Review Article
Volume 1 Issue 6 - 2017
Occurrence of Paracetamol in Aquatic Environments and Transformation by Microorganisms: A Review
1Medicinal Chemistry Department, Faculty of Pharmacy, Sana'a University, Yemen
2Chemistry Department, Faculty of Science, Sana'a University, Yemen
3Biology Department, Faculty of Science, Sana'a University, Yemen
2Chemistry Department, Faculty of Science, Sana'a University, Yemen
3Biology Department, Faculty of Science, Sana'a University, Yemen
*Corresponding Author: Wadhah Hassan A. Edrees. Biology Department, Faculty of Science, Sana'a University, Sana’a City, Yemen.
Received: December 15, 2017; Published: December 23, 2017
Abstract
Paracetamol is a widely used for relieving pain and reducing fever worldwide as a non-prescription drug. It is one of the most pharmaceutical products often detected in sewage treatment plant effluents, surface water, and drinking water, so it has emerged as an imperative aquatic environmental pollutant, originating from pharmaceutical industries and human use. This review summarizes the pathways of paracetamol reach to the aquatic environments, its concentration, and proposed metabolic pathways of biotransformation. Paracetamol reaches water bodies via various paths.
The most important routes are the excretion by human beings and the industrial effluents originating from paracetamol active ingredients production. It was noticed that during the last years the paracetamol concentration increased in the aquatic environments, which indicates that it is not completely removed from wastewater by wastewater treatment plant (WWTP) as well as not entirely eliminated during ground water infiltration. Therefore, most of the paracetamol comes and leaches into the aquatic environment, groundwater and drinking water, through the discharges from this wastewater. On the other hand, biodegradation of paracetamol by microorganisms was used as a complement the method for transforming paracetamol into simpler constituents in the aquatic environment. The biodegradation mechanism varies from one microorganism to another. It was observed that the intermediate products are closely similar to each other. The comprehensive understanding of the metabolic pathways and enzyme systems involved in the utilization of paracetamol will be helpful for optimizing and allowing the rational design of biodegradation systems for paracetamol-contaminated wastewater, which expected to more efficient and will reduce the cost of treatment the pharmaceutical wastes in the aquatic environments.
Keywords: Paracetamol Occurrence; Drinking Water; Waste water; Biotransformation; Microorganisms and Aquatic environment
Introduction
Paracetamol or acetaminophen is a widely used for relieving pain and reducing fever sold as over-the-counter (OTC) drug worldwide [1]. It is one of the most often detected pharmaceutical products in sewage treatment plant effluents, surface water, and drinking water [2]. Detection of this compound is greater in highly populated areas such as urban centers where drug usage is expected to reach elevated proportions [3]. The Frequent occurrence of paracetamol in aquatic environments and drinking water has raised a concern about their potential effects on the environment and human health [4].
Paracetamol consumption throughout the world has increased. It is ranked as one of the top three drugs prescribed in England and one of the top 200 prescriptions in the USA [5,6]. In Yemen, it is ranked as first of the top ten drugs produced by local industry and one of the top ten drugs imported [7]. Also, it was consumed as the second prescription during the year 2008 in Kuwait [8].
In the UK, about 3.2 × 109 tablets of paracetamol are consumed each year, which is an average of 55 tablets/person [9]. In the Nordic countries, the rates in some developed countries exceeded 20 g/person/year [10]. In 2002, the USA produced 3.6 × 109g of paracetamol [11]. It is easily accumulated in aquatic environment due to their high solubility and hydrophilicity, which have been detected in drinking water, surface water, and wastewater throughout the world [4].
The occurrence of this compound in the aquatic environment has stimulated investigations into the biodegradation and biotransformation by different microorganisms. Some microorganisms are capable of using paracetamol as carbon and energy source as well as capable of degrading and converting it to nontoxic compounds [4]. The strategies pathway for the paracetamol biotransformation by some microorganisms have been described [4,12,13].
The production thousand tons of paracetamol annually have recently begun to receive a large amount of attention for its potential effects on the environment and human health. The increased use of this substance for long times will increase the presence in water sources. The aim of this work is to attempt to summarize the knowledge about the possible pathways of paracetamol reaches the aquatic environment from different sources. However, the presence of paracetamol concentration in drinking water, surface water, groundwater, wastewater, and sewage water around the world during the period from 1998 to 2016. The biotransformation mechanism for paracetamol with a different type of microorganisms.
Pathways of paracetamol reach the aquatic environment
According to previous studies about the possible sources and pathways of paracetamol reach the aquatic environment as shown in scheme (1). It is continuously introduced into the aquatic environment as a parent compound, metabolites or conjugate of both by pharmaceutical industrial effluents and human use [14,15]. These industries are releasing their effluents containing paracetamol substance originating from paracetamol active ingredients or formulation factories to wastewater. In addition, 58-68% of unchanged paracetamol is excreted from the human body during therapeutic use and disposed to sewage water [16,17].
According to previous studies about the possible sources and pathways of paracetamol reach the aquatic environment as shown in scheme (1). It is continuously introduced into the aquatic environment as a parent compound, metabolites or conjugate of both by pharmaceutical industrial effluents and human use [14,15]. These industries are releasing their effluents containing paracetamol substance originating from paracetamol active ingredients or formulation factories to wastewater. In addition, 58-68% of unchanged paracetamol is excreted from the human body during therapeutic use and disposed to sewage water [16,17].
The effluents containing paracetamol substance maybe discharge to the sewer, sewage treatment plant (STP), or wastewater treatment plant (WWTP). Paracetamol is known to be exhibited virtually no sorption and no retardation in aquifer sand which can eventually reach ground water as sources of drinking water via the sewer system [18].
Recent studies detected the paracetamol concentration in water discharged from STP and WWTP that is constantly released to surface water or be subjected to groundwater recharges [19]. Furthermore, the sludge from these treatment units which may contain paracetamol may be used as fertilizer in agricultural land. Over time, remains of this substance accumulate in the soil or drain into groundwater or surface water resources. The residues of paracetamol presence in water bodies especially drinking water reveal that STP and WTP are the major sources of entry of this substance into aquatic environment resulting from insufficient treatment the contaminated substance [20].
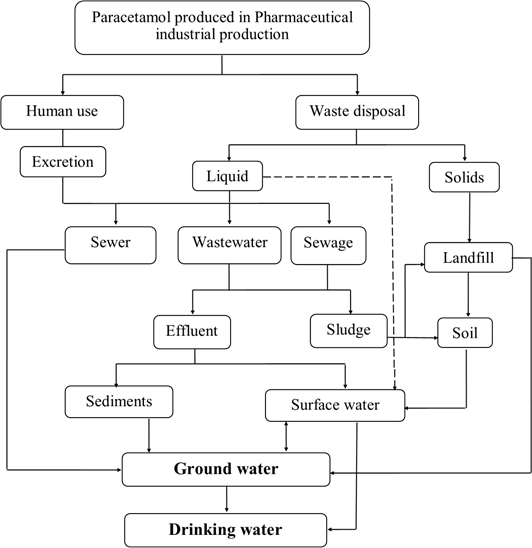
Scheme 1: The possible sources and pathways for the occurrence of paracetamol residues in the aquatic environment
Paracetamol in aquatic environment
The occurrence of paracetamol in drinking waters
Paracetamol is a pollutant that has been found in the drinking water that could be used as drinking water sources in major cities such as Atlanta, Minneapolis, New York City, Oklahoma, and Minnesota City [21,22]. The paracetamol concentration was recorded between 0.0003-0.298 µg/L in drinking water. [23,24].
The occurrence of paracetamol in drinking waters
Paracetamol is a pollutant that has been found in the drinking water that could be used as drinking water sources in major cities such as Atlanta, Minneapolis, New York City, Oklahoma, and Minnesota City [21,22]. The paracetamol concentration was recorded between 0.0003-0.298 µg/L in drinking water. [23,24].
The first detected of this substance in finished drinking water was observed in samples collected near Atlanta, Georgia [25]. In the USA, the paracetamol was reported in drinking water samples with level > 0.02 µg/L in Nevada [26], 0.12 µg/L and 0.0003 µg/L in source and finished drinking water, respectively [23] and 0.002 μg/L in 7% of drinking water samples [27].
Moreover, the paracetamol was recorded in France at 0.211 µg/L in a drinking water in Herault watershed [28], 0.210 µg/L in drinking water in Marseilles area [29], and 0.045 µg/L in finished drinking water [30]. Also, 0.298 µg/L and 0.017 µg/L of paracetamol were reported in source and finished drinking water, respectively, in Ontario, Canada [24]. Moreover, it was 0.260 µg/L and 0.010 µg/L recorded in source and finished drinking water, respectively, in Spain [31]. Table (1) summarizes the paracetamol concentrations detected in drinking water samples.
| Country | Concentration (μg/L) | References |
| USA | > 0.02 | 26 |
| 0.12–0.0003 | 23 | |
| 0.002 | 27 | |
| Canada | 0.298–0.017 | 24 |
| France | 0.211 | 28 |
| 0.210 | 29 | |
| 0.045 | 30 | |
| Spain | 0.260–0.010 | 31 |
Table 1: Paracetamol occurrence in the drinking water.
The occurrence of paracetamol in groundwater
The presence of paracetamol in groundwater is due to the infiltration of sewage effluent and surface water to the groundwater. During the soil passage, it is not efficiently sorbed to soil particles or biodegraded and still persistent [32].
The presence of paracetamol in groundwater is due to the infiltration of sewage effluent and surface water to the groundwater. During the soil passage, it is not efficiently sorbed to soil particles or biodegraded and still persistent [32].
Paracetamol has been detected in groundwater using for drinking water supplies in the USA. It was 0.036 μg/L and 6.5 μg/L detected, respectively, in public and private supplies wells in Massachusetts [33], 0.015 µg/L in groundwater wells in Nebraska [34], 0.16 µg/L in different groundwater samples [35], 0.38 µg/L in groundwater samples [36], 1.89 μg/L in samples of groundwater in California [37], and 0.12 μg/L in groundwater samples in Minnesota [38]. However, it was 0.034 µg/L detected in groundwater wells in Spain [39] and 0.010 µg/L (17%) in groundwater in Rhônee-Alpes, France [40]. Table (2) summarizes the occurrence of paracetamol concentrations detected in groundwater samples.
| Country | Concentration (μg/L) | References |
| USA | 0.16 | 35 |
| 0.015 | 34 | |
| 0.036 – 6.5 | 33 | |
| 0.12 | 38 | |
| 1.89 | 37 | |
| 0.38 | 36 | |
| Spain | 0.034 | 39 |
| France | 0.010 | 40 |
Table 2: Paracetamol occurrence in the groundwater.
Paracetamol in surface waters
The presence of paracetamol in the surface waters such as lakes and rivers waters is due to the uncontrolled discharge of wastewater effluent to an environment containing this substance. It is one of the most frequently detected pharmaceutical products in approximately 75% of natural water such as rivers and lakes [23].
The presence of paracetamol in the surface waters such as lakes and rivers waters is due to the uncontrolled discharge of wastewater effluent to an environment containing this substance. It is one of the most frequently detected pharmaceutical products in approximately 75% of natural water such as rivers and lakes [23].
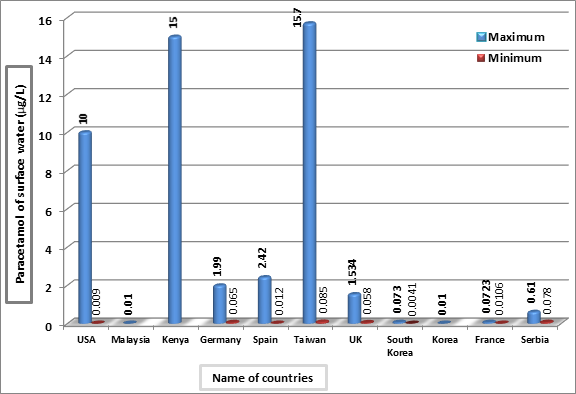
In the USA surface water, the paracetamol was detected at range 0.026 µg/L in Las Vegas Wash water, Nevada and 0.012 µg/L in Lake Mead water, Nevada and Arizona [41], between 1.95-10 µg/L in streams [42,43], 0.110 µg/L in surface water [44], 0.009 µg/L in surface water [45], 1.78 µg/L in surface water [46], 0.031 µg/L in New Jersey stream water [47], 0.117 µg/L in the Colorado River [48], 0.065 µg/L in the Mississippi River, New Orleans, Louisiana [49], and 0.012 µg/L in 13.3% of surface and subsurface samples of Tennessee River [50] (Figure 1).
However, in Germany, the paracetamol was found with concentration 0.065 µg/L in Elbe River water [51] and 1.99 µg/L in the Leine River [52]. In Spain surface water, it was 0.250 µg/L recorded in Ebro River basin water in Croatian [53], from 0.012 to 0.030 µg/L in three river waters (Ebre, Llobregat, and Ter) [54], 2.42 µg/L in Llobregat River [55], 0.043 µg/L in the Henares-Jarama-Tajo River in Madrid [56], 1.968 µg/L in the Mediterranean rivers in the Castellon province [57], and 0.021 µg/L in reservoir water, 0.243 µg/L in Onyar River, and 0.023 µg/L in Mediterranean Sea in Barcelona and Catalonia [58] (Figure 1).
In the UK, the paracetamol was found between 0.112-0.555 µg/L in surface water the South–East of England [59], 1.388 µg/L in River Taff, in Wales, and 0.058 µg/L in River Warta, Poland [60], and 1.534 µg/L in River Taff and 0.716 µg/L in River Ely in South Wales [61]. Also, it was ranged between 0.0041-0.073 µg/L in surface water in South Korea [2], 0.0348 µg/L in the Han River, North Korea [62], and 0.10 μg/L in the river water samples in Busan city, North Korea [63] (Figure 1).
In France, the paracetamol was reported between 0.0106-0.0723 µg/L in surface water in Marseilles area [29], 0.071 µg/L in surface water [30], and 0.014 µg/L in surface water sampled from Rhônee-Alpes [40]. Also, it was ranged 0.078–0.610 µg/L in 15% of river water samples in Serbia [64], 3.35 µg/L in Sindian River, 15.7 µg/L in Dahan River, and 0.085 µg/L in Gaoping River in Taiwan [65], 0.01 µg/L in Langat River dam in Selangor, Malaysia [66], and up to 15 µg/L in the Nairobi River, Kenya [67] (Figure 1).
Paracetamol in wastewater
Paracetamol is one of the ubiquitous pharmaceuticals detected in wastewater at different concentrations throughout the world [68,69]. It was recorded between range 1.746-43.223 µg/L in WWTP influent samples and between 0.025-4.319 µg/L in 83% of WWTP effluent samples. Also, it was found between 13.874-177.674 µg/L in Ulleval university effluent samples and 5.421-1368.47 µg/L in Rikshospitalet wastewater in Oslo, Norway [68].
Paracetamol is one of the ubiquitous pharmaceuticals detected in wastewater at different concentrations throughout the world [68,69]. It was recorded between range 1.746-43.223 µg/L in WWTP influent samples and between 0.025-4.319 µg/L in 83% of WWTP effluent samples. Also, it was found between 13.874-177.674 µg/L in Ulleval university effluent samples and 5.421-1368.47 µg/L in Rikshospitalet wastewater in Oslo, Norway [68].
In the UK, the paracetamol concentration was reported from 5.529 to 69.57 µg/L in WWTP influent in Howdon [70], between 0.129-0.555 µg/L in WWTP effluent in England [59], 211.38 µg/L in influent and 11.733 µg/L in effluent of Cilfynydd WWTP as well as 178.116 µg/L in influent and 0.353 µg/L in effluent of Coslech WWTP in South Wales [61].
In Spain, the paracetamol was identified from 0.5-29 µg/L in hospital wastewater in Almerıa [71], from 0.130-26.09 µg/L in WWTP influent and 5.99 µg/L in effluent in Croatian [53], 0.123 µg/L in WWTP influent [72], 16.72 µg/L in WWTP influent and 0.338 µg/L in effluent in Barcelona and Catalonia [58], between 1.13-201 µg/L in WWTP influent in Castellon [69], and between 109.3-114.4 μg/L in hospital wastewater in Girona [73].
In the USA, the paracetamol was observed at level 1.06 µg/L in WWTP effluent [46], 0.96 µg/L in the Back River WWTP influent in Baltimore [74], 61 µg/L in WWTP influent and 0.86 µg/L in effluent in New York [75], 140 µg/L in hospital WWTP influent in San Marcos, Texas [76], 182-233 µg/L at five WWTPs influent in the Pacific Northwest [77], and 150.079 µg/L in effluents from fifty WWTPs [78]. Also, it was 1000 μg/L found in influent of WWTP in Wisconsin [79].
In the France, the paracetamol was up to 11.3 µg/L reported in a WWTP effluent [28], between 0.108–11.308 µg/L in WWTP effluent in Marseilles area [29], and 292 µg/L in WWTP influent [80]. Also, it was 23.3 µg/L recorded in wastewater influent in Sydney, Australia [81] and 1.7 µg/L in the WWTPs influent in Japan [82]. In South Korea, it was observed between 0–0.26 µg/L in influents and 0–0.16 µg/L in effluents of WWTP [83], between 0.0018–0.019 µg/L in WWTP effluents [2], and 21.95 µg/L in WWTP influent and 0.017 µg/L ultrafiltration effluents [84]. However, it was recorded with 20.6 µg/L in influent and 0.9 µg/L in effluent of WWTP as well as 9.3 µg/L in hospital WWTP influent and 3.6 µg/L effluent in Western Greece [85].
In North Korea, the paracetamol was 41.9 μg/L and 6.76 μg/L detected, respectively, in hospital WWTP influent and effluent. Also, it was 6.80 μg/L reported in the municipal WWTP influent [63] and 10.234 µg/L in influent of WWTP in Ulsan [86]. In Taiwan, it was recorded up to 186.5 µg/L in hospital wastewater influent and up to 417.5 µg/L in drug production facility wastewater influent [65], from 1.80–30.967 µg/L in six WWTPs effluents [87], and 2.695 µg/L in influent and 0.33 µg/L in effluent of WWTP [88].
In Italy, the paracetamol was 246 μg/L found in the raw WWTP influent [89] and from 1.4 to 5.9 μg/L in two hospital wastewater influents as well as 1.2 μg/L in WWTP influent and 0.058 μg/L in effluent [90]. However, it was 107 µg/L recorded in hospital wastewater influent in Switzerland [91], 150 µg/L in hospital wastewater in China [4], and up to 58.857 µg/L in hospital wastewater influent, 9.286 µg/L in WWTP influent and 0.106 µg/L in effluent in Coimbra, Portugal [92].
Furthermore, the paracetamol was recorded between 57.5‒77.4 µg/L in the WWTP influent and 90.2 µg/L in the hospital wastewater influent in Québec, Canada [93]. In Kuwait, it was detected with highest concentration 2.086 μg/L in WWTP influent samples and 0.0521 μg/L in WWTP effluent in the 2011 year [8]. Also, it was 12 ug/L reported in hospital WWTP influent and 0.073 ug/L in WWTP effluent in Saudi Arabia [94]. Table (3) summarizes the paracetamol concentrations detected in influent (inf) and effluent (eff) of WWTP samples.
| Country | Minimum (μg/L) |
References | Maximum (μg/L) |
References |
| Norway | 1.746 (inf) | 68 | 1368.47 (inf) | 68 |
| 0.025 (eff) | 68 | 4.319 (eff) | 68 | |
| UK | 5.529 (inf) | 70 | 211.38 (inf) | 61 |
| 0.129 (eff) | 59 | 11.733 (eff) | 61 | |
| Spain | 0.123 (inf) | 72 | 201 (inf) | 69 |
| 0.338 (eff) | 58 | 5.99 (eff) | 53 | |
| USA | 0.96 (inf) | 74 | 1000 (inf) | 79 |
| 0.86 (eff) | 75 | 150.079 (eff) | 78 | |
| France | 0.108 (eff) | 29 | 11.308 (eff) | 29 |
| 292 (inf) | 80 | |||
| South Korea | 0.26 (inf) | 83 | 21.95 (inf) | 84 |
| 0.0018 (eff) | 2 | 0.16 (eff) | 83 | |
| Western Greece | 9.3 (inf) | 85 | 20.6 (inf) | 85 |
| 0.9 (eff) | 85 | 3.6 (eff) | 85 | |
| North Korea | 10.234 (inf) | 86 | 41.9 (inf) | 63 |
| 6.76 (eff) | 63 | |||
| Taiwan | 2.695 (inf) | 88 | 417.5 (inf) | 65 |
| 0.33 (eff) | 88 | 30.967 (eff) | 87 | |
| Italy | 1.2 (inf) | 90 | 246 (inf) | 89 |
| 0.058 (eff) | 90 | |||
| Portugal | 9.286 (inf) | 92 | 58.857 (inf) | 92 |
| 0.106 (eff) | 92 | |||
| Canada | 57.5 (inf) | 93 | 90.2 (inf) | 93 |
| Kuwait | 0.0521 (eff) | 8 | 2.086 (inf) | 8 |
| Saudi Arabia | 0.073 (eff) | 94 | 12 (inf) | 94 |
| Japan | - | - | 1.7 (inf) | 82 |
| Australia | - | - | 23.3 (inf) | 81 |
| Switzerland | - | - | 107 (inf) | 91 |
| China | - | - | 150 (inf) | 4 |
Table 3: The minimum and maximum concentrations of influent and effluent paracetamol in WWTP.
inf: inffluent, eff: effluent
inf: inffluent, eff: effluent
Paracetamol in sewage waters
Paracetamol is reported as one of the most frequently detected pharmaceuticals in sewage treatment plant effluents [19]. The first occurrence of paracetamol was detected with concentration 6 µg/L in the STP effluent in German [95]. In Sydney, Australia, it was 148 µg/L recorded in STP influent [96]. Also, it was 1.9 μg/L found in the final effluents of eight STP in Atlantic Canada [97], 4.8 µg/L in STP influent and 1 µg/L in activated sludge effluent in the south of England, UK [98].
Paracetamol is reported as one of the most frequently detected pharmaceuticals in sewage treatment plant effluents [19]. The first occurrence of paracetamol was detected with concentration 6 µg/L in the STP effluent in German [95]. In Sydney, Australia, it was 148 µg/L recorded in STP influent [96]. Also, it was 1.9 μg/L found in the final effluents of eight STP in Atlantic Canada [97], 4.8 µg/L in STP influent and 1 µg/L in activated sludge effluent in the south of England, UK [98].
In Spain, the highest concentrations of paracetamol were found from 29–246 µg/L in STP influent and 4.3 µg/L in STP effluent in Almerıa [99], 37.458 µg/L in STP influent in Madrid [100], and 19.850 µg/L in STP influent in Catalonia [101]. Also, it was observed at level 56.9 µg/L in STP influent in Seoul, North Korea [62], 84 µg/L found in samples of STP influent situated in Stockholm, Sweden [102], and 0.07 µg/L in STP effluent in Selangor, Malaysia [66]. Table (4) summarizes the occurrence of paracetamol concentrations detected in sewage water samples.
| Country | Concentration (µg/L) | References |
| German | 6 | 95 |
| Australia | 148 | 96 |
| Canada | 1.9 | 97 |
| UK | 4.8 | 98 |
| Sweden | 84 | 102 |
| Spain | 37.458 | 100 |
| 29–246 | 99 | |
| 4.3 | ||
| 19.850 | 101 | |
| Malaysia | 0.07 | 66 |
| Korea | 56.9 | 62 |
Table 4: Paracetamol occurrence in the sewage waters.
Microbial degradation and transformation of paracetamol
The strategy for aromatic degradation includes hydroxylation and cleavage of the aromatic ring. Hydroxylation into the dihydroxylated intermediates is catalyzed by oxygenases belonging to three groups: hydroxylating dioxygenases, activated-ring monooxygenases, or nonactivated-ring monooxygenases. The main intermediates such as catechol, protocatechuic acid, hydroxyquinol, or gentisic acid are formed as a result of hydroxylation. These products are substrates for ring-cleaving dioxygenases [103,104].
The strategy for aromatic degradation includes hydroxylation and cleavage of the aromatic ring. Hydroxylation into the dihydroxylated intermediates is catalyzed by oxygenases belonging to three groups: hydroxylating dioxygenases, activated-ring monooxygenases, or nonactivated-ring monooxygenases. The main intermediates such as catechol, protocatechuic acid, hydroxyquinol, or gentisic acid are formed as a result of hydroxylation. These products are substrates for ring-cleaving dioxygenases [103,104].
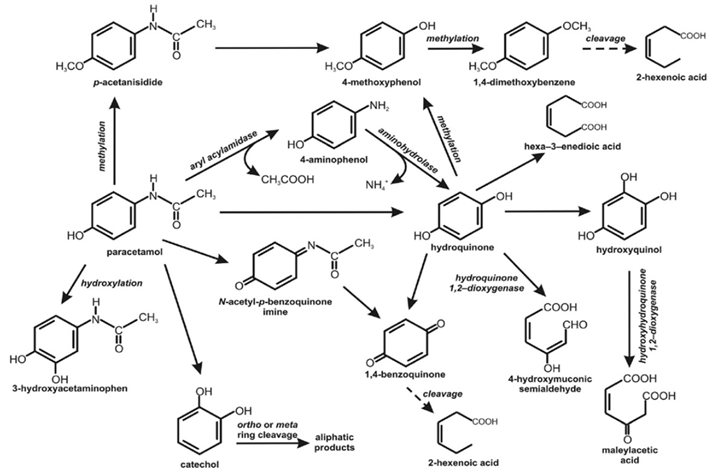
Many of the researchers were focused on summarizing the studies on paracetamol biodegradation in the following aspects: paracetamol degrading bacteria, and proposed metabolic/biodegrading pathways in microorganisms, enzymes, and possible intermediates. It was recorded the ability of isolated Penicillium sp. to transform paracetamol to 4-aminophenol and acetate, maybe with the use of aryl acylamidase. 4-aminophenol is a dead-end metabolite [105] (Figure 2). Also, it was reported the ability of Rhodococcus strains to degrade paracetamol with three detectable metabolites: 4-aminophenol, catechol, and hydroquinone [106].
Further degradation of 1,4-hydroxybenzene could proceed in two ways. Hydroquinone may be directly cleaved by hydroquinone 1,2-dioxygenase with 4-hydroxymuconic semialdehyde as an aliphatic product [107]. Methylation of hydroquinone may further result in the mono and di O-methylated intermediates 4-methoxyphenol and 1, 4-dimethoxybenzene, as observed in the microbial transformation of paracetamol by two bacterial strains, Delftia tsuruhatensis and Pseudomonas aeruginosa [108]. Paracetamol was likely also metabolized via an amidohydrolase reaction and the cleavage of the bond between nitrogen and carbon from the carbonyl group would yield 4-aminophenol, from which nitrogen elimination followed by hydroxylation would lead to the formation of hydroquinone [109].
However, the Burkholderia sp. strain AK-4 was recorded to convert 4-aminophenol to 1,4-hydroxybenzene and further to 1,2,4-trihydroxybenzene. Then 1,2,4-trihydroxybenzene was cleaved by hydroxyhydroquinone 1,2-dioxygenase to maleylacetic acid, which is introduced to the basic metabolism [110,111] (Figure 2).
In further details, the conversion of paracetamol to hydroquinone was next transformed to an aliphatic product hexa-3-enedioic acid which seems that it was a product of aromatic ring fission or, if not, it that means some intermediate metabolites between aromatic and aliphatic compounds were passed over. Hexa-3-enedioic acid is similar to muconic acid-a product of ortho ring cleavage of catechol. Based on reported intermediates a primary pathway of paracetamol degradation could be proposed. The mechanism may be based on cutting off two carbon atoms in the form of formic acid [13] (Figure 2).
In addition, Akay and Tezel, (2016) reported that the R. erythropolis can convert paracetamol to phenols and organic acids by a series of hydroxylation reactions. During the biotransformation, paracetamol was initially converted to 4-aminophenol which was then transformed to hydroquinone by substitution of the amino group with hydroxyl. Hydroquinone then goes into ring fusion.
Furthermore, it was described the formation of glucoside conjugates with paracetamol by soil filamentous fungi via O- and N-linkages [113]. This is a similar way to the human detoxication routes of xenobiotics in phase II of detoxication [114].
Also, the degradation pathway of paracetamol in soil microorganisms was proposed and described. It was shown that in the first step, the aromatic ring of paracetamol is hydroxylated to 3-hydroxyparacetamol, oxygenated to N-acetyl-p-benzoquinone imine, or methylated to p-acetanisidide. N-acetyl-p-benzoquinone imine is then metabolized to 1,4-benzoquinone which is more stable and critical toxic metabolite. p-acetanisidide is transformed to 4-methoxyphenol and in the next step to the 1,4-dimethoxybenzene. The presence of 2-hexenoic acid in the soil extract suggests the cleavage of the aromatic ring of paracetamol [115].
In soil, monooxygenases such as flavin-containing hydroxylases are widely distributed among microorganisms and catalyze various oxidative reactions such as hydroxylation of phenols to catechols [116].
Conclusion
The pharmaceutical industry holds a major role in polluting water resources. Paracetamol substance is constantly introduced into the aquatic environment by several discharges from manufacturing facilities, consumer use and disposal, and hospital waste. Nowadays the pharmaceutical industries are widely producing a thousand tons of paracetamol which are extensively using as non-prescription drugs worldwide. The wastewater containing a high concentration of paracetamol resulting from the production processes and excretion from human body during use is the main source for contaminating the aquatic environment. The detection of paracetamol in the surface water indicates that the WWTPs and STPs are insufficient to remove this substance completely from wastewater containing-paracetamol. Also, the existence of this substance in groundwater even at low concentration is the evidence that the paracetamol persists for degrading throughout the soil filtration. So, this substance is easily leaching into the aquatic environment as parent compound throughout the contaminated wastewater with paracetamol.
The high concentration of paracetamol occurrence in the aquatic environment is explained by the relationship between pharmaceutical consumption and WWTP efficiency. On the other hand, the microorganisms existing in the aquatic environment play an important role in degradation and transformation the paracetamol to nontoxic compounds. The biodegradation method for removing this substance have been investigated in advance. The enzymes produced from microorganisms are contributing to degrade and transform the paracetamol to intermediate compounds which are less harmful to aquatic environment. The development of innovative and cost-effective approaches is essential to ensure the complete elimination of paracetamol for the further protection of water quality and ecological health.
References
- Martindale W. “The complete drug reference, cough suppressants, expectorants, mucolytics and nasal decongestants”. 36th edition. The pharmaceutical Press, London, England (2009): 967-1082.
- Kim S., et al. “Occurrence and removal of pharmaceuticals and endocrine disruptors in South Korean surface, drinking and wastewaters”. Water Research 41.5 (2007a): 1013-1021.
- Luna MG., et al. “Acetaminophen degradation by electro-Fenton and photoelectro-Fenton using a double cathode electrochemical cell”. Journal of Hazardous Materials217 (2012): 200-207.
- Wu S., et al. “Paracetamol in the environment and its degradation by microorganisms”. Applied Microbiology and Biotechnology96.4 (2012): 875-884.
- Sebastine IM and Wakeman RJ. “Consumption and environmental hazards of pharmaceutical substances in the UK”. Process Safety and Environmental Protection81.4 (2003): 229-235.
- Zhang X., et al. “Photodegradation of acetaminophen in TiO2 suspended solution”. Journal of Hazardous Materials 157.2-3 (2008): 300-307.
- Edrees WH., et al. “A review on comparative study between the physicochemical and biological processes for paracetamol degradation”. Universal Journal of Pharmaceutical Research2.2 (2017): 9-13.
- Alajmi HM. “Effect of physical, chemical and biological treatment on the removal of five pharmaceuticals from domestic wastewater in laboratory-scale reactors and a full-scale plan”. Ph.D. thesis. University of Newcastle Upon Tyne. (2014): 50-87.
- Jones AL. “Mechanism of action and value of N-acetylcysteine in the treatment of early and late acetaminophen poisoning: A critical review”. Clin Toxicology36.4 (1998): 277-285.
- Sheen L., et al. “Paracetamol toxicity: Epidemiology, prevention and costs to the health-care system”. QJM 95.9 (2002):609-619.
- Bedner M and Maccrehan WA. “Transformation of acetaminophen by chlorination produces the toxicants 1,4-benzoquinone and N-acetyl-p-benzoquinone imine”. Environ Science Technology 40.2 (2006): 516-522.
- Fang W., et al. Study on Bacterial Function of High-Efficiency Paracetamol-Degrading Aerobic Granule. Master's thesis. Zhejiang University of Technology, Microbiology, China (2011): 1-15.
- Zhang L., et al. “Degradation of paracetamol by pure bacterial cultures and their microbial consortium”. Applied Microbiology and Biotechnology 97 (2013): 3687-3698.
- Langford K and Thomas K. “Determination of pharmaceutical compounds in hospital effluents and their contribution to wastewater treatment works”. Environment International35.5 (2009): 766-770.
- Philips P., et al. “Pharmaceutical formulation facilities as sources of opioids and other pharmaceuticals to wastewater treatment plant effluents”. Environmental Science & Technology44.13 (2010): 4910-4916.
- Muir N., et al. “Comparative bioavailability of aspirin and paracetamol following single dose administration of soluble and plain tablets”. Current Medical Research and Opinion 13.9 (1997): 491-500.
- Ikehata K., et al. “Degradation of aqueous pharmaceuticals by ozonation and advanced oxidation processes: A Review”. Ozone: Science & Engineering28.6 (2006): 353-414.
- Lorphensri O., et al. “Sorption and transport of acetaminophen, 17-alpha-ethynyl estradiol, nalidixic acid with low organic content aquifer sand”. Water Research 41 (2007): 2180-2188.
- Kim Y., et al. “Aquatic toxicity of acetaminophen, carbamazepine, cimetidine, diltiazem and six major sulfonamides, and their potential ecological risks in Korea”. Environment International33.3 (2007b): 370-375.
- Caliman F and Gavrilescu M. “Pharmaceuticals, personal care products and endocrine disrupting agents in the environment: A review”. Clean-Soil Air Water 37.4-5 (2009): 277-303.
- CBS News and Associated Press. Probe: Pharmaceuticals in drinking water, (2008): 1-3. November 6, (2009).
- Minnesota Department of Health (MDH). Acetaminophen in drinking water, (2014): 1-2. Available from Accessed March 17, (2016).
- Stackelberg EP., et al. “Efficiency of conventional drinking-water-treatment processes in removal of pharmaceuticals and other organic compounds”. Science of the Total Environment377.2-3 (2007): 255-272.
- Kleywegt S., et al. “Pharmaceuticals, hormones and bisphenol A in untreated source and finished drinking water in Ontario, Canada: Occurrence and treatment efficiency”. Science of the Total Environment409.8 (2011): 1481-1488.
- Frick E., et al. “Presence of pharmaceuticals in treated wastewater effluent and surface water supply systems, metropolitan Atlanta, Georgia, July–September 1999”. Proceedings of the March 26–27, 2001 Georgia Water Resources Conference, at the university of Georgia, Athens, Institute of Ecology (2001): 82-83.
- Westerhoff P., et al. “Fate of endocrine-disruptor, pharmaceutical, and personal care product chemicals during simulated drinking water treatment”. Environmental Science & Technology 39.17 (2005): 6649-6663.
- Intertox Inc. “Pharmaceuticals and endocrine disrupting compounds in water: A primer for public outreach”. Water Research Foundation 4387 (2015): 1–73.
- Rabiet M., et al. “Consequences of treated water recycling as regards pharmaceuticals and drugs in surface and ground waters of a medium-sized Mediterranean catchment”. Environmental Science & Technology 40.17 (2006): 5282-5288.
- Togola A and Budzinski H. “Multi-residue analysis of pharmaceutical compounds in aqueous samples”. Journal of Chromatography A1177.1 (2008): 150-158.
- Vulliet E., et al. “Occurrence of pharmaceuticals and hormones in drinking water treated from surface waters”. Environmental Chemistry Letters9.1 (2011): 103-114.
- Boleda MR., et al. “Behavior of pharmaceuticals and drugs of abuse in a drinking water treatment plant (DWTP) using combined conventional and ultrafiltration and reverse osmosis (UF/RO) treatments”. Environmental Pollution 159.6 (2011): 1584-1591.
- Heberer T. “Occurrence, fate and removal of pharmaceutical residues in the aquatic environment: A review of recent research data”. Toxicology Letters131.1-2 (2002): 5-17.
- Zimmerman MJ. “Occurrence of organic wastewater contaminants, pharmaceuticals, and personal care products in selected water supplies”. Geological Survey Open-File Report 1206 (2005): 1-16.
- Verstraeten IM., et al. “Use of tracers and isotopes to evaluate vulnerability of water in domestic wells to septic waste”. Ground Water Monitoring and Remediation 25.2 (2005): 107-117.
- Focazio MJ., et al. “A national reconnaissance for pharmaceuticals and other organic wastewater contaminants in the United States - II) Untreated drinking water sources”. Science of the Total Environment 402.2-3 (2008): 201-216.
- Barnes KK., et al. “A national reconnaissance of pharmaceuticals and other organic wastewater contaminants in the United States –I) Groundwater”. Science of the Total Environment402.2-3 (2008): 192-200.
- Fram MS and Belitz K. “Occurrence and concentrations of pharmaceutical compounds in groundwater used for public drinking-water supply in California”. Science of the Total Environment 409.18 (2011): 3409-3417.
- Erickson ML., et al. “Contaminants of emerging concern in ambient groundwater in urbanized areas of Minnesota, 2009–12”. Geological Survey Scientific Investigations Report 5096 (2014): 38.
- Radjenovic J., et al. “Rejection of pharmaceuticals in nanofiltration and reverse osmosis membrane drinking water treatment”. Water Research42.14 (2008): 3601-3610.
- Vulliet E and Cren-Olivé C. “Screening of pharmaceuticals and hormones at the regional scale, in surface and ground waters intended to human consumption”. Environmental Pollution 159 (2011): 2929-2934.
- Boyd RA and Furlong ET. “Human-health pharmaceutical compounds in Lake Mead, Nevada and Arizona, and Las Vegas Wash, Nevada, October 2000–August 2001”. US Geological Survey, Reston (2002): 1-18.
- Kolpin DW., et al. “Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999– 2000: A national reconnaissance”. Environmental Science & Technology36.6 (2002): 1202-1211.
- Kolpin DW., et al. “Urban contribution of pharmaceuticals and other organic wastewater contaminants to streams during differing flow conditions”. Science of the Total Environment 328.1-3 (2004): 119-130.
- Cahill DJ., et al. “Determination of pharmaceutical compounds in surface-and ground-water samples by solid-phase extraction and high-performance liquid chromatography–electrospray ionization mass spectrometry”. Journal of Chromatography A1041.1-2 (2004): 171-180.
- Stackelberg PE., et al. “Persistence of pharmaceutical compounds and other organic wastewater contaminants in a conventional drinking water treatment plant”. Science of the Total Environment 329.1-3 (2004): 99-113.
- Glassmeyer ST and Shoemaker JA. “Effects of chlorination on the persistence of pharmaceuticals in the environment”. Bulletin of Environmental Contamination and Toxicology74.1 (2005): 24-31.
- Alvarez D., et al. “Comparison of a novel passive sampler to standard water-column sampling for organic contaminants associated with wastewater effluents entering a New Jersey stream”. Chemosphere 61.5 (2005): 610-622.
- Snyder SA., et al. “Ozone oxidation of endocrine disruptors and pharmaceuticals in surface water and wastewater”. Science and Engineering: The Journal of the International Ozone Association28.6 (2006): 445-460.
- Zhang S., et al. “Simultaneous quantification of polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), and pharmaceuticals and personal care products (PPCPs) in Mississippi river water, in New Orleans, Louisiana, USA”. Chemosphere 66.6 (2007): 1057-1069.
- Conley JM., et al. “Spatial and temporal analysis of pharmaceutical concentrations in the upper Tennessee River basin”. Chemosphere 73.8 (2008): 1178-1187.
- Wiegel S., et al. “Pharmaceuticals in the river Elbe and its tributaries”. Chemosphere 57 (2004): 107-126.
- Nödlera K., et al. “Development of a multi-residue analytical method, based on liquid chromatography–tandem mass spectrometry, for the simultaneous determination of 46 micro-contaminants in aqueous samples”. Journal of Chromatography A 1217.42 (2010): 6511-6521.
- Gros M., et al. “Development of a multi-residue analytical methodology based on liquid chromatography-tandem mass spectrometry (LC-MS/MS) for screening and trace level determination of pharmaceuticals in surface and wastewaters”. Talanta 70.4 (2006): 678-690.
- Pedrouzo M., et al. “Pharmaceutical determination in surface and wastewaters using high-performance liquid chromatography-(electrospray)-mass spectrometry”. Journal of Separation Science30.3 (2007): 297-303.
- Ginebreda A., et al. “Environmental risk assessment of pharmaceuticals in rivers: Relationships between hazard indexes and aquatic macroinvertebrate diversity indexes in the Llobregat River (NE Spain)”. Environment International 36.2(2009): 1-10.
- Fernández C., et al. “Occurrence of pharmaceutically active compounds in surface waters of the Henares-Jarama-Tajo river system (Madrid, Spain) and a potential risk characterization”. Science of the Total Environment 408.3 (2010): 543-551.
- Gracia-Lor E., et al. “Multi-class determination of around 50 pharmaceuticals, including 26 antibiotics, in environmental and wastewater samples by ultra-high performance liquid chromatography–tandem mass spectrometry”. Journal of Chromatography A 1218.16 (2011): 2264-2275.
- Gros M., et al. “Fast and comprehensive multi-residue analysis of a broad range of human and veterinary pharmaceuticals and some of their metabolites in surface and treated waters by ultra-high-performance liquid chromatography coupled to quadrupole-linear ion trap tandem mass spectrometry”. Journal of Chromatography A 1248 (2012): 104-121.
- Bound J and Voulvoulis N. “Predicted and measured concentrations for selected pharmaceuticals in UK rivers: Implications for risk assessment”. Water Research40.15 (2006): 2885–2892.
- Kasprzyk-Hordern B., et al. “Multi-residue method for the determination of basic/neutral pharmaceuticals and illicit drugs in surface water by solid-phase extraction and ultra-performance liquid chromatography–positive electrospray ionisation tandem mass spectrometry”. Journal of Chromatography A 1161.1.2 (2007): 132-145.
- Kasprzyk-Hordern B., et al. “The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters”. Water Research43.2 (2009): 363-380.
- Choi K., et al. “Seasonal variation of several pharmaceutical residues in surface water and sewage treatment plants of Han River, Korea”. Science of the Total Environment405.1.3 (2008): 120-128.
- Sim WJ., et al. “Occurrence and fate of pharmaceuticals in wastewater treatment plants and rivers in Korea”. Environmental Pollution 158.5 (2010): 1938-1947.
- Grujic S., et al. “Determination of multiple pharmaceutical classes in surface and ground waters by liquid chromatographyeion trapetandem mass spectrometry”. Journal of Chromatography A 1216.25 (2009): 4989-5000.
- Lin YA and Tsai T. “Occurrence of pharmaceuticals in Taiwan's surface waters: Impact of waste streams from hospitals and pharmaceutical production facilities”. Science of the Total Environment407.12 (2009): 3793-3802.
- Al-Odaini NA., et al. “Multi-residue analytical method for human pharmaceuticals and synthetic hormones in river water and sewage effluents by solid-phase extraction and liquid chromatography–tandem mass spectrometry”. Journal of Chromatography A 1217.44 (2010): 6791-6806.
- Koreje K., et al. “From multi-residue screening to target analysis of pharmaceuticals in water: Development of a new approach based on magnetic sector mass spectrometry and application in the Nairobi River basin, Kenya”. Science of the Total Environment437 (2012): 153-164.
- Thomas KV., et al. “Source to sink tracking of selected human pharmaceuticals from two Oslo city hospitals and a wastewater treatment works”. Journal of Environmental Monitoring Home-A9.12 (2007): 1410-1418.
- Gracia-Lor E., et al. “Occurrence and removal of pharmaceuticals in wastewater treatment plants at the Spanish Mediterranean area of Valencia”. Chemosphere 87.5 (2012): 453-462.
- Roberts PH and Thomas K. “The occurrence of selected pharmaceuticals in wastewater effluent and surface waters of the lower Tyne catchment”. Science of the Total Environment356.1.3 (2006): 143-153.
- Gomez MJ., et al. “Determination of pharmaceuticals of various therapeutic classes by solid-phase extraction and liquid chromatography–tandem mass spectrometry analysis in hospital effluent wastewaters”.Journal of Chromatography A 1114.2 (2006): 224-233.
- Radjenovic J., et al. “Analysis of pharmaceuticals in wastewater and removal using a membrane bioreactor”. Analytical and Bioanalytical Chemistry 387.4 (2007): 1365-1377.
- Cruz-Morató C., et al. “Hospital wastewater treatment by fungal bioreactor: removal efficiency for Pharmaceuticals and Endocrine Disruptor Chemicals”. Science of the Total Environment493 (2014): 365-376.
- Yu JT., et al. “Occurrence and biodegradability studies of selected pharmaceuticals and personal care products in sewage effluent”. Agricultural Water Management 86.1.2 (2006): 72-80.
- Benotti JM and Brownawell JB. “Distributions of pharmaceuticals in an urban estuary during both dry and wet weather conditions”. Environmental Science & Technology41.16 (2007): 5795-5802.
- Foster AL. “Occurrence and fate of endocrine disruptors through the San Marco Wastewater treatment plant”. Thesis of Master of Science (2007): 6-23.
- Lubliner B., et al. Pharmaceuticals and personal care products in municipal wastewater and their removal by nutrient treatment technologies. Washington State Department of Ecology, Olympia, WA, (2010): 2-35.
- Kostich MS., et al. “Concentrations of prioritized pharmaceuticals in effluents from 50 large wastewater treatment plants in the U.S. and implications for risk estimation”. Environmental Pollution 184 (2014): 354-359.
- Wilcox JD., et al. “Removal of organic wastewater contaminants in septic systems using advanced treatment technologies”. Journal of Environmental Quality38.1 (2009): 149-156.
- Miege C., et al. “Removal efficiency of pharmaceuticals and personal care products with varying wastewater treatment processes and operating conditions–conception of a database and first results”. Water Science & Technology57.1 (2008): 49-56.
- Al-Rifai JH., et al. “Occurrence of pharmaceutically active and non-steroidal estrogenic compounds in three different wastewater recycling schemes in Australia”. Chemosphere 69.5 (2007): 803-815.
- Okuda T., et al. “Removal efficiency of 66 pharmaceuticals during wastewater treatment process in Japan”. Water Science & Technology57.1 (2008): 65-71.
- Han GH., et al. “Ecotoxicological risk of pharmaceuticals from wastewater treatment plants in Korea: Occurrence and toxicity to Daphnia magna”. Environmental Toxicology and Chemistry 25.1 (2006): 265-271.
- Snyder SA., et al. “Role of membranes and activated carbon in the removal of endocrine disruptors and pharmaceuticals”. Desalination 202.1.3 (2007): 156-181.
- Kosma IC., et al. “Occurrence and removal of PPCPs in municipal and hospital wastewaters in Greece”.Journal of Hazardous Materials 179.1.3 (2010): 804-817.
- Behera SK., et al. “Occurrence and removal of antibiotics, hormones and several other pharmaceuticals in wastewater treatment plants of the largest industrial city of Korea”. Science of the Total Environment409.20 (2011): 4351-4360.
- Lin AC., et al. “Fate of selected pharmaceuticals and personal care products after secondary wastewater treatment processes in Taiwan”. Water Science & Technology62.10 (2010): 2450-2458.
- Dutta K., et al. “Removal of pharmaceuticals and organic matter from municipal wastewater using two-stage anaerobic fluidized membrane bioreactor”. Bioresource Technology165 (2014): 42-49.
- Verlicchi P., et al. “Occurrence of pharmaceutical compounds in urban wastewater: Removal, mass load and environmental risk after a secondary treatment: A review”. Science of the Total Environment429 (2012b): 123-155.
- Verlicchi P., et al. “Hospital effluent: Investigation of the concentrations and distribution of pharmaceuticals and environmental risk assessment”. Science of the Total Environment430 (2012): 109-118.
- Kovalova L., et al. “Hospital wastewater treatment by membrane bioreactor: Performance and efficiency for organic micropollutant elimination”. Environmental Science & Technology46.3 (2012): 1536-1545.
- Santos HL., et al. “Contribution of hospital effluents to the load of pharmaceuticals in urban wastewaters: Identification of ecologically relevant pharmaceuticals”. Science of the Total Environment461.462 (2013): 302-316.
- Ba S., et al. “Synthesis and characterization of combined cross-linked laccase and tyrosinase aggregates transforming acetaminophen as a model phenolic compound in wastewaters”. Science of the Total Environment487 (2014): 748-755.
- Al-Qarni H., et al. “Investigating the removal of some pharmaceutical compounds in hospital wastewater treatment plants operating in Saudi Arabia”. Environmental Science and Pollution Research23.13 (2016): 13003-13014.
- Ternes TA. “Occurrence of drugs in German treatment plants and rivers”. Water Research32.11 (1998): 3245-3260.
- Khan SJ and Ongerth JE. “Occurrence and removal of pharmaceuticals at an Australian sewage treatment plant”. J Austral Water 32 (2005): 80-85.
- Brun GL., et al. “Pharmaceutically active compounds in Atlantic Canadian sewage treatment plant effluents and receiving waters, and potential for environmental effects as measured by acute and chronic aquatic toxicity”. Environmental Toxicology and Chemistry25.8 (2006): 2163-2176.
- Jones OAH., et al. “The occurrence and removal of selected pharmaceutical compounds in a sewage treatment works utilizing activated sludge treatment”. Environmental Pollution145.3 (2007): 738-744.
- Gomez MJ., et al. Pilot survey monitoring pharmaceuticals and related compounds in a sewage treatment plant located on the Mediterranean coast”. Chemosphere 66.6 (2007): 993-1002.
- Rosal R., et al. “Occurrence of emerging pollutants in urban wastewater and their removal through biological treatment followed by ozonation”. Water Research 44.2 (2010): 578-588.
- Pedrouzo M., et al. “Presence of pharmaceuticals and hormones in waters from sewage treatment plants”. Water, Air, & Soil Pollution217.1.4 (2011): 267-2681.
- Ganiyat M. The toxicological evaluation of sewage effluents and pharmaceuticals with the use of zebrafish as a model organism. Thesis of Master of Science Programme in Veterinary Medicine, Swedish University of Agricultural Sciences (2013): 1-50.
- Guzik U., et al. Intradiol dioxygenases–the key enzymes in xenobiotics degradation. In Chamy R, Rosenkranz F, eds. Biodegradation of Hazardous and Special Products (2013): 129-153.
- Guzik U., et al. “Microbial degradation non-steroidal anti-inflammatory drugs”. Postepy Mikrobiologii 53 (2014): 61-69.
- Hart A and Orr DL. “The degradation of paracetamol (4-hydroxyacetanilide) and other substituted acetanilides by a Penicillium species”. Antonie van Leeuwenhoek 41.3 (1975): 239-247.
- Ivshina IB., et al. “Catalysis of the biodegradation of unusable medicines by Alkanotrophic rhodococci”. Applied Biochemistry and Microbiology42.4 (2006): 392-395.
- Daubaras DL., et al. “Purification of hydroxyquinol 1, 2 dioxygenase and maleylacetate reductase: The lower pathway of 2, 4 and 5- trichlorophenoxyacetic acid metabolism by Burkholderia cepacia AC1100”. Applied and Environmental Microbiology62.11 (1996): 4276-4279.
- Gusseme BD., et al. “Degradation of acetaminophen by Delftia tsuruhatensis and Pseudomonas aeruginosa in a membrane bioreactor”. Water Research45.4 (2011): 1829-1837.
- Takenaka S., et al. “Metabolic pathway of 4-aminophenol in Burkholderia sp. strain AK-5 differs from that of aniline and aniline with C-4 substituents”. Applied and Environmental Microbiology69.9 (2003): 5410-5413.
- Moonen MJ., et al. “Hydroquinone dioxygenase from Pseudomonas fluorescens ACB: A novel member of the family of nonheme-iron (II)-dependent dioxygenases”. Journal of Bacteriology190.15 (2008): 5199-5209.
- Kolvenbach BA., et al. “Purification and characterization of hydroquinone dioxygenase from Sphingomonas sp. strain TTNP3”. AMB Express 1 (2011): 2-11.
- Akay C and Tezel U. “Biotransformation of acetaminophen by four phylogenetically distinct bacteria”. Proceedings CRETE 2016, Fifth International Conference on Industrial and Hazardous Waste Management. Chania – Crete – Greece; (2016): 27-30.
- Huang HH., et al. “Formation of glucoside conjugate of acetaminophen by fungi separated from soil”. European Journal of Drug Metabolism and Pharmacokinetics31.2 (2006): 103-108.
- Halling-Sorensen B., et al. “Occurrence, fate, and effects of pharmaceutical substances in the environment: A review”. Chemosphere 36.2 (1998): 357-393.
- Li J., et al. “Degradation and transformation products of acetaminophen in soil”. Water Research49 (2014): 44-52.
- Sariaslani FS. “Microbial enzymes for oxidation of organic molecules”. Critical Reviews in Biotechnology9.3 (1989): 171-257.
- Marchlewicz A., et al. “Over-the-counter monocyclic non-steroidal anti-inflammatory drugs in environment-Sources, risks, biodegradation”. Water, Air, & Soil Pollution(2015): 226-355.
Citation:
Wadhah Hassan A Edrees., et al. “Occurrence of Paracetamol in Aquatic Environments and Transformation by Microorganisms:
A Review”. Chronicles of Pharmaceutical Science 1.6 (2017): 341-355.
Copyright: © 2017 Wadhah Hassan A Edrees., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.