- Lees AJ., et al. “Opicapone as Adjunct to Levodopa Therapy in Patients with Parkinson Disease and Motor Fluctuations: A randomized clinical trial”. JAMA Neurology 74.2 (2017): 197-206.
- Meiser J., et al. “Complexity of dopamine metabolism”. Cell Communication and Signaling 11.1 (2013): 34.
- Millan MJ., et al. “Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes”. Journal of Pharmacology and Experimental Therapeutics 303.2 (2002): 791-804.
- Merck Index Online Piperine. Monograph ID: M8854. Royal Society of Chemistry. (2013).
- Morgan LA and Grundmann O. “Preclinical and potential applications of common western herbal supplements as complementary treatment in Parkinson's disease”. Journal of Dietary Supplements 14.4 (2017): 453-466.
- Perez Lloret S and Rascol O. “The safety and efficacy of safinamide mesylate for the treatment of Parkinson's disease”. Expert Review of Neurotherapeutics 16.3 (2016): 245-258.
- Pietta PG. “Flavonoids as antioxidants”. Journal of Natural Products 63.7 (2000): 1035-1042.
- Sarrafchi A., et al. “Oxidative stress and Parkinson's disease: New hopes in treatment with herbal antioxidants”. Current Pharmaceutical Design 22.2 (2016): 238-246.
- Segura Aguilar J., et al. “Dopamine and L-dopa as Selective Endogenous Neurotoxins, in: R. M. Kostrzewa (Ed.). Handbook of Neurotoxicity”. Springer New York (2014): 199-218.
- Shrivastava P., et al. “Anti-apoptotic and anti-inflammatory effect of Piperine on 6-OHDA induced Parkinson's rat model”. The Journal of Nutritional Biochemistry 24.4 (2013): 680-687.
- Srinivasan K. “Black pepper and its pungent principle-piperine: a review of diverse physiological effects”. Critical Reviews in Food Science and Nutrition 47.8 (2007): 735-748.
- Tournaire C., et al. “Antioxidant activity of flavonoids: efficiency of singlet oxygen (1Δg) quenching”. Journal of Photochemistry and Photobiology B 19.3 (1993): 205-215.
- Wood M., et al. “Rotigotine is a potent agonist at dopamine D1 receptors as well as at dopamine D2 and D3 receptors”. British Journal of Pharmacology 172.4 (2015): 1124-1135.
- Yuan Y., et al. “Plasma antioxidant status and motor features in de novo Chinese Parkinson's disease patients”. International Journal of Neuroscience 126.7 (2016): 641-646.
Citation:
Peter Kovacic and Wil Weston. “Treatment of Parkinson’s Disease with Phenolic Antioxidant Drugs: Oxidative Stress, Reactive
Oxygen Species and Selectivity.” Chronicles of Pharmaceutical Science 1.4 (2017): 193-198.
Copyright: © 2017 Peter Kovacic and Wil Weston. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.