Research Article
Volume 1 Issue 4 - 2017
Coating and Evaluation of Compressed Tablets using two Different Concentrations of Gelatin.
Department of Pharmaceutical Technology and Industrial Pharmacy Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka, 410001, Nigeria
*Corresponding Author: Ugwu Calister Elochukwu, Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka, 410001, Nigeria.
Received: July 18, 2017; Published: September 18, 2017
Abstract
Coating is an application of coating solution to the surface of the tablet to confer specific benefits over uncoated tablet. The goal of the research is to determine the effects of two different concentrations of gelatin coating solutions and tablet properties. Compressed tablet was coated with 4 and 8% of gelatin using dip method. The coated tablets were evaluated using the following parameters: Hardness, friability, disintegration, weight uniformity and release profile. The result showed that the hardness test of 4% batch had an average hardness of 5.33 ± 0.26 Kgf while 8% showed an average hardness of 8.91 ± 0.34 Kgf. Average disintegration tests of 50.70 ± 5.39 and 33.47 ± 0.37 min were obtained for 8 and 4% respectively.
The coated tablets were less friable than the uncoated one with the percent weight deviation being within the acceptable rang. The results showed significant differences between the two concentrations (p < 0.001) in both hardness and disintegration tests. The uncoated tablet had an immediate and faster release of drugs than the coated batches because it has no outer coating film, while the 8% coated batch had more prolonged release of drug due to the thickness of the coat than the 4% batch. Therefore, as the coating concentration increases, the hardness and the disintegration time of tablet increases but less friable the batches and more prolong the drug release.
Introduction
Coating is a process of applying coating solution to the surface of tablets to confer specific benefits over uncoated tablet. Tablet coating can be described as a process of applying polymer solution on the surface of a pharmaceutical dosage form to achieve specific benefits. Coating is applicable to several kinds of solid dosage forms like tablets, pellets, pills, drug crystals, etc. Tablet surfaces get covered with a tacky polymeric film when a coating solution is applied to tablets in a coating pan. The tablets are then allowed to dry forming films with non-sticky dry surface. The coating technique involves parameters such as the spray pattern, drop size, and nozzle spacing and also multiple other non-spray related parameters, which must all be precisely controlled in order to ensure uniform distribution of the coating material [1,2].
- Rationales of coating Pharmaceutical products [3]:
- There so many reasons why tablet coating occurs. These include the follows:
- To mask the objectionable odor, color or taste of the tablet.
- To offer a physicochemical protection to the drug.
- To protect an acid-labile drug from the gastric environment.
- To control and sustain drug release from the dosage form.
- To incorporate another drug which create incompatibility issues.
- Increasing the mechanical strength of the dosage form, etc.
Coating process
It is of importance that the coating should be uniform and should not be liable to crack under stress or transportation. There are various techniques which are designed for the application of the coating on the tablet surface. Commonly, the coating solutions are applied onto the uncoated tablets as the tablets are being rotated in a pan, fluid bed, etc. As the solution is being sprayed, a thin film is formed which sticks to each tablet surface. The liquid portion of the coating solution is tackled through evaporation by passing air over the surface of the tumbling pans. A single application or multiple spraying cycles in layers forms the coating films of the tablet. Rotating coating pans are commonly used in the Pharmaceutical Industry. There are different coating methods that are applicable in pharmaceutical sciences.
It is of importance that the coating should be uniform and should not be liable to crack under stress or transportation. There are various techniques which are designed for the application of the coating on the tablet surface. Commonly, the coating solutions are applied onto the uncoated tablets as the tablets are being rotated in a pan, fluid bed, etc. As the solution is being sprayed, a thin film is formed which sticks to each tablet surface. The liquid portion of the coating solution is tackled through evaporation by passing air over the surface of the tumbling pans. A single application or multiple spraying cycles in layers forms the coating films of the tablet. Rotating coating pans are commonly used in the Pharmaceutical Industry. There are different coating methods that are applicable in pharmaceutical sciences.
These include: Sugar Coating
Sugar coating is originally developed for masking of the bitter taste of some drugs and also to provide aesthetic appeal to the tablet core. The process of sugar coating comprises of multiple steps.
Sugar coating is originally developed for masking of the bitter taste of some drugs and also to provide aesthetic appeal to the tablet core. The process of sugar coating comprises of multiple steps.
These include: Sealing
A sealing coat is applied over the tablet to waterproof the tablet core. It prevents moisture penetration into the tablet core. Zein is a currently used sealant, a corn protein derivative, which has replaced previously used shellac due to polymerization problems.
A sealing coat is applied over the tablet to waterproof the tablet core. It prevents moisture penetration into the tablet core. Zein is a currently used sealant, a corn protein derivative, which has replaced previously used shellac due to polymerization problems.
Sub coating: This step is done to round the edges and increase the tablet weight. It a very critical stage and therefore care should be taken.
Colour coating: This covered up imperfections in tablet surface and the predetermined size is achieved. It gives the tablet its final colour. This step requires the maximum skill too.
Polishing: Powdered wax (beeswax or carnauba) is applied to provide a desired luster and shininess.
Film coating
This is another method of tablet coating. Tablet coating technique has been majorly replaced by film coating technology due to very time consumption and dependency on the skills of the coating operator found in sugar coating process,. The process involves application of polymer solution of polymer which contains pigments and plasticizer onto a rotating tablet bed to form a thin, uniform film on the tablet core.
This is another method of tablet coating. Tablet coating technique has been majorly replaced by film coating technology due to very time consumption and dependency on the skills of the coating operator found in sugar coating process,. The process involves application of polymer solution of polymer which contains pigments and plasticizer onto a rotating tablet bed to form a thin, uniform film on the tablet core.
Selection of polymer mainly depends on the desired site of drug release whether in the stomach, intestine, colon or any desired release rate. Examples of the coating polymers for non-enteric usage are Ethylcellulose, Hydroxyproply methyl cellulose (HPMC), Methyl hydroxyethyl cellulose, Povidone, etc, while the commonly used enteric coating polymers are Cellulose acetate phthalate, Acrylate polymers (Eudragit copolymer), HPMC phthalate, etc. An ideal film coating material should possess the following qualities (2):
- It should be soluble in a solvent of choice.
- It should be inert and non-toxic.
- It must produce an elegant coat.
- It should be stable in presence of heat, light or moisture.
- It should not possess disagreeable color, taste or odor.
- It should be compatible with coating additives.
Organic film coating
Recently, the most common technology for coating solid dosage forms is the liquid coating technology (aqueous based organic based polymer solutions). A mixture of polymers, pigments and excipients is dissolved in an organic solvent (for water insoluble polymers) or water (for water soluble polymers) to form a solution, or dispersed in water to form a dispersion in liquid coating. This is sprayed onto the tablet batches in a pan coater and dried continuously by providing heat, typically using hot air, until a dry coating film is formed [4].
Recently, the most common technology for coating solid dosage forms is the liquid coating technology (aqueous based organic based polymer solutions). A mixture of polymers, pigments and excipients is dissolved in an organic solvent (for water insoluble polymers) or water (for water soluble polymers) to form a solution, or dispersed in water to form a dispersion in liquid coating. This is sprayed onto the tablet batches in a pan coater and dried continuously by providing heat, typically using hot air, until a dry coating film is formed [4].
Organic solvent based coating provides a variety of useful polymer alternatives, because most of the polymers are soluble in the numerous ranges of organic solvents. In contrast, there are some disadvantages of organic solvents which include flammability, toxicity, costly and possess environmental issues [5]. Due to products safety profile, ICH guidelines prefer the avoidance of organic solvents involvement in pharmaceutical dosage preparations. Therefore, the attentions of Pharmaceutical Industries are now drawn in developing formula with aqueous film coating.
Aqueous Film Coating
Due to the problems associated with organic solvents resulted to the use of water as the preferred coating solvent. The use of aqueous based coating makes the coating process more economical than organic solvent based, though it requires a little investment to upgrade the coating facility. This up-gradation of equipment is important due to the need of higher drying capacity of aqueous solvent as the latent heat of water is 2200 kJ when compared to methylene chloride with 550 kJ. This entails that 4 times more energy is required when compared to organic solvent (5).
Due to the problems associated with organic solvents resulted to the use of water as the preferred coating solvent. The use of aqueous based coating makes the coating process more economical than organic solvent based, though it requires a little investment to upgrade the coating facility. This up-gradation of equipment is important due to the need of higher drying capacity of aqueous solvent as the latent heat of water is 2200 kJ when compared to methylene chloride with 550 kJ. This entails that 4 times more energy is required when compared to organic solvent (5).
Recent Technologies in Tablet Coating
Electrostatic coating: This is an effective way of applying a coat on conductive substances. Strong electrostatic charge is applied to a substrate. The coating material comprising of conductive ionic species with opposite charges is sprayed onto the charged substrate. A complete and uniform coating of corners on the substrate is usually achieved. This has two kinds of spraying units, based on the charging mechanisms which include corona charging and tribo charging.
Electrostatic coating: This is an effective way of applying a coat on conductive substances. Strong electrostatic charge is applied to a substrate. The coating material comprising of conductive ionic species with opposite charges is sprayed onto the charged substrate. A complete and uniform coating of corners on the substrate is usually achieved. This has two kinds of spraying units, based on the charging mechanisms which include corona charging and tribo charging.
Magnetically assisted impaction coating (MAIC): Some dry coating methods have been discovered. These include compression coating, plasticizer dry coating, heat dry coating and electrostatic dry coating. The methods are generally used for the application of high hearing stresses or high impaction forces or exposure to higher temperature in order to achieve coating. In these methods, strong mechanical forces are obtained with the accompanying heat generated which cause layering and even embedding of the guest particles onto the surface of the host particles.
However, some food and pharmaceutical ingredients, being organic and relatively soft, are very sensitive to heat and can quite easily be deformed by severe mechanical forces. Therefore, soft coating methods that can attach the guest (coating material) particles onto the host (material to be coated) particles with a minimum degradation of particle size, shape and composition caused by the buildup of heat are the better candidates for such applications.
In the coating with magnetically assisted impaction coating (MAIC) devices, coating of soft organic host and guest particles can occur without causing major changes in the material shape and size. There is negligible generated heat on a microscale due to the collisions of particles during MAIC process. In pharmaceutical products this is of an advantage when dealing with heat sensitive powders [6,7].
Compression coating: Compression coating is not commonly used. It does not require use of organic solvents or water. It is mainly used for coating of two incompatible ingredients whereby one is formulated with the core and the second excipient is incorporated in the compression coating solid materials. It makes uses of a specialized tablet machine [2].
Vacuum film coating: This is a new coating technology that employs specially designed baffled pan. The pan of vacuum film coating is hot and water jacketed and it can be sealed to achieve a vacuum system. The air in the pan is displaced by nitrogen before the desired vacuum level is obtained when coating. The coating solution is applied through airless spray system, while the vapors of the evaporated solvents are removed by vacuum system. This coating technique could make uses of organic solvents effectively with high environment safety (2).
Dip Coating
This coating method involves the application of coating by dipping them into coating solution. The wet tablets are dried in conventional coating pans. Intermittent dipping and drying steps may be repeated several times to achieve the coating of desired weight though the process lacks the speed, versatility, and the reliability of spray coating techniques (2).
This coating method involves the application of coating by dipping them into coating solution. The wet tablets are dried in conventional coating pans. Intermittent dipping and drying steps may be repeated several times to achieve the coating of desired weight though the process lacks the speed, versatility, and the reliability of spray coating techniques (2).
Materials and Methods
Gelatin (Sigma Aldrich), Tartrazine (ChemIDplus), water (Lion water, UNN), all other reagents were of analytical grades and used without further purification.
Gelatin (Sigma Aldrich), Tartrazine (ChemIDplus), water (Lion water, UNN), all other reagents were of analytical grades and used without further purification.
Coating of the tablets
Two gelatin solutions of 4 and 8% were prepared by dissolving 4 and 8g of gelatin in warm water. Then, two 50 tablets were randomly selected from the uncoated tablet batch and coated with the two gelatin concentrations by dipping method using a forceps respectively. The dipping method was done up to four times and each dipping was followed by intermittent drying in an oven set at 50°C for about 15 min.
Two gelatin solutions of 4 and 8% were prepared by dissolving 4 and 8g of gelatin in warm water. Then, two 50 tablets were randomly selected from the uncoated tablet batch and coated with the two gelatin concentrations by dipping method using a forceps respectively. The dipping method was done up to four times and each dipping was followed by intermittent drying in an oven set at 50°C for about 15 min.
Evaluation of the coated tablets
Hardness test
Ten tablets were randomly selected from each batch of the formulation and their hardness was determined using an electronic hardness tester (Erweka TBH 2B, Frankfurt, Germany). The average of this determination was taken as the hardness for the batch of that formulation.
Hardness test
Ten tablets were randomly selected from each batch of the formulation and their hardness was determined using an electronic hardness tester (Erweka TBH 2B, Frankfurt, Germany). The average of this determination was taken as the hardness for the batch of that formulation.
Friability test
Twenty tablets from each batch were selected randomly and the adhering particles blown off. They were weighed together using an analytical balance (Adventurer, Ohaus, China) and the tablets subjected to abrasion using an Erweka friabilator set at 25 rpm for 4 min after which the tablets were recovered, dedusted and reweighed again. The percentage friability was obtained using the formula:
Twenty tablets from each batch were selected randomly and the adhering particles blown off. They were weighed together using an analytical balance (Adventurer, Ohaus, China) and the tablets subjected to abrasion using an Erweka friabilator set at 25 rpm for 4 min after which the tablets were recovered, dedusted and reweighed again. The percentage friability was obtained using the formula:
Distintegration test
The disintegration time was done using the British Pharmacopoeia method (BP, 1988) and was adopted by using disintegration test apparatus (Erweka, Germany). A 500 ml of distilled water was used as the disintegration medium maintained at a temperature of 37 ± 1°C the time it took all the tablet particles to pass completely through the mesh-screen into the medium was noted as the disintegration time.
The disintegration time was done using the British Pharmacopoeia method (BP, 1988) and was adopted by using disintegration test apparatus (Erweka, Germany). A 500 ml of distilled water was used as the disintegration medium maintained at a temperature of 37 ± 1°C the time it took all the tablet particles to pass completely through the mesh-screen into the medium was noted as the disintegration time.
Weight uniformity test
Ten tablets from each batch were selected randomly weighed individually and together using an analytical balance (Adventurer, Ohaus, China). The mean tablet weight was calculated and compared with the individually weighed tablet for different batches.
Ten tablets from each batch were selected randomly weighed individually and together using an analytical balance (Adventurer, Ohaus, China). The mean tablet weight was calculated and compared with the individually weighed tablet for different batches.
In vitro release profile
The matrix tablets of each batch of uncoated, 4 and 8% coated tablets were subjected to the paddle dissolution method using 900 ml of phosphate buffer solution (pH 6.8 ± 0.2) as the dissolution medium, placed in a dissolution apparatus set to rotate at 100 rpm and the temperature was set at 37 ± 1°C. At intervals of 2, 5, 10, 15, 20, 25, 30, 40, 50, 60, 90, 120, 150 and 180 min, a 5 ml aliquots of the dissolution medium was collected and immediately replaced with 5 ml of fresh phosphate buffer solution. The withdrawn samples were analyzed using a UV-Vis spectrophotom¬eter (Jenway, 6405, Germany) at 420 nm wavelength of the Tartrazine. The procedure was repeated with three times and the mean determined.
The matrix tablets of each batch of uncoated, 4 and 8% coated tablets were subjected to the paddle dissolution method using 900 ml of phosphate buffer solution (pH 6.8 ± 0.2) as the dissolution medium, placed in a dissolution apparatus set to rotate at 100 rpm and the temperature was set at 37 ± 1°C. At intervals of 2, 5, 10, 15, 20, 25, 30, 40, 50, 60, 90, 120, 150 and 180 min, a 5 ml aliquots of the dissolution medium was collected and immediately replaced with 5 ml of fresh phosphate buffer solution. The withdrawn samples were analyzed using a UV-Vis spectrophotom¬eter (Jenway, 6405, Germany) at 420 nm wavelength of the Tartrazine. The procedure was repeated with three times and the mean determined.
Data and statistical analysis
All experiments were performed at least in triplicates for validity of statistical analysis. Results were expressed as mean ± SD. ANOVA and students t-tests were performed on the data sets generated using Microsoft Excel. Differences were considered significant for p values < 0.05.
All experiments were performed at least in triplicates for validity of statistical analysis. Results were expressed as mean ± SD. ANOVA and students t-tests were performed on the data sets generated using Microsoft Excel. Differences were considered significant for p values < 0.05.
Results and Discussion
The results of hardness and disintegration tests
The results of hardness and disintegration tests of tablets coated with 4 and 8% gelatin solution are shown in Tables 1 and 2. The results of the hardness test as indicated from the table showed that the hardness of 4% batch had an average hardness of 5.33 ± 0.26 Kgf, while 8% showed an average hardness of 8.91 ± 0.34 Kgf. This showed that 8% batch had a significant variation (p < 0.05) from 4% in terms of hardness. This is indicating that the 8% coated batch had greater mechanical strength with more resistance to crushing. Average disintegration tests of 50.70 ± 5.39 and 33.47 ± 0.37 min were obtained for 8 and 4% respectively. There was also a significant difference between the two concentrations at (p < 0.001).
The results of hardness and disintegration tests of tablets coated with 4 and 8% gelatin solution are shown in Tables 1 and 2. The results of the hardness test as indicated from the table showed that the hardness of 4% batch had an average hardness of 5.33 ± 0.26 Kgf, while 8% showed an average hardness of 8.91 ± 0.34 Kgf. This showed that 8% batch had a significant variation (p < 0.05) from 4% in terms of hardness. This is indicating that the 8% coated batch had greater mechanical strength with more resistance to crushing. Average disintegration tests of 50.70 ± 5.39 and 33.47 ± 0.37 min were obtained for 8 and 4% respectively. There was also a significant difference between the two concentrations at (p < 0.001).
| Tablet | Hardness test (Kgf) | Disintegration test (min) |
| A1 | 5.2 | 33.50 |
| A2 | 5.3 | 33.72 |
| A3 | 5.5 | 32.98 |
| A4 | 5.0 | 33.15 |
| A5 | 5.1 | 34.01 |
| A6 | 5.2 | 33.62 |
| A7 | 5.2 | 33.20 |
| A8 | 5.5 | 33.32 |
| A9 | 5.4 | 34.03 |
| A10 | 5.9 | 33.15 |
Table 1: The results of hardness test, and disintegration test of tablets coated with 4% gelatin solution.
| Tablet | Hardness test (Kgf) | Disintegration test (min) |
| A1 | 8.6 | 54.50 |
| A2 | 8.6 | 44.69 |
| A3 | 8.9 | 55.12 |
| A4 | 9.4 | 43.95 |
| A5 | 8.7 | 55.36 |
| A6 | 8.4 | 55.05 |
| A7 | 8.9 | 55.01 |
| A8 | 9.0 | 54.11 |
| A9 | 9.2 | 44.13 |
| A10 | 9.4 | 45.05 |
Table 2: The results of hardness test and disintegration test of tablets coated with 8% gelatin solution.
The results of Friability and Weight uniformity tests
The results of Friability and Weight uniformity tests as presented in Table 3. The results showed that friability losses of the batches were within the acceptable range (0.8–1%). There was very minimum loss in 8% coated tablets with significant variation (p < 0.05) from other batches. This effect was due to the effect of the coating film which conferred more stability to the batch. This indicated that the batches will withstand any shock or stress during packaging and transportation.
The results of Friability and Weight uniformity tests as presented in Table 3. The results showed that friability losses of the batches were within the acceptable range (0.8–1%). There was very minimum loss in 8% coated tablets with significant variation (p < 0.05) from other batches. This effect was due to the effect of the coating film which conferred more stability to the batch. This indicated that the batches will withstand any shock or stress during packaging and transportation.
All the batches equally passed the uniformity of weight test and deviations as they complied with BP standards of not more than 5% deviation for tablets weighing 250 mg or more (9). This test is important because differences in tablet weight leads to variation in drug content which will affect the therapeutic efficiency and then the bioavailability of the drug.
| Tablet | 4% coated tablet | 8% coated tablet | Uncoated tablet |
| Friability lost (%) | 0.38 | 0.02 | 0.89 |
| Weight uniformity (%) | < 5 | < 5 | < 5 |
Table 3: The Friability and weight uniformity test of uncoated tablet, and tablets coated with 4 and 8% gelatin solution.
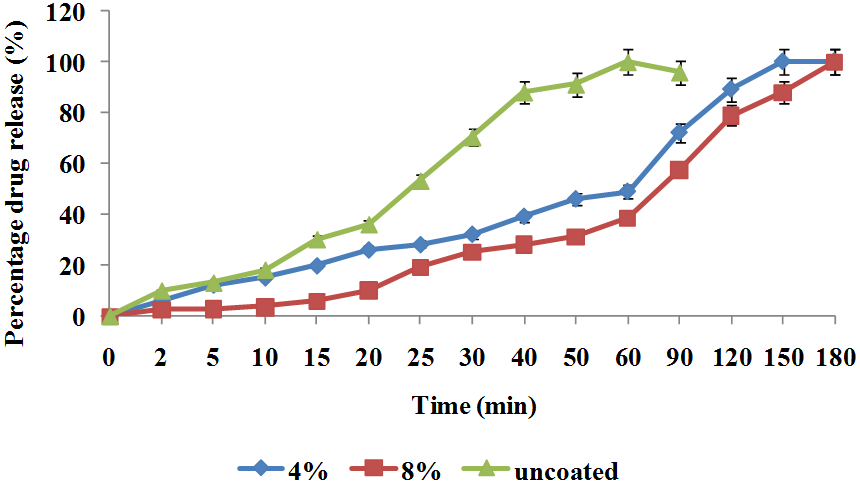
In vitro release profile
The release profiles of the uncoated, 4 and 8% coated tablets are shown in Figure 1. The value of T45 and T90 were given as 25, 50 and 90 for T45 and 50, 150 and 180 for T90 respectively for uncoated, 4 and 8% coated tablet batches. The uncoated tablet had an immediate and faster release of drugs than the coated batches because it has no outer coating film, while the 8% coated batch had more prolonged release of drug due to the thickness of the coated than the 4% batch. This showed that if more prolonged drug released is desired in tablet batch it is better to applied thicker coats to the formulation especially in the treatment of diseases that are associated with rhythmic disorder.
The release profiles of the uncoated, 4 and 8% coated tablets are shown in Figure 1. The value of T45 and T90 were given as 25, 50 and 90 for T45 and 50, 150 and 180 for T90 respectively for uncoated, 4 and 8% coated tablet batches. The uncoated tablet had an immediate and faster release of drugs than the coated batches because it has no outer coating film, while the 8% coated batch had more prolonged release of drug due to the thickness of the coated than the 4% batch. This showed that if more prolonged drug released is desired in tablet batch it is better to applied thicker coats to the formulation especially in the treatment of diseases that are associated with rhythmic disorder.
Conclusion
Coating of Pharmaceutical formulation is of some importance as it enhances stability, prolongs drug release, inhibits volatility, prevents bitterness, etc. The coating technologies have been subject to remarkable developmental efforts aiming to ensure and enhance the final product quality. This contribution significantly improved safety profiles. In future there is enormous possibility of developments in the area of tablet coating to achieve specific benefits.
The combined effect of these tests was to ensure that all tablets in a batch are within reasonable limits. All the batches passed the uniformity of weight test and deviations as they complied with BP standards of not more than 5% deviation for tablets weighing 250 mg or more [8,9].
The 8% batch had the highest mean hardness followed by 4% and then uncoated, but the least friability was obtained with 8% coated due to the effect of the coating film on the batch. Therefore, as the coating concentration increases, the hardness and the disintegration time of tablet increases but less friable the batch.
References
- Kamble ND., et al. “Innovations in tablet coating technology: A review”. International Journal of Applied Biology and Pharmaceutical Technology 2.1 (2011): 214-218 .
- Leon L and Herbert AL. The Theory and Practice of Industrial Pharmacy. Second edition, Fourth Indian Reprint, Published by Varghese Publishing house, Bombay. (1991): 346-372.
- Remington’s The Science and Practice of Pharmacy. Volume-I. 21st ed. Indian Edition, Lippincot Williams and Wilkins. (2005): 929-938.
- Cole G., et al. “Pharmaceutical Coating Technology”. Taylor and Francis, London (1995): 1-5.
- Thomas M. “Solvent film coating, aqueous vs. organic”. Midwest Regional Meeting, Academy of Pharmaceutical Sciences. Industrial Pharmaceutical Technology Section (1978).
- Singh P., et al. “Estimation of Coating Time in the Magnetically Assisted Impaction Coating Process”. Powder Technology 121.2.3 (2001): 159-167.
- Tamahane P.M. Enteric Aqueous Film Coating, Wincoat Colours & Coatings Pvt. Ltd (2011).
- British Pharmacopoeia, Vol. 1 and 11, HMSO, London, 2001. A 223-324, 1113-1343, 1523.
- Ofoefule S.I. A Text Book of Pharmaceutical Technology and Industrial Pharmacy, Samakin (Nig.) Enterprises, (2002).
Citation:
Ugwu Calister Elochukwu. “Coating and Evaluation of Compressed Tablets using two Different Concentrations of Gelatin.”.
Chronicles of Pharmaceutical Science 1.4 (2017): 254-261.
Copyright: © 2017 Ugwu Calister Elochukwu. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































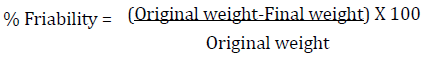
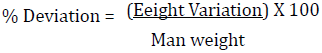
 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.