Research Article
Volume 2 Issue 1 - 2018
Locomotor inhibitory Activity of Some Murrayanine-Chalcone based 2, 3-dihydrobenzo[b] [1, 4] thiazepine Derivatives: Exploring Anxiolytic Potentials
1Department of Pharmaceutical Chemistry, Dadasaheb Balpande College of Pharmacy, Nagpur 440037, Maharashtra, India
2Department of Pharmaceutical Chemistry, Kamla Nehru College of Pharmacy, Nagpur 441108, Maharashtra, India
3Department of Pharmaceutical Chemistry, NRI Institute of Pharmacy, Bhopal 462021, Madhya Pradesh, India
2Department of Pharmaceutical Chemistry, Kamla Nehru College of Pharmacy, Nagpur 441108, Maharashtra, India
3Department of Pharmaceutical Chemistry, NRI Institute of Pharmacy, Bhopal 462021, Madhya Pradesh, India
*Corresponding Author: Debarshi Kar Mahapatra, Department of Pharmaceutical Chemistry, Dadasaheb Balpande College of Pharmacy, Nagpur 440037, Maharashtra, India.
Received: February 15, 2018; Published: February 27, 2018
Abstract
The seven-membered ring candidate benzothiazepine is a well-known scaffold that finds the majority of applications in hypnotic, sedative, anxiolytic, and other CNS associated therapeutics. Marketed drugs like diltiazem, clentiazem, and several other emerging molecules are the best example of this scaffold prototype. Murrayanine is the highly explored carbazole compound present in Indian curry plant Murraya koenigii L. (Rutaceae). A lot of heterocyclic hybrids have been synthesized and their pharmacotherapeutic significance has been revealed by our research group.
The current study involved the development of a number of seven-membered containing heterocycle; murrayanine-2-(substituted)-phenyl-2,3-dihydrobenzo[b] [1,4 ]thiazepine derivatives from murrayanine-chalcone, a previously reported component by our research group by cyclization reaction. The locomotor inhibitory activity was explored in Swiss albino rat which may be translated with anti-anxiety or hypnotic effects by the fabricated molecules. The compound 3g displayed the highest inhibition (60.17%) of the locomotor activity followed by compounds 3h and 3f. The analytical tools helped to determine the structure of proposed compounds. The position, number, and the type of substituent exerted a decisive role in mediating the biological activity. However, a crystal-clear SAR cannot be predicted from this study. The mode of action of the benzothiazepine analogs may be considered by enhancing the effect of GABA neurotransmitter at GABAA receptor to produce anxiolysis and hypnosis. The present research will definitely attract researchers across the world by providing clues for rational designing of natural product-based inhibitors with multifarious pharmacological activities.
Keywords: Murrayanine; Chalcone; Benzothiazepine; Heterocycle; Locomotor; Murraya koenigii
Introduction
The six-membered ring fused seven-membered ring candidates such as benzodiazepine, benzothiazepine, and benzoxazepine are the well-known scaffold that finds the majority of applications in hypnotic, sedative, anxiolytic, and other CNS associated therapeutics. [1] Benzothiazepines are the class of drugs which have multifarious pharmacological potentials such as anti-inflammatory [2], anti-retroviral [3], anti-cancer [4], anti-bacterial [5], anti-fungal [6], anti-malarial [7], anti-viral [8], anti-convulsant [9], anti-diabetic [10], anti-depressant [11], anti-oxidant [12], anti-hypertensive [13], hypolipidemic [14], etc. Marketed drugs like diltiazem, clentiazem, and several other emerging molecules are the best example of this scaffold prototype.
Murraya koenigiiL. is also known as Indian curry plant, belonging to the family Rutaceae. [15] Ethnopharmacological importance of the plant extracts of theroot, leaf, and stem bark include febrifuge, carminative, anti-helminthic, stomachic, purgative, astringent, etc. [16] Scientific studies over the years have reported the presence of therapeutically active carbazole moieties such as mahanine, bismahanine, isomahanine, euchrestine B, bispyrayafoline, bismurrayafoline, koenimbine, O-methylmahanine, O-methylmurrayamine A, mahaninebicine, mahaninebine, and murrayanine which displayed potent anti-oxidant, anti-bacterial, anti-fungal, anti-ulcerogenic, and immunomodulatory activities. [17] Murrayanine is the highly explored compound of this series and a lot of heterocyclic hybrids have been synthesized and their pharmacotherapeutic significance has been revealed by our research group. [18-25]
The current study involved the development of a number of seven-membered containing heterocycle; murrayanine-2-(substituted)-phenyl-2,3-dihydrobenzo[b] [1,4] thiazepine derivatives from murrayanine-chalcone, a previously reported component by our research group by cyclization reaction. The locomotor inhibitory activity was explored in Swiss albino rat which may be translated with anti-anxiety or hypnotic effects by the fabricated molecules.
Materials and Methods
Chemical and Instrumentation
The chemical reaction was initiated from “Murrayanine-Chalcone”, a chemical component previously reported by our research group. The chemical reagents and solvents of analytical grade were procured from Merck, Sigma-Aldrich, and HiMedia. The progress of the reaction was monitored by Merck Pre-coated silica gel G TLC plates. The CHN analyses were performed using PerkinElmer 2400 model Elemental Analyzer. The FT-IR spectra were recorded on Shimadzu® IRAffinity-1 infrared spectrometer using KBr methods and expressed in cm-1. The mass spectra were recorded on MICROMASS Q-TOF instrument. The proton (1H) NMR was recorded using Bruker Avance-II instrument using tetramethylsilane (TMS) as the internal standard and expressed in ppm relative to the internal standard.
The chemical reaction was initiated from “Murrayanine-Chalcone”, a chemical component previously reported by our research group. The chemical reagents and solvents of analytical grade were procured from Merck, Sigma-Aldrich, and HiMedia. The progress of the reaction was monitored by Merck Pre-coated silica gel G TLC plates. The CHN analyses were performed using PerkinElmer 2400 model Elemental Analyzer. The FT-IR spectra were recorded on Shimadzu® IRAffinity-1 infrared spectrometer using KBr methods and expressed in cm-1. The mass spectra were recorded on MICROMASS Q-TOF instrument. The proton (1H) NMR was recorded using Bruker Avance-II instrument using tetramethylsilane (TMS) as the internal standard and expressed in ppm relative to the internal standard.
Animals
The experiment was performed after receiving approval from the Department Ethical Committee (DEC) and CPCSEA (1389/a/10/CPCSEA) on Swiss albino rat (6 rats per group) of age 5-6 weeks and weight 180-280 g range. The animals were housed under good hygienic and controlled conditions where 24–25ºC temperature, humidity 50–60%, and 12 hr light and dark cycle was maintained. The rats were provided free access to water and standard rodent pellets.
The experiment was performed after receiving approval from the Department Ethical Committee (DEC) and CPCSEA (1389/a/10/CPCSEA) on Swiss albino rat (6 rats per group) of age 5-6 weeks and weight 180-280 g range. The animals were housed under good hygienic and controlled conditions where 24–25ºC temperature, humidity 50–60%, and 12 hr light and dark cycle was maintained. The rats were provided free access to water and standard rodent pellets.
Synthesis of target compounds
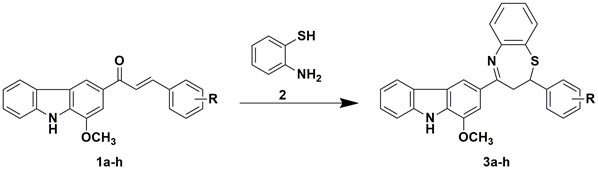
The fabrication of 2, 3-dihydrobenzo [b] [1, 4] thiazepine derivatives (3a-h) involved the transformation of murrayanine-chalcone (1a-h), the compound reported previously by our research group, by reacting with 2-aminothiophenol (2). The chemical reaction involved immediate modification of the carbonyl segment into the closed ring benzothiazepine form. The outline of chemical transformation is described in Scheme 1.
The fabrication of 2, 3-dihydrobenzo [b] [1, 4] thiazepine derivatives (3a-h) involved the transformation of murrayanine-chalcone (1a-h), the compound reported previously by our research group, by reacting with 2-aminothiophenol (2). The chemical reaction involved immediate modification of the carbonyl segment into the closed ring benzothiazepine form. The outline of chemical transformation is described in Scheme 1.
Synthetic protocol for 4-(1-methoxy-9H-carbazol-3-yl)-2-(substituted)-phenyl-2, 3-dihydrobenzo[b] [1,4] thiazepine(3a-h). The murrayanine-chalcone derivatives (1a-h) (0.1 M) was dissolved in ethanol media and reacted with equimolar quantity of 2-aminothiophenol (2) in presence of 7-8 drops of glacial acetic acid. The content was refluxed for 8-10 hrs and the progress of the reaction was inspected using TLC. After validating the completion of reaction, crushed ice was added into the reaction mixture to acquire the crude solid product. The product was filtered using Buchner’s funnel, washed carefully with cold water, and recrystallized from raw ethanol to achieve pure compound.
2-(2-fluorophenyl)-4-(1-methoxy-9H-carbazol-3-yl)-2, 3-dihydrobenzo[b] [1, 4] thiazine (3a) 37% yield; FTIR (KBr) υ (cm-1): 3247 (-NH), 3152 (C-H, aromatic), 1684 (C = N, aromatic), 1621 (C = C, aromatic), 1592 (-NH, bending), 1326 (C-N), 1258 (C-O), 1197 (C-F); 1H NMR (δ, ppm, CDCl3): 10.14 (9, 1H), 7.0-8.4 (Aromatic, 14H), 3.91 (13, 1H), 3.89 (1, 3H), 2.18 (12, 1H); MS: M+ 452. Anal. Calcd. For C28H21FN2OS: C, 74.31; H, 4.68; N, 6.19. Found: C, 73.92; H, 4.23; N, 5.88.
2-(4-fluorophenyl)-4-(1-methoxy-9H-carbazol-3-yl)-2,3-dihydrobenzo[b] [1,4] thiazepine (3b) 52% yield; FTIR (KBr) υ (cm-1): 3295 (-NH), 3129 (C-H, aromatic), 1644 (C = N, aromatic), 1638 (C = C, aromatic), 1554 (-NH, bending), 1314 (C-N), 1225 (C-O), 1150 (C-F); 1H NMR (δ, ppm, CDCl3): 10.19 (9, 1H), 7.1-8.6 (Aromatic, 14H), 3.88 (13, 1H), 3.81 (1, 3H), 2.13 (12, 1H); MS: M+ 452. Anal. Calcd. For C28H21FN2OS: C, 74.31; H, 4.68; N, 6.19. Found: C, 73.81; H, 4.15; N, 5.82.
2-(2-iodophenyl)-4-(1-methoxy-9H-carbazol-3-yl)-2,3-dihydrobenzo[b] [1,4] thiazepine (3c) 45% yield; FTIR (KBr) υ (cm-1): 3274 (-NH), 3135 (C-H, aromatic), 1661 (C = N, aromatic), 1617 (C = C, aromatic), 1579 (-NH, bending), 1331 (C-N), 1279 (C-O), 691 (C-I); 1H NMR (δ, ppm, CDCl3): 10.12 (9, 1H), 7.0-8.6 (Aromatic, 14H), 3.93 (13, 1H), 3.84 (1, 3H), 2.17 (12, 1H); MS: M+ 560. Anal. Calcd. For C28H21IN2OS: C, 60.01; H, 3.78; N, 5.00. Found: C, 59.26; H, 3.18; N, 4.43.
2-(4-iodophenyl)-4-(1-methoxy-9H-carbazol-3-yl)-2,3-dihydrobenzo[b] [1,4] thiazepine (3d) 61% yield; FTIR (KBr) υ (cm-1): 3232 (-NH), 3148 (C-H, aromatic), 1673 (C = N, aromatic), 1624 (C = C, aromatic), 1565 (-NH, bending), 1309 (C-N), 1262 (C-O), 658 (C-I); 1H NMR (δ, ppm, CDCl3): 10.16 (9, 1H), 7.0-8.4 (Aromatic, 14H), 3.96 (13, 1H), 3.79 (1, 3H), 2.11 (12, 1H); MS: M+ 560. Anal. Calcd. For C28H21IN2OS: C, 60.01; H, 3.78; N, 5.00. Found: C, 59.34; H, 3.27; N, 4.39.
2-(4-bromophenyl)-4-(1-methoxy-9H-carbazol-3-yl)-2,3-dihydrobenzo[b] [1,4] thiazepine (3e) 49% yield; FTIR (KBr) υ (cm-1): 3261 (-NH), 3127 (C-H, aromatic), 1651 (C = N, aromatic), 1631 (C = C, aromatic), 1583 (-NH, bending), 1335 (C-N), 1243 (C-O), 637 (C-Br); 1H NMR (δ, ppm, CDCl3): 10.11 (9, 1H), 7.0-8.4 (Aromatic, 14H), 3.87 (13, 1H), 3.73 (1, 3H), 2.21 (12, 1H); MS: M+ 512, M+2 514. Anal. Calcd. For C28H21BrN2OS: C, 65.50; H, 4.12; N, 5.46. Found: C, 64.76; H, 3.87; N, 5.08.
4-(1-methoxy-9H-carbazol-3-yl)-2-(2-(trifluoromethyl) phenyl)-2, 3-dihydrobenzo[b] [1, 4] thiazine (3f) 33% yield; FTIR (KBr) υ (cm-1): 3258 (-NH), 3166 (C-H, aromatic), 1649 (C = N, aromatic), 1611 (C = C, aromatic), 1598 (-NH, bending), 1317 (C-N), 1251 (C-O), 1118 (C-F); 1H NMR (δ, ppm, CDCl3): 10.21 (9, 1H), 7.2-8.4 (Aromatic, 14H), 3.83 (13, 1H), 3.75 (1, 3H), 2.14 (12, 1H); MS: M+ 502. Anal. Calcd. For C29H21F3N2OS: C, 69.31; H, 4.21; N, 5.57. Found: C, 68.57; H, 3.83; N, 5.18.
2-(3,5-bis(trifluoromethyl)phenyl)-4-(1-methoxy-9H-carbazol-3-yl)-2,3-dihydrobenzo[b] [1,4] thiazepine (3g) 40% yield; FTIR (KBr) υ (cm-1): 3244 (-NH), 3139 (C-H, aromatic), 1672 (C = N, aromatic), 1640 (C = C, aromatic), 1559 (-NH, bending), 1322 (C-N), 1216 (C-O), 1135 (C-F); 1H NMR (δ, ppm, CDCl3): 10.15 (9, 1H), 7.2-8.5 (Aromatic, 13H), 3.86 (13, 1H), 3.80 (1, 3H), 2.19 (12, 1H); MS: M+ 520. Anal. Calcd. For C29H20F4N2OS: C, 66.91; H, 3.87; N, 5.38. Found: C, 65.82; H, 3.59; N, 4.93.
2-(2,4-dichloro-5-fluorophenyl)-4-(1-methoxy-9H-carbazol-3-yl)-2,3-dihydrobenzo[b] [1,4] thiazepine (3h) 72% yield; FTIR (KBr) υ (cm-1): 3277 (-NH), 3163 (C-H, aromatic), 1656 (C = N, aromatic), 1607 (C = C, aromatic), 1564 (-NH, bending), 1312 (C-N), 1234 (C-O), 1141 (C-F), 753 (C-Cl); 1H NMR (δ, ppm, CDCl3): 10.13 (9, 1H), 7.1-8.7 (Aromatic, 12H), 3.97 (13, 1H), 3.89 (1, 3H), 2.17 (12, 1H); MS: M+ 520, M+2 522. Anal. Calcd. For C28H19FN2OS: C, 64.50; H, 3.67; N, 3.64. Found: C, 64.11; H, 3.24; N, 3.17.
Acute toxicity studies
Acute toxicity study was performed to determine the in vivo safety profile of the experimental compounds and to approximate the dose which will express the highest promising biological activity with no manifestation of any toxicity signs and symptoms. The compounds were injected at gradually escalating quantity ranging from 10 mg/kg to 100 mg/kg. The dose was calculated based on the death of 50% animals.
Acute toxicity study was performed to determine the in vivo safety profile of the experimental compounds and to approximate the dose which will express the highest promising biological activity with no manifestation of any toxicity signs and symptoms. The compounds were injected at gradually escalating quantity ranging from 10 mg/kg to 100 mg/kg. The dose was calculated based on the death of 50% animals.
Inhibition of locomotor activity: Exploring anti-anxiety effect
Using an actophotometer, the locomotor activity was studied comprehensively with little modification. For the experiment, each animal was placed separately in the actophotometer system containing photocells. For each experimental animal, the basal activity score was calculated after 10 and 20 min of drug administration. The locomotor activity on each rat was retested for 10 min. The difference in the locomotor activity values was recorded before and after the drug treatment. Finally, the reduction in locomotor activity was calculated and expressed in percentage.
Using an actophotometer, the locomotor activity was studied comprehensively with little modification. For the experiment, each animal was placed separately in the actophotometer system containing photocells. For each experimental animal, the basal activity score was calculated after 10 and 20 min of drug administration. The locomotor activity on each rat was retested for 10 min. The difference in the locomotor activity values was recorded before and after the drug treatment. Finally, the reduction in locomotor activity was calculated and expressed in percentage.
Statistical treatment
The obtained data were compared with the control group and analyzed statistically by one-way ANOVA method followed by Dunnett’s multiple comparisons test. A value P < 0.01 was considered statistically significant.
The obtained data were compared with the control group and analyzed statistically by one-way ANOVA method followed by Dunnett’s multiple comparisons test. A value P < 0.01 was considered statistically significant.
Result and Discussion
Chemistry
The alteration of chalcone into the seven-membered heterocycle benzothiazepine was supported by sophisticated analytical techniques. The conversion of the ketonic C = O moiety of the murrayanine-chalcone into the cyclic form was strongly confirmed by the disappearance of the characteristic ketonic peak in the FT-IR spectra, which appeared previously at 1670-1780 cm-1. The amide components present in carbazole and benzothiazepine moiety were largely characterized by absorption frequencies at (stretching) 3232-3298 cm-1 and 1554-1598 (bending) cm-1, respectively.
The alteration of chalcone into the seven-membered heterocycle benzothiazepine was supported by sophisticated analytical techniques. The conversion of the ketonic C = O moiety of the murrayanine-chalcone into the cyclic form was strongly confirmed by the disappearance of the characteristic ketonic peak in the FT-IR spectra, which appeared previously at 1670-1780 cm-1. The amide components present in carbazole and benzothiazepine moiety were largely characterized by absorption frequencies at (stretching) 3232-3298 cm-1 and 1554-1598 (bending) cm-1, respectively.
The features of the aromatic ring were noticed by stretching of C-H and C = C components at 3127-3166 cm-1 and 1607-1640 cm-1, respectively. The 1H-NMR studies demonstrated the discrete attributes of the prepared benzothiazepine molecules. The proton associated with the carbazole nitrogen appeared principally at 10 ppm. Chiefly, the aromatic ring hydrogens were positioned in the spectral range of 7.0-8.6 ppm. The protons of the methoxy group were perceived at 3.8-3.9 ppm in the NMR spectra. The mass spectra of the compounds revealed that base peaks matched exactly with the theoretical molecular mass of the molecules. The isotope forms of the chlorine and bromine were identified by the base peak + 2 molecular mass. Additionally, a few fragment peaks were noticed in the range of m/z 100-200. The determined ratios of carbon, hydrogen, and nitrogen illustrated the probable composition of the derivatives which indeed supported the fabrication of the molecules. These above spectroscopical results authenticate the formation of seven-membered heterocycles from murrayanine-chalcone.
Determination of LD50 value
The benzothiazepine derivatives were found to be relatively safe as no symptoms and signs of toxicity were detected over the administrated dose range of 10-100 mg/kg b.w. For carrying out the experiment, a fixed dose of 30 mg/kg b.w. was used for determining the locomotor inhibitory activity in Swiss albino rats.
The benzothiazepine derivatives were found to be relatively safe as no symptoms and signs of toxicity were detected over the administrated dose range of 10-100 mg/kg b.w. For carrying out the experiment, a fixed dose of 30 mg/kg b.w. was used for determining the locomotor inhibitory activity in Swiss albino rats.
Locomotor inhibitory activity
The experimental compounds demonstrated moderate to fairly high inhibitory effect in the treated animals by acting directly on the CNS interface. All the molecules displayed a nearly same degree of inhibitory potentials. The compound 3g displayed the highest inhibition (60.17%) of the locomotor activity, followed by compounds 3h and 3f which displayed inhibition of 57.97% and 54.98%, respectively. On focusing the structure-activity-relationships (SARs), it was observed that the number, position, and the type of substituent has a critical role in imparting pharmacological activity. In fluorine substituents (3a and 3b), the para-position was found to be more prevalent for locomotion inhibitory effect. In contrast, the iodine substituents (3c and 3d), the ortho-position was found to be more privileged. The phenomenon observed in the case of benzothiazepine was entirely opposite with that of data obtained in the case of benzodiazepine. In the case of bromo-substituent (3e), a moderate inhibitory activity in the rats was detected (48.22%). However, none of the compounds exhibited better or higher pharmacological activity than the standard drug, benzodiazepine (diazepam). The lipophilicity may be believed to be the crucial factor in the reduced biological activity of some of the analogs. The fall in the activity may be explained on the basis that the drug molecules distributed to all compartments of the body and are available in the least quantity in the CNS interface.[26] In other ways, it may be enlightened that the formation of micelles of the molecules or binding to the amino acid residues may produce obstruction in crossing the biological barrier.[27] The mode of action of the benzothiazepine analogs may be considered by enhancing the effect of GABA neurotransmitter at GABAA receptor to produce anxiolysis and hypnosis. The mechanism may be supposed to be pretty similar to that of benzodiazepine (diazepam).
The experimental compounds demonstrated moderate to fairly high inhibitory effect in the treated animals by acting directly on the CNS interface. All the molecules displayed a nearly same degree of inhibitory potentials. The compound 3g displayed the highest inhibition (60.17%) of the locomotor activity, followed by compounds 3h and 3f which displayed inhibition of 57.97% and 54.98%, respectively. On focusing the structure-activity-relationships (SARs), it was observed that the number, position, and the type of substituent has a critical role in imparting pharmacological activity. In fluorine substituents (3a and 3b), the para-position was found to be more prevalent for locomotion inhibitory effect. In contrast, the iodine substituents (3c and 3d), the ortho-position was found to be more privileged. The phenomenon observed in the case of benzothiazepine was entirely opposite with that of data obtained in the case of benzodiazepine. In the case of bromo-substituent (3e), a moderate inhibitory activity in the rats was detected (48.22%). However, none of the compounds exhibited better or higher pharmacological activity than the standard drug, benzodiazepine (diazepam). The lipophilicity may be believed to be the crucial factor in the reduced biological activity of some of the analogs. The fall in the activity may be explained on the basis that the drug molecules distributed to all compartments of the body and are available in the least quantity in the CNS interface.[26] In other ways, it may be enlightened that the formation of micelles of the molecules or binding to the amino acid residues may produce obstruction in crossing the biological barrier.[27] The mode of action of the benzothiazepine analogs may be considered by enhancing the effect of GABA neurotransmitter at GABAA receptor to produce anxiolysis and hypnosis. The mechanism may be supposed to be pretty similar to that of benzodiazepine (diazepam).
| Group | R | Photocell count in 10 min | % inhibition | Photocell count in 20 min | % inhibition |
| Control* | - | 402.8 ± 2.14 | - | 408.2 ± 2.36 | - |
| Standard# | - | 112.2 ± 0.29 | 72.15 | 103.6 ± 0.41 | 74.63 |
| 3a& | 2-F | 214.8 ± 1.72** | 46.68 | 202.2 ± 2.84* | 50.47 |
| 3b | 4-F | 205.4 ± 2.38* | 49.01 | 193.4 ± 1.55* | 52.63 |
| 3c | 2-I | 234.6 ± 1.63* | 41.76 | 219.2 ± 1.37** | 46.31 |
| 3d | 4-I | 244.6 ± 2.24* | 39.28 | 234.2 ± 2.61** | 42.63 |
| 3e | 4-Br | 226.2 ± 1.94** | 43.85 | 211.4 ± 1.43** | 48.22 |
| 3f | 2-CF3 | 196.4 ± 1.17** | 51.25 | 183.8 ± 1.76** | 54.98 |
| 3g | 3,5-CF3 | 173.6 ± 1.46** | 56.91 | 162.6 ± 2.99* | 60.17 |
| 3h | 2,4-Cl; 5-F | 185.6 ± 2.66* | 53.93 | 171.6 ± 1.21** | 57.97 |
Table 1: Locomotor inhibitory potential of benzothiazepine derivatives in rats.
*0.9% saline; #Benzodiazepine – 3 mg/kg b.w.; &Dose of 20 mg/kg b.w.; **P < 0.01, *P < 0.05; Values expressed as mean ± SEM, from 6 rats.
*0.9% saline; #Benzodiazepine – 3 mg/kg b.w.; &Dose of 20 mg/kg b.w.; **P < 0.01, *P < 0.05; Values expressed as mean ± SEM, from 6 rats.
Conclusion
The current research revealed the method to fabricate semi-synthetic heterocycles from natural product murrayanine having high limits of safety and efficacy. The compound 3g displayed the highest inhibition (60.17%) of the locomotor activity followed by compounds 3h and 3f. The analytical tools helped to determine the structure of proposed compounds. The position, number, and the type of substituent exerted a decisive role in mediating the biological activity. However, a crystal-clear SAR cannot be predicted from this study. The mode of action of the benzothiazepine analogs may be considered by enhancing the effect of GABA neurotransmitter at GABAA receptor to produce anxiolysis and hypnosis. The present research will definitely attract researchers across the world by providing clues for rational designing of natural product-based inhibitors with multifarious pharmacological activities.
Acknowledgement
Authors are highly thankful to Savitribai Phule Pune University, Pune, and Maharashtra, India for providing research grants (Grant No. 13PHM000126).
Authors are highly thankful to Savitribai Phule Pune University, Pune, and Maharashtra, India for providing research grants (Grant No. 13PHM000126).
References
- Mahapatra DK and Bharti SK. Handbook of Research on Medicinal Chemistry. 1st ed. Apple Academic Press, New Jersey, 2017.
- Mhaske GR., et al. “Synthesis and evaluation of novel 1, 5-benzothiazepine derivatives as antiinflammatory agents”. International Journal of Innovative Research in Science, Engineering and Technology 3.6 (2014): 13208-13215.
- Grandolini G., et al. “Synthesis of some new 1, 4-benzothiazine and 1, 5-benzothiazepine tricyclic derivatives with structural analogy with TIBO and their screening for anti-HIV activity”. European journal of medicinal chemistry 34.9 (1999): 701-709.
- Ameta KL., et al. “Synthesis and in vitro anti breast cancer activity of some novel 1, 5-benzothiazepine derivatives”. Journal of the Serbian Chemical Society 77.6 (2012): 725-731.
- Ceylan M., et al. “Synthesis, carbonic anhydrase I and II isoenzymes inhibition properties, and antibacterial activities of novel tetralone‐based 1, 4‐benzothiazepine derivatives”. Journal of biochemical and molecular toxicology 31.4 (2017).
- Dandia A., et al. “Efficient microwave enhanced solvent-free synthesis of potent antifungal agents: Fluorinated benzothiazepine fused β-lactam derivatives”. Journal of fluorine chemistry 128.5 (2007): 524-529.
- Dong CK., et al. “Identification and validation of tetracyclic benzothiazepines as Plasmodium falciparum cytochrome bc1 inhibitors”. Chemistry & biology 18.12 (2011): 1602-1610.
- Li T., et al. “Design, synthesis, and antiviral activities of 1, 5-benzothiazepine derivatives containing pyridine moiety”. European journal of medicinal chemistry 5 (2017): 657-662.
- Pandeya SN., et al. “Newer applications of 1, 5-benzothiazepines and their anticonvulsant activity”. Der Pharma Chemica 4.5 (2012): 1853-1855.
- Pei Y., et al. “Efficient Syntheses of Benzothiazepines as Antagonists for the Mitochondrial Sodium− Calcium Exchanger: Potential Therapeutics for Type II Diabetes”. The Journal of organic chemistry 68.1 (2003): 92-103.
- Díaz JA., et al. “Synthesis and antidepressant evaluation of new hetero [2, 1] benzothiazepine derivatives”. Archiv der Pharmazie 329.7 (1996): 352-360.
- Ansari FL., et al. “Syntheses and Biological Activities of Chalcone and 1, 5‐Benzothiazepine Derivatives: Promising New Free‐Radical Scavengers, and Esterase, Urease, and α‐Glucosidase Inhibitors”. Chemistry & biodiversity 2.4 (2005): 487-496.
- Inoue H., et al. “Synthesis of halogen-substituted 1, 5-benzothiazepine derivatives and their vasodilating and hypotensive activities”. Journal of medicinal chemistry 34.2 (1991): 675-687.
- Starke I., et al. “Benzothiazepine derivatives for the treatment of hyperlipidemia”. United States patent US (2006).
- Kumar VS., et al. “Murraya koenigii: A review”. Journal of Applied Research on Medicinal and Aromatic Plants 21 (1999):1139-1144.
- Bhandari PR. “Curry leaf (Murraya koenigii) or cure leaf: review of its curative properties”. Journal of Medical Nutrition and Nutraceuticals 1.2 (2012): 92-97.
- Anupam N., et al. “Review on chemistry and pharmacology of Murraya koenigii Spreng (Rutaceae)”. Journal of Chemical and Pharmaceutical Research 2.2 (2010): 286-299.
- Mahapatra DK., et al. “Substituted thiazole linked murrayanine-Schiff’s base derivatives as potential anti-breast cancer candidates: Future EGFR Kinase inhibitors”. International Journal of Pharmaceutical Science and Drug Research 9.3 (2017): 139-144.
- Mahapatra DK., et al. “Development of Murrayanine-Chalcone hybrids: An effort to combine two privilege scaffolds for enhancing hypoglycemic activity”. International journal of pharmaceutical chemistry and analysis 4.2 (2017): 30-34.
- Mahapatra DK., et al. “Design and characterization of Murrayanine linked Isoxazole derivatives: Novel class of bacteriocidal agents”. International Journal of Research in Drug & Pharmaceutical Science 1.1 (2017): 11-15.
- Mahapatra DK., et al. “Novel Murrayanine based Pyrazole analogs as emerging anti-fungal candidates: Design, synthesis, characterization, and in vitro evaluation”. Research Pharmaceutica 1.1 (2017): 1-5.
- Mahapatra DK and Shivhare RS. “Synthesizing an anti-oxidant principle 2-(((1-methoxy-9H-carbazol-3-yl) methylene) amino) isoindoline-1, 3-dione from N-aminophthalimide and murrayanine”. Inventi Med Chem 4 (2017): 1-3.
- Shivhare RS., et al. “Schiff’s base derivatives of murrayanine demonstrated enhanced anti-oxidant activity than its parent moiety”. Indian Journal of Pharmaceutical Education and Research 50.4 (2016): 9-15.
- Mahapatra DK., et al. “Murrayanine-hydantoin and -thiohydantoin analogs as promising anti-convulsant agents: Synthesis, Characterization and Molecular Docking Studies”. MOJ Bioorg Org Chem (2018).
- Mahapatra DK., et al. “Murrayanine-chalcone transformed into novel pyrimidine compounds demonstrated promising anti-inflammatory activity”. Asian Journal of Pharmaceutical Research (2018).
- Lemke TL and Williams DA. “Foye’s Principles of Medicinal Chemistry”. Lippincott Williams & Wilkins, (2012).
- Beale JM and Block JH. “Wilson and Gisvold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry”. Lippincott Williams & Wilkins (2011).
Citation:
Debarshi Kar Mahapatra., et al. “Locomotor inhibitory Activity of Some Murrayanine-Chalcone based 2, 3-dihydrobenzo[b]
[1, 4] thiazepine Derivatives: Exploring Anxiolytic Potentials”. Chronicles of Pharmaceutical Science 2.1 (2018): 462-468.
Copyright: © 2018 Debarshi Kar Mahapatra., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.